| Sahyadri Conservation Series: 23 |
ENVIS Technical Report: 53, May 2013 |
 |
Status of Forest in Shimoga District, Karnataka |
 |
1Energy and Wetlands Research Group, Centre for Ecological Sciences, Indian Institute of Science, Bangalore – 560012, India.
2Member, Western Ghats Task Force, Government of Karnataka, 3Member, Karnataka Biodiversity Board, Government of Karnataka
*Corresponding author: cestvr@ces.iisc.ac.in
STATUS OF WILDLIFE
Wild fauna diversity is one of the most gracious gifts of nature to the region.Wildlife has been diminishing at an alarming rate during recent years, particularly during the last 20-25 years as a result of deforestation, fragmentation of animal habitats, etc.Wildlife and wildlife habitat play a vital role in the ecological and biological processes that is essential to life itself. The functioning of the biosphere, and hence the maintenance and enhancement of human life, depends on countless inter actions among plants, animals and micro organisms.These ecological processes are essential for agriculture, forestry, fisheries and other endeavours necessary to human life. They also help maintain environmental quality by degrading and otherwise removing some pollutants and by preventing waste accumulation. Some of the biological processes in which wild species play a key role are pollination, germination seed dispersal, soil generation, nutrient cycling, predation, habitat maintenance, waste break down and pest control. Wildlife habitat regardless of whether it is upland or wetland habitat, is significant because of a number of functions it performs to support wildlife. Wildlife needs adequate space and habitat for the basic life requirements (Sameer Ali et.al 2007).
The primary step taken towards conservation and management measures is to preserve a small proportion of forest and declare it as bioreserve, wildlife sanctuary or national park. The criteria followed in this regard, involved prioritising regions based on naturalness, diversity, rarity and or uniqueness, and size. Such planned actions were aimed at preserving and conserving biodiversity and natural resources of a region/nation or at larger scale contributing to global biodiversity. At the same time, it helps in improving local biodiversity and the environment in and around such areas in a natural and protected environment (Sameer Ali et.al 2007). The areas having significant conservation value are declared as national parks and wildlife sanctuaries under the Wildlife (Protection) Act, 1972, which was amended in 1991. The Act specifies that, the state governments are empowered to declare any area as a sanctuary or a national park as per the procedures, for the purpose of protecting, propagating or developing wildlife or its environment. The National Parks and Wildlife Sanctuaries have been studied for ecological significance and to implement measures to conserve endemic and endangered species of flora and fauna.
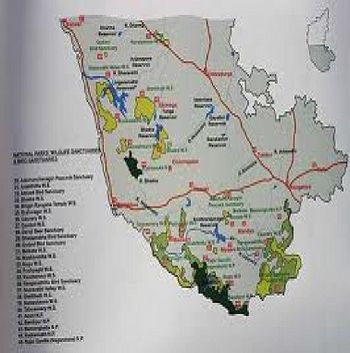
There are two wildlife sanctuaries and one bird sanctuary in Shimoga forest circle.
- Shettihalli Wildlife Sanctuary
- Sharavathi Valley Wildlife Sanctuary
- Gudavi Bird Sanctuary
1. SHETTIHALLI WILDLIFE SANCTUARY
Shettihalli wildlife Sanctuary with a spatial extent of 395.6 Sq.km is spread over parts of three taluks of Shimoga district: Shimoga, Hosnagara and Thirthahalli taluks (Figure 1). It is situated between 13° 40' to 14° 5' N and 75° 10' to 75° 35' E (Karnataka forest department, 2006). The vegetation in the region mainly consists of dry deciduous, moist deciduous and semi evergreen types. This Sanctuary was constituted under the government notification No.Afd.47.F.W.L.74 dt 31st October 1974. After declaration of Shettihalli wildlife Sanctuary, protection and development activities towards better management of wildlife had been initiated by wildlife wing of forest department.
The Karnataka Forest Act, 1963 and Rules 1969 regulate working in the forest areas. The State has 5 National Parks and 22 Wildlife Sanctuaries covering an area of 6576.76 sq. kms, which forms nearly 15.17% of the total forest area as protected area(http://karnatakaforest.gov.in). Wildlife (Protection) Act was enacted during 1972 by Government of India to provide for the protection of wild animals, birds and plants and with a view to ensuring the ecological and environmental security of the country.
 |
North: From Ayanur along the southern portion of Ayanur-Hosnagara road upto Rippenpet running from East to West. |
| South: From Konandur the boundaries of Riponpet, Hanagere and Thirthahalli range forest area upto Mandagadde running from West to East. |
| West: From Ripponpet along eastern portion of Ripponpet-Tirthahalli road including Mugudthi state forest upto Konandur which runs north to South. |
South-East: From Mandagadde along the western portion of Shimoga running from South to
North-East: Starting from Shimoga, the line runs along the western portion of Bangalore-Honnavar road upto Ayanur (Karnataka forest Department, 2006) |
| Figure 1: Shettihalli wildlife sanctuary, Shimoga |
The overall area of the sanctuary is plain to undulating with a few pockets consisting of very steep and undulating terrains and hillocks. These consist of perennial nalas and a number of small streams. The highest peak is Shankaragudda with an altitude of 1031 meters. The Sanctuary receives rainfall from south west monsoon. The intensity of rainfall is more during June to September with an average rainfall of 2000 mm. The average minimum and maximum temperatures are 12° C and 38º C respectively (Karnataka forest Department, 2006).
Figure 1: Shettihalli Wildlife Sanctuary
History
During early 20th century the forest within Sanctuary were under the control of Previously State of Mysore. For better management of forests and wildlife these forests were declared as ‘protected forests’ during 1905-1920. Table 1 details the spatial extent of forests in Shettihalli Wildlife Sanctuary.
Table 1: Forests in Shettihalli wildlife sanctuary
| Name of Forest |
Extent (in Ha) |
| Hanagere RF |
6755.0 |
| Kudi RF |
2730.0 |
| Harohitlu RF |
1795.00 |
| Masaruru RF-Block vii 1,2,3,6,7,8,9 |
1060.0 |
| Kumadhwathi RF |
3817.0 |
| Baruve RF- vii 12 to 14 |
807.0 |
| Mugudthi RF |
194.0 |
| Anesara RF |
1819.0 |
| Puradal RF |
2591.0 |
| Shankar RF |
9330.0 |
| Sacrebyle RF |
3886.0 |
| Arakere MF |
368.0 |
| Anupinakatte MF |
497.0 |
| Basavapure MF-XII 1 |
317.0 |
| Bedankalmatti MF-XII 2,3 |
693.0 |
| Keegadi MF-XIII 20 |
144.0 |
| Talale MF-XIV13 |
242.0 |
| Kullunde MF-XIV14 |
204.0 |
| Halasavala MF- XIV10 |
325.0 |
| Kanagalakoppa MF- XIV11 |
150.0 |
| Mandagadde MF-XIV8 |
223.0 |
| Bommenahalli XIV15 |
50.0 |
| Mandagatta MF |
942.0 |
| Kittanduru MF VIII23 |
294.0 |
| Bide MF VIII24 |
209.0 |
| Muniyur MF VIII 25 |
332.0 |
| Anupinakatte Pltn |
87.0 |
| Sacrebyle Pltn |
100.0 |
| TOTAL |
39560.00 |
During 1960-1965 Linganamakki reservoir was constructed in Sharavathi river valley, which led to submersion of many villages. The people affected by this Hydro-Electric project were shifted from protected area and allowed to settle in Shettihalli Wildlife Sanctuary. In this way more settlements of rehabilitated village come into existence in the sanctuary leading to encroachments and clearing of forests. The animals found in this sanctuary are Tiger, Panther, Wild elephant, Bison, Sambar, Spotted Deer, Barking deer, Mouse deer, wild pig, Porcupine, Sloth bear, Wild Cats etc. In Shettihalli Wildlife Sanctuary there are both natural forests and artificial plantations. It consists of 11 reserve forests, 14 minor forests and 3 plantation areas (Karnataka forest Department, 2006).
Vegetation:
Forest types:
- Southern tropical dry deciduous type: This type of forests is seen in Puradal, Anesara, Sacrebyle, Shankargudda, Kudi and part of Hanagere state forest. The top canopy consists of Terminalia tomentosa, Terminalia bellerica, Tectona grandis, Anogeissus latifolia, Lagerstroemia lanceolata etc. The second canopy consists of Wrightia tinctoria, Zizyphus zuzuba, Santalum album, Emblica officinalis, Cassia fistula, Shorea talura, Randia domatorum and bambbos etc. The ground floor consists of grassy patches.
- Southern tropical moist deciduous type: This type of forest is seen on the western side of the sanctuary i.e, part of Hanagere state forest, Kumadwathi state forest, Mugudthi state forest etc. Bambusa aurundanasea and Dendrocalamus strictus occur throughout the area.Terminalia tomentosa, Tectona grandis, Lagerstroemea lanceolata, Adina cardifolia, Dalbergia latifolia, Xylia xylocarpa, Grewia tiliafolia are the other species.
- Semi evergreen type: This type of forests is seen in parts of Hanagere state forests and Kumadwathi state forests. The importantspecies found areDipterocarpus, Hopea, Terminalia, Hopea, Xylia, Michelia and Bambusa species.
Plantations:The sanctuary has about 6000 Ha of Teak plantations.
Aquatic habitat:The Tunga reservoir bordering the sanctuary has a good population of otter, fish, and some crocodiles, water birds like Cormorants and Snake birds visit the river island near Mandagadde.
The butterfly diversityinvestigations in Tiger-Lion safari Thyaverekoppa, revealed the presence of 57 species of butterflies, representing 5 familiesPramod Kumar et.al, 2007. Papilionidae is represented by 5 genera and 10 species; Lycaenidae by 8 genera each with one species. Nymphalidae by 21 genera with 28 species; Pieridae by 7 genera and 8 species and hesperidae by 3 genera each with one species. The checklist of all species observed is given in Table 2.
Table 2: Butterflies along with their status in the Tiger-Lion safari, Thyavarekoppa
| Sl.No. |
Scientific name |
Common name |
Status |
|
Family Papilionidae |
| 1 |
Graphium agamemnon (Linnaeus) |
Tailed Jay |
C |
| 2 |
Graphium nomius (Esper) |
Spot Swordtail |
C |
| 3 |
Graphium sapedon (Linnaeus) |
Common Blue Bottle |
R |
| 4 |
Pachlioopta aristolochiae(Linnaeus) |
Common Rose |
R |
| 5 |
Pachlioopta hector (Linnaeus) |
Crimson Rose* |
VC |
| 6 |
Pachlioopta pandiyana (Moore) |
Malabar Rose* |
R |
| 7 |
Papilio demoleus (Linnaeus) |
Lime Butterfly |
VC |
| 8 |
Papilio polymnestor (Cramer) |
Blue Mormon** |
R |
| 9 |
Papilio polytes (Linnaeus) |
Common Mormon |
C |
| 10 |
Troides minos (Cramer) |
Southern Birdwing* |
R |
|
Family : Lycaenidae |
| 11 |
Alphnaeus vulcanus (Fabricius) |
Common Silverline |
R |
| 12 |
Arhopala amantes (Hewitson) |
Large Oak Blue |
R |
| 13 |
Castalius rosimon (Fabricius) |
Common Pierrot |
VC |
| 14 |
Discolampa ethion (Westwood) |
Banded Blue Pierrot |
C |
| 15 |
Jamides bochus (Stoll) |
Dark Cerulean |
C |
| 16 |
Lampides boeticus (Linnaeus) |
Pea blue |
C |
| 17 |
Talicada nyseus (Guerin-Meneville) |
Red Pierrot |
C |
| 18 |
Zizeeria karsandra (Moore) |
Common dark Grass Blue |
VC |
|
Family: Nymphalidae |
| 19 |
Acraea violae (Fabricius) |
Tawny Coster |
VC |
| 20 |
Ariadne merione (Cramer) |
Common Castor |
R |
| 21 |
Byblia ilithyia (Drury) |
Jocker |
C |
| 22 |
Cupha erymanthis (Drury) |
Rustic |
C |
| 23 |
Danaus chrysippus (Linnaeus) |
Plain Tiger |
R |
| 24 |
Danaus genutia (Cramer) |
Striped Tiger |
C |
| 25 |
Euploea core(Cramer) |
Common Indian Crow |
VC |
| 26 |
Hypolomnas bolina (Linnaeus) |
Great Eggfly |
R |
| 27 |
Hypolomnas misippus(Linnaeus) |
Danaid eggfly** |
C |
| 28 |
Junonia almana (Linnaeus) |
Peacock Pansy |
R |
| 29 |
Junonia atlites (Linnaeus) |
Gray Pansy |
R |
| 30 |
Junonia hierta (Fabricius) |
Yellow Pansy |
C |
| 31 |
Junonia iphita (Cramer) |
Chocolate Pansy |
C |
| 32 |
Junonia lemonias (Linnaeus) |
Lemon Pansy |
VC |
| 33 |
Junonia orithya (Linnaeus) |
Blue Pansy |
C |
| 34 |
Lethe rohria (Fabricius) |
Common tree brown |
R |
| 35 |
Melanitis leda (Linnaeus) |
Common Evening Brown |
VC |
| 36 |
Moduza procris (Cramer) |
Commander |
R |
| 37 |
Mycalesis patnia (Moore) |
Glade eye Bush brown** |
R |
| 38 |
Mycalesis perseus (Fabricius) |
Common Bush brown |
C |
| 39 |
Neptis hylas (Moore) |
Common Sailer |
VC |
| 40 |
Orsotrioena medus( Fabricius) |
Nigger |
C |
| 41 |
Phalanta phalantha (Drury) |
Common Leopard |
VC |
| 42 |
Polyura athamas (Drury) |
Common Nawab |
R |
| 43 |
Symphaedta nais (Forster) |
Baronet** |
VC |
| 44 |
Tanaecial lepidea (Butler) |
Grey Count |
R |
| 45 |
Tellewo limniace (Cramer) |
Blue Tiger |
VC |
| 46 |
Ypthima baldus (Fabricius) |
Common Four Ring |
VC |
|
Family: Pieridae |
| 47 |
Anaphaeis aurota(Fabricius) |
Pioneer |
VC |
| 48 |
Catopsilia pomona (Fabricius) |
Common Emigrant |
C |
| 49 |
Catopsilia pyranthe(Linnaeus) |
Mottled Emigrant |
VC |
| 50 |
Colotis danae (Fabricius) |
Crimson Tip |
R |
| 51 |
Delias eucharis (Drury) |
Common Jezebel** |
R |
| 52 |
Eurema hecabe (Linnaeus) |
Common Grass Yellow |
VC |
| 53 |
Hebomoea glaucippe (Linnaeus) |
Great Orange Tip |
R |
| 54 |
Valeria valeria (Joicey & Talbot) |
Common Wanderer |
C |
|
Family : Hesperiidae |
| 55 |
Borbo cinnara (Wallace) |
Rice Swift |
C |
| 56 |
Gangara thyrsid (Fabricius) |
Gaint Red Eye |
R |
| 57 |
Spialia galba (Fabricius) |
Indian Skipper |
R |
| VC - Very Common; C-Common; R- Rare |
|
|
| * - Endemic to Western Ghats; ** - Endemic to Peninsular India and Sri Lanka. |
Animals (Karnataka forest Department, 2006): 22 species of mammals (Table 3), 42 birds (Table 4), 10 reptiles (Table 5), 6 Amphibians (Table 6) and 16 fishes (Table 7) have been reported from the Sanctuary (Source: Shimoga wildlife division).
- Prey animals: Spotted deer, Sambar, Indian Gaur,Indian wild Boar, Indian porcupine, Hare and common langurs.
- Predators: Panthers, Tigers, Indian wild dogs, Pythons and King Cobras, Jackals, Hyenas and vultures.
- Other Animals:Elephants, Sloth bear, Malbar Squirrel, Monkeys, Tortoise.
Table 3: Mammals of Shettihalli wildlife Sanctuary
| Sl. No. |
Species name |
Common names |
| 1 |
Macaca sinica |
The Bonnet monkey |
| 2 |
Pithecus entellus |
Hanuman monkey |
| 3 |
Loris lydekkerianus |
Slender loris |
| 4 |
Felis affinis |
The tiger |
| 5 |
Felis affinis |
The Jungle cat |
| 6 |
Acinonyx venaticus |
The hunting leopard |
| 7 |
Mangos mungo mungo |
Indian mungoose |
| 8 |
Canis indicus |
The Indian Jackal |
| 9 |
Lutra lutra |
The common Otter |
| 10 |
Melurus ursinus |
The sloth bear |
| 11 |
Tragullus meminna |
Mouse deer |
| 12 |
Pteropus giganteus |
The Indian flying fox |
| 13 |
Lyroderma lyra lyra |
Vampire bat |
| 14 |
Petaurista philippensis |
South Indian flying squirrel |
| 15 |
Seiurus malabaricus |
The Malabar Squirrel |
| 16 |
Seirus |
The Bison |
| 17 |
Muntiacus vaginalis |
The barking deeer |
| 18 |
Rosa unicolor |
The sandbur |
| 19 |
Axis |
The spotted deer |
| 20 |
Sues cryostats |
The Indian Wild Boar |
| 21 |
Hystrix leucra |
The Indian Porcupine |
| 22 |
Manis crassicaudata |
The Indian Pangolin |
Table 4: Birds of Shettihalli Wildlife Sanctuary
| Sl. No. |
Species name |
Common Name |
| 1 |
Corvus macrorhynchos |
Jungle Crow |
| 2 |
Palaeornis torquatus |
Common Indian parrot |
| 3 |
Neopharon ginginianus |
Vulture |
| 4 |
Haliastur indus |
Brahminy kite |
| 5 |
Crocopus chlorogaster |
Green pigeon |
| 6 |
Columba intemedia |
Blue rock pigeon |
| 7 |
Pavo cristatus |
Pen fowl |
| 8 |
Gallus sonnerati |
Gray jungle fowl |
| 9 |
Gallooerdix spadicea |
Red Sour Fowl |
| 10 |
Francolinus pondicerians |
Gray patridge |
| 11 |
Sarkidiornis melanotos |
Comb Duck |
| 12 |
Dendrocygna javanica |
The Whistling teal |
| 13 |
Nettium crecea |
Common teal |
| 14 |
Gallus bankiva murgi |
Red jungle fowl |
| 15 |
Dendrocitta rufa |
Tree pie |
| 16 |
Dumetia hyperithra |
The Rufous-Hellied Babbler |
| 17 |
Otocompusa jocose fascucaudata |
Southern Red Whiskered Bul Bul |
| 18 |
Saxicolodes cambaiensis |
India Robin |
| 19 |
Pycnonotus luteolus |
White browed bulbul |
| 20 |
Terpsiphone paradisi |
Paradise flycatcher |
| 21 |
Cyornis tickellioe |
Blue flycatcher |
| 22 |
Tephrodornis pondiceriana |
common woodshrike |
| 23 |
Pericocotus speciosus |
The Scarlet minivet |
| 24 |
Dicururus macrocerus |
The king Crow |
| 25 |
Dissemurus sctorius |
The Rocket tailed drongo |
| 26 |
Orthotomus sctoricus |
The Tailor Bird |
| 27 |
Acredotheres trestis |
The Common myna |
| 28 |
Gymnoris xanthocolis |
Yellow throated Sparrow |
| 29 |
Hirindo rustica |
The Common Sallow |
| 30 |
Hirundo filifera |
Wire tailed Swallow |
| 31 |
Dicoem erythrorthyncum |
Tikells Flower peacker |
| 32 |
Leopicus blanfordil |
Yellow frinted pied wood pecker |
| 33 |
Centropus parroti |
Southern crow pheasant |
| 34 |
Alcedo benghalensis |
Common king fisher |
| 35 |
Sarcogyps calvus |
Black Vulture |
| 36 |
Astur dussumier |
The Indian shikhara |
| 37 |
Oenopopelia transquebarica |
The red turtle dove |
| 38 |
Amauromis phoenicurus |
The white breasted water hen |
| 39 |
Bulbulcus coromandus |
Cattle egret |
| 40 |
Nettion crecca |
The common teal |
| 41 |
Demdrocygna javanica |
common whistling teal |
| 42 |
Niroca rufa |
The white Bye |
Table 5: Reptiles found in Shettihalli Wildlife Sanctuary
| Sl. No. |
Species name |
Common Name |
| 1 |
Crocodilus palustris |
The Mugger |
| 2 |
Testudo elegans |
The land Tortoise |
| 3 |
Gonotodes mysorenisi |
The Monitor lizard |
| 4 |
Calotes versicolor |
|
| 5 |
Chameleon calcaratus |
Chameleon |
| 6 |
Python molures |
Python |
| 7 |
Tropidinotus stolatus |
Common Green Snake |
| 8 |
Bugarus coeruleus |
Krait |
| 9 |
Naja tripudians |
Cobra |
| 10 |
Vipera resselli |
Viper King cobra |
Table 6: Amphibians of Shettihalli Wildlife Sanctuary
| Sl. No. |
Species name |
Common Name |
| 1 |
Rana hexadactyla |
Green tank frog |
| 2 |
Rana tigrina |
Bull frog |
| 3 |
Rana cyanophlyctis |
Skipper frog |
| 4 |
Rana malabarica |
The Tree frog |
| 5 |
Rhacophorus pleuroxtictus |
Tree frog |
| 6 |
Rana verrucosa kalloula |
The Plantain frog |
Table 7: Fishes of Shettihalli Wildlife SanctuarySanctuary
| Sl. No. |
Species name |
Common Name |
| 1 |
Clarias batrachus |
The Black cat fish |
| 2 |
Saccobranchus fossillis |
Scorpin fish |
| 3 |
Wallago attu |
|
| 4 |
Callichrous bimaculatus |
Butter fish |
| 5 |
Pseudotropius atheronoides |
Lady fish |
| 6 |
Macrones vittatus |
Pidler |
| 7 |
Macrenes ao |
|
| 8 |
Macrenes kelitius |
|
| 9 |
Barbus tor |
|
| 10 |
Barbus neilli |
|
| 11 |
Berbus sarana |
|
| 12 |
Labeo kontius |
|
| 13 |
Labeo boga |
|
| 14 |
Mastocembalus armatus |
|
| 15 |
Ophioce halus puntatus |
|
| 16 |
Oleucopunctatus gachua |
|
Social Aspects: The sanctuary has 32 enclosures and 70 villages inside the sanctuary. The size of the revenue enclosure varies from few house hold to a maximum of 110 household.95% of the people are dependent on agriculture. There are about 383 families and 616.18 Ha of encroachment before 1978 and 1292 families and 989.43 Ha after 1978 within the sanctuary, totally occupying 1605.61 Ha of the forest. (Karnataka forest Department, 2006).
Encroachment: There are about 383 families and 616.18 Ha of encroachment before 1978 and 1992. 989.46 Ha after 1978 within the sanctuary who occupied 1605.61 Ha. (Karnataka forest Department, 2006)
Park Zonations:The Sanctuary is classified into zones as per the norms, for better management of the sanctuary. The details of Zonations are as follows:
- Core Zone: This Zone comprises part of Hanagere state forest and part of Shankar state forest, excluding the enclosures. The area of core zone is 100.60 Sq.Km.
- Buffer Zone: This Zone includes Purdal state forest, part of Anesara and Shankar state forest, entire Sacrebyle, Kudi and Kumadwathi state forests, Harohithlu, Masarur, Baruve, Mugudthi state forests. Excluding enclosure the total area of buffer zone is 237.4 Sq.Km.
- Tourism Zone: It includes parts of Shankar, Kudi, Sacrebyle and Kumdwathi state forests. Tourism zone also includes Lion safari at Thyavarekoppa, Elephant camp at Sacrebyle and Bird Sanctuary at Mandagadde. The total area is 57.60 Sq.Kms.
Tourism: The following are the existing tourism facilities:
- Tiger and Lion safari, Thyavarekoppa: Tiger and lion Safari was established in the year 1988 at Thyavarekoppa. A safari park is a place of education, enterainment and enlightment and a breeding place of endangered species. The total extent of safari is 195.0 hectares.
- Sacrebyle elephant camp: It is situated on Shimoga-Mangalore highway and it is 14 Kms from Shimoga city. There are 19 elephants and 2 calves at present.
- Mandagadde Bird Sanctuary: It is about 30 Kms from Shimoga on the left side of Shimoga-Tirthahalli main road.This is an island in Thunga River and 1.14 Acre in extent. This ia a place for the migratory birds which come for breeding and feeding.
6.3 SHARAVATHI VALLEY WILDLIFE SANCTUARY
Geographically Sharavathi Valley Wildlife Sanctuarylocated between 13° 54’ to 14˚ 12’ North and 74° 38’ to 75˚ 00’ Eastin central Western Ghats region of Karnataka state (Figure 2).Sharavathi Valley Wildlife Sanctuary was notified vide Government order No. AFD70/FWL71/ Dated 20.04.1972 and has an area of 431.23 Sq. Kms. with a final notification No. AFD/12/FWL/74 Dated 27.06.1974. It is spread over in the Sharavathi River Valley of Sagar Taluk in Shimoga District. The area of the Sanctuary is 431.23 Sq. Kms out of which an area of 123.63 Sq. Kms is under the water spread of Sharavathi Reservoir. The Sanctuary lies in the Western Ghats, mainly covered with evergreen and semi-evergreen forests in the valleys and grassy patches on hill tops, and are immensely rich in flora and fauna both in variety and diversity. The boundaries of the sanctuary are as follows: Jog S.F., Thalakalale Reservoir and Karagal S.F. form the northern boundary of the Sanctuary.Eastern boundary of Sharavathi Reservoir forms the Eastern boundary of the Sanctuary. The southern part consists of Mukambika Wildlife Sanctuary and North Canara District boundary. Common boundary of Shimoga and North Canara district forms Western boundary of the Sanctuary. The area is highly undulating with altitudinal range of 94 mts. MSL at Nagavalli to 1102 mts. MSL at Edigudda and consists of valleys and hillocks. The area is marked by perennial nalas and a large number of small streams. The forests are rich with evergreen and semi evergreen species and dense undergrowth.The climate is of monsoon type. The intensity of rain fall is more during June to September by the regular south west monsoon. The break of the monsoon is attended by high velocity wind. The temperature varies from 11˚ C to 38˚ C depending upon the factor of elevation.The rainfall, particularly during monsoon, is very heavy. The sanctuary is exposed to torrential showers during April, May and October with heavy showers in June, July and August. The erosive action of the torrential rain can be noticed in open areas. In areas devoid of vegetation cover the, top soil gets washed out with water resulting in unproductive, barren lateritic surfaces. The average rainfall of the area is 4500 mm.
Brief history
This sanctuary area previously came under the control of the princely State of Mysore. The MysoreKingdom had shown keen interest towards the protection of forests, and for better management of forests and wild-life, all the forest areas had been declared as State Forests during 1905-1940. Since then, these forests have been managed in a systematic way for fulfilling the needs of people.During 1964-65, Linganamakki dam was constructed across the Sharavathi River which led to submersion of many villages and forest areas. The people affected by this hydro-electric project were shifted from the project area and allowed to settle in various other places. After the submersion of forest area many big and small Islands has created. The study of these Islands shows good vegetation due to least biotic pressure and inaccessible. There are 31 Islands found in the sanctuary (Karnataka forest Department, 2006).
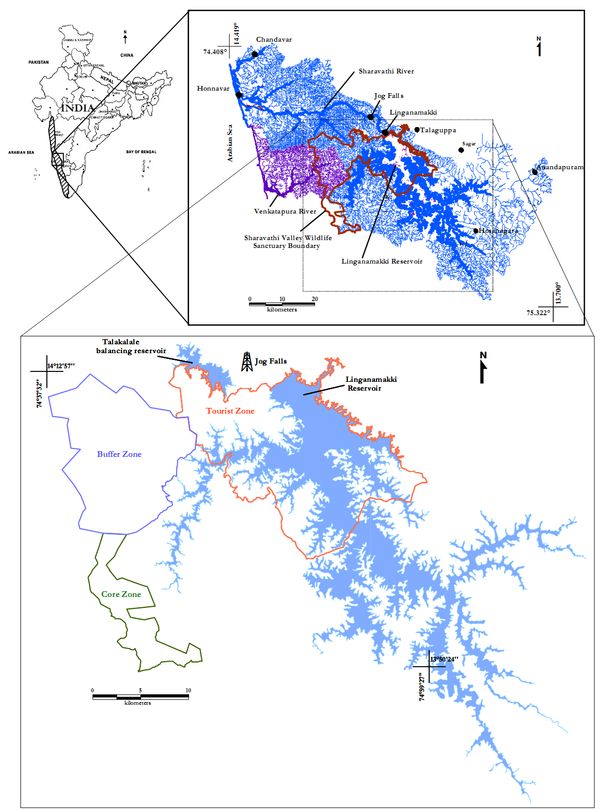
Figure 2.1: Sharavathi Valley Wildlife Sanctuary
After the enactment of the Wildlife (protection) Act, 1972 and Forest Conservation Act, 1980, more emphasis has been given for protection of wildlife and in creating awareness among the people about the need to conserve wildlife. Then onwards the protection and development activities towards the better management of wildlife in the sanctuary were commenced and continued by the wildlife wing of the Forest Department. With the handing over of the sanctuary areas to the wildlife wing by the territorial wing, these activities have been further intensified in a systematic way.Thewildlife division has been functioning independently since 01.08.1993 after taking over of 6 State Forests, submersion area and islands, from Sagar Territorial Division. Details of State Forests and other areas coming under Sharavathi Valley Wildlife Sanctuary are given in Table 81.
Table 8.1:Details of forests in Sharavathi valley wildlife sanctuary
| Sl. No. |
Name of the forest |
Legal status |
Block No. |
Compartment No. |
Area in Ha. |
| 1 |
Govardhanagiri |
SF |
XX |
1–34 (34) |
13473.68 |
| 2 |
Karini |
SF |
XXI |
1-17 (17) |
5102.53 |
| 3 |
Muppane Bl. A |
SF |
XIX |
4,5,6,7 (4) |
961.77 |
| 4 |
Muppane Bl. B |
SF |
XIX |
8,9,10,11 (4) |
629.16 |
| 5 |
Channagonda (part) |
SF |
XIX |
13 (part) |
701.05 |
| 6 |
Attigodu |
SF |
XIX |
1,2,3 (3) |
763.70 |
| 7 |
Submerged area |
|
|
|
12363.00 |
| 8 |
Islands |
|
|
|
507.00 |
| 9 |
Others |
|
|
|
8621.11 |
| |
TOTAL |
|
|
|
43123.00 |
Land-use analysis was done using maximum likelihood classifier and percentage compositions of various categories of land-use are listed in Table 8.2; the same is depicted in Figure 2.2. The forest cover in the sanctuary is about 49.5% and 17.6% is water body at full level of the reservoir.
Table 8.2: Land-use analysis (%area) in SVWS.
| Classification |
Area (%) |
| Built-up |
8.62 |
| Evergreen to semi-evergreen |
35.63 |
| Moist-deciduous |
13.84 |
| Plantation (Areca/Acacia/Casuarina) |
15.27 |
| Water body |
17.64 |
| Agriculture |
2.66 |
| Open land |
6.35 |

Figure 2.2: Land-use in Sharavathi Valley Wildlife Sanctuary
Vegetation types inside the sanctuary area including islands (in the reservoir) vary from grassland to evergreen forest. The vegetation type in the core zone and buffer zone varies from moist-deciduous to evergreen forest. But in few places, grasslands, especially on hill tops, are interspersed with evergreen forests. Scrub jungles to semi-evergreen forests are more prevalent in the tourist zone of the sanctuary. The species richness suggests that semi-evergreen forests have more species due to the combined presence of both evergreen and deciduous species. The evergreen forest in the sanctuary area is more fragmented and disturbed and this is clearly depicted in the Shannon’s diversity index. The percentage evergreenness and endemic plants are more in the evergreen forest area.
The sanctuary has a variety of habitats that support rich flora of herbs, shrubs and climbers of which about 215 species have been recorded. Evergreen to semi-evergreen forests and grasslands of the Western Ghats have the largest congregations of endemic herbs. Some of the herbs are exclusive to specialised habitats like tree trunks and wet rocks. The increasing human impact and openings in forest canopy as well as over grazing are posing threats to many of these rare plants.
Evergreen to semi-evergreen forests are the major source of perennial waters. On the other hand in the deciduous tract, the streams mostly dry up in the summer months. Therefore conservation of evergreen forests and restoration of such forests are of paramount importance. Bulk of the water flow into reservoir comes from natural forests. Unfortunately, in some parts of the sanctuary area, monoculture plantations have been raised causing the drying up of streams and impoverishment of the ecosystems as a whole. Since the plantations do not yield any fodder or NTFP, the rural population is put to great hardship. Therefore such land-uses are not desirable in the sanctuary area. The numerous streams and the banks of Sharavathi and Venkatapura rivers and their tributaries in the evergreen to semi-evergreen forest belt are lined with characteristic riparian vegetation of which the notable tree species are Calophyllum apetalum, Elaeocarpus tuberculatus, Mastixia arborea, Hydnocarpus laurifolia, Madhuca neriifolia, etc. Towards the drier forests, water bodies are lined with tree species such as Pongamia pinnata, Madhuca neriifolia, Hopea wightiana, Bambusa sp., etc. The riparian vegetation plays a crucial role in protecting the water bodies from siltation, creating shade conditions to maintain appropriate temperature regime for sustaining populations of endemic fishes, amphibians, phytoplankton, zooplankton and aquatic insects. Of late there has been numerous instances of misuse of the banks of streams and rivers in the catchment area causing severe upsets in the characteristic biota associated with them. Stream waters are often diverted to newly created horticultural farms, thereby, affecting the water flow into the reservoir. During field observations, it was noticed that the endemic vegetation patches were associated with perennial streams. The estimated basal area per hectare is highest for evergreen forests and is decreasing from semi-evergreen to scrub. But higher Shannon diversity for semi-evergreen compared to evergreen, may be due to disturbances and canopy openings, which pave way for the addition of some pioneers and other secondary species. Plant species of the sanctuary are listed in Table 8.3 and the percentage evergreens and percentage endemics range from evergreen towards moist deciduous (Table 8.4).
Table 8.3: Plant species in SVWS
| Species name |
Family |
Habit |
Distribution |
| Abrus pulchellus |
Faboideae |
Climber |
Oriental and Paleotropic |
| Abutilon persicum |
Malvaceae |
Shrub |
Oriental-Indomalaysia |
| Acanthospermum hispidum |
Asteraceae |
Herb |
Neotropic |
| Achyranthus aspera |
Amaranthaceae |
Herb |
Pantropical |
| Acronychia pedunculata |
Rutaceae |
Tree |
Oriental-Indomalaysia |
| Actinodaphne hookeri |
Lauraceae |
Tree |
Oriental-W. Ghats |
| Adhatoda zeylanica |
Acanthaceae |
Shrub |
Oriental-Indomalaysia |
| Aeginetia indica |
Orobanchaceae |
Herb |
Indomalaysia to Japan |
| Aerides maculosum |
Orchidaceae |
Herb |
Oriental-W. Ghats |
| Aeschynomene aspera |
Faboideae |
Herb |
Paleotropics |
| Aganosma cymosa |
Apocynaceae |
Climber |
Oriental-India, Sri Lanka |
| Aglaia anamallayana |
Meliaceae |
Tree |
Oriental-W. Ghats |
| Aglaia roxburghiana |
Meliaceae |
Tree |
Oriental-Indomalaysia |
| Aglaia sp(bark not red) |
Meliaceae |
Tree |
|
| Aglaia sp (red bark big leaf) |
Meliaceae |
Tree |
|
| Alangium salvifolium |
Alangiaceae |
Straggler |
Oriental-W. Ghats |
| Allophylus cobbe |
Sapindaceae |
Shrub |
Oriental-S. India, Sri Lanka, |
| Alpinia malaccansis |
Zingiberaceae |
Herb |
Oriental-Indomalaysia |
| Alseodaphne semicarpifolia |
Lauraceae |
Tree |
Oriental-W. Ghats, Sri Lanka |
| Alstonia scholaris |
Apocynaceae |
Tree |
Oriental to Australian |
| Alysicarpus bupleurifolius |
Faboideae |
Herb |
Indomalaysia, China |
| Ammannia baccifera |
Lythraceae |
Herb |
Paleotropics |
| Amoora polystachia |
Meliaceae |
Tree |
Oriental-India, Sumatra |
| Amorphophallus bulbifer |
Araceae |
Herb |
India, Burma |
| Anamirta cocculus |
Menispermaceae |
Climber |
Oriental-Indomalaysia |
| Ancistrocladus heyneanus |
Ancistrocladaceae |
Climber |
Oriental-W. Ghats |
| Andrographis ovata |
Acanthaceae |
Herb |
|
| Species name |
Family |
Habit |
Distribution |
| Angelonia biflora |
Scrophulariaceae |
Herb |
S America |
| Anisomeles indica |
Lamiaceae |
Undershrub |
Indomalaysia, China |
| Annonaceae member |
Annonaceae |
Climber |
|
| Antidesma menasu |
Euphorbiaceae |
Tree |
Oriental-W. Ghats |
| Apama siliquosa |
Aristolochiaceae |
Shrub |
Oriental-W. Ghats, Sri Lanka |
| Aphyllorchis montana |
Orchidaceae |
Herb |
Oriental-W. Ghats |
| Ardisia solanacea |
Myrsinaceae |
Shrub |
Oriental-Peninsular India |
| Arenga wightii |
Arecaceae |
Palm |
Oriental-W. Ghats |
| Argostemma courtallense |
Rubiaceae |
Herb |
Oriental-W. Ghats |
| Argostemma verticillatum |
Rubiaceae |
Herb |
Oriental-India |
| Arisaema tortuosum |
Araceae |
Herb |
Oriental- Himalayas, W.Ghats |
| Aristolochia indica |
Aristolochiaceae |
Climber |
Oriental-India, Sri Lanka |
| Artabotrys zeylanicus |
Annonaceae |
Sca.shrub |
Oriental-W. Ghats, Sri Lanka |
| Artocarpus gomezianus |
Moraceae |
Tree |
Oriental-W. Ghats, Sri Lanka |
| Artocarpus heterophyllus |
Moraceae |
Tree |
Oriental-W. Ghats |
| Artocarpus hirsutus |
Moraceae |
Tree |
Oriental-W. Ghats |
| Asclepiadaceae member_1 |
Asclepiadaceae |
Climber |
|
| Asclepias curassavica |
Asclepiadaceae |
Herb |
Neotropic |
| Asparagus racemosus |
Liliaceae |
Climber |
Palaeotropics |
| Asystasia crispata |
Acanthaceae |
Herb |
|
| Atalantia wightii |
Rutaceae |
Tree |
Oriental-W. Ghats, Sri Lanka |
| Bacopa monnieri |
Scrophulariaceae |
Herb |
Tropics |
| Bambusa arundinaceae |
Poaceae |
Reed |
Oriental-Throughout India |
| Bauhinia racemosa |
Faboideae |
Tree |
Oriental-Indomalaysia, China |
| Begonia integrifolia |
Begoniaceae |
Herb |
Oriental-W. Ghats |
| Begonia malabarica |
Begoniaceae |
Herb |
Oriental-W. Ghats |
| Beilschmiedia fagifolia |
Lauraceae |
Tree |
Oriental-W. Ghats |
| Bhidea burnsiana |
Poaceae |
Herb |
Oriental-Peninsular India |
| Bidens biternata |
Asteraceae |
Herb |
Asiatic |
| Biophytum sensitivum |
Oxalidaceae |
Herb |
Western peninsular India, Sri Lanka |
| Bischofia javanica |
Euphorbiaceae |
Tree |
Oriental-Indomalaysia |
| Species name |
Family |
Habit |
Distribution |
| Blachia denudata |
Euphorbiaceae |
Shrub |
Oriental-W. Ghats |
| Blepharis asperrima |
Acanthaceae |
Herb |
Western India |
| Boehmeria glomerulifera |
Urticaceae |
Climber |
Oriental-Indomalaysia |
| Boehmeria platyphylla |
Urticaceae |
Herb |
South west India, Sri Lanka |
| Boesenbergia pulcherrima |
Zingiberaceae |
Herb |
Oriental-W. Ghats |
| Bombax ceiba |
Bombacaceae |
Tree |
Oriental-Indomalaysia |
| Breynia retusa |
Euphorbiaceae |
Shrub |
Oriental-India, Sri Lanka |
| Bridelia scandens |
Euphorbiaceae |
Shrub |
Oriental-W. Ghats |
| Buchanania lanzan |
Anacardiaceae |
Tree |
Oriental-India, Myanmar |
| Burmannia coelestis |
Burmanniaceae |
Herb |
Oriental-Indomalaysia |
| Butea monosperma |
Faboideae |
Tree |
Indomalaya |
| Calamus sp. |
Arecaceae |
Climber |
Oriental-W. Ghats |
| Calicopteris floribunda |
Combretaceae |
Climber |
Oriental-Indomalaysia |
| Callicarpa tomentosa |
Verbenaceae |
Tree |
Oriental-South India |
| Calophyllum apetalum |
Clusiaceae |
Tree |
Oriental-W. Ghats |
| Calophyllum tomentosum |
Clusiaceae |
Tree |
Oriental, Paleoarctic |
| Calotropis gigantea |
Asclepiadaceae |
Climber |
Tropical Asia |
| Calycopteris floribunda |
Combretaceae |
Straggler |
Oriental-Indomalaysia |
| Canarium strictum |
Burseraceae |
Tree |
Oriental-W. Ghats |
| Canscora decurrens |
Gentianaceae |
Herb |
Oriental-W. Ghats |
| Canscora decussata |
Gentianaceae |
Herb |
Tropical Africa, Madagascar, India |
| Canscora perfoliata |
Gentianaceae |
Herb |
Oriental-W. Ghats |
| Canthium dicoccum |
Rubiaceae |
Tree |
South India, Myamnar |
| Canthium parviflorum |
Rubiaceae |
Shrub |
Oriental-W. Ghats |
| Capparis rheedei |
Capparaceae |
Sca.shrub |
Oriental-W. Ghats |
| Carallia brachiata |
Rhizophoraceae |
Tree |
Oriental to Australian |
| Careya arborea |
Lecythidaceae |
Tree |
Oriental-Himalayas to Sri Lanka |
| Carissa inermis |
Apocynaceae |
Sca.shrub |
Oriental-Peninsular India |
| Caryota urens |
Arecaceae |
Tree |
Oriental-W. Ghats |
| Casearia Sp. |
Flacourtiaceae |
Tree |
|
| Caseria rubescens |
Flacourtiaceae |
Tree |
Oriental-W. Ghats |
| Species name |
Family |
Habit |
Distribution |
| Cassia fistula |
Faboideae |
Tree |
Oriental-China, Indomalaysia |
| Cassia mimosoides |
Faboideae |
Herb |
Tropics |
| Cassia tora |
Faboideae |
Herb |
Tropics |
| Cassine glauca |
Celastraceae |
Tree |
Oriental-Indomalaysia |
| Cayratia trifolia |
Vitaceae |
Climber |
India, Ceylon, Malacca |
| Celosia argentea |
Amaranthaceae |
Herb |
Tropics |
| Celtis cinnamomea |
Ulmaceae |
Tree |
Oriental-Indomalaysia |
| Centella asiatica |
Apiaceae |
Herb |
Tropics |
| Centranthera indica |
Scrophulariaceae |
Herb |
Oriental-Indomalaysia |
| Cestrum nocturnum |
Solanaceae |
Sca.shrub |
West Indies |
| Chasalia ophioxyloides |
Rubiaceae |
Shrub |
South India, Sri Lanka |
| Chlorophytum orchidastrum |
Liliaceae |
Herb |
India, Tropical Africa |
| Chrysophyllum roxburghii |
Sapotaceae |
Tree |
Oriental-Indomalaysia |
| Cinnamomum macrocarpum |
Lauraceae |
Tree |
Oriental-W. Ghats |
| Cinnamomum zeylanicum |
Lauraceae |
Tree |
Oriental-Indomalaysia |
| Cissus discolor |
Vitaceae |
Climber |
Oriental-Indomalaysia |
| Cissus repens |
Vitaceae |
Climber |
Indomalaysia, Nepal to Taiwan, Java |
| Cleidion javanicum |
Euphorbiaceae |
Tree |
Oriental-Indomalaysia |
| Cleisostoma tenuifolium |
Orchidaceae |
Herb |
Oriental-W. Ghats |
| Clerodendrum paniculatum |
Verbenaceae |
Shrub |
Oriental-Indomalaysia |
| Clerodendrum serratum |
Verbenaceae |
Shrub |
Oriental-India, Sri Lanka |
| Clerodendrum viscosum |
Verbenaceae |
Shrub |
Oriental-Indomalaysia |
| Coldenia procumbens |
Boraginaceae |
Herb |
Pantropical |
| Combretum latifolium |
Combretaceae |
Climber |
Oriental-Indomalaysia |
| Commelina benghalensis |
Commelinaceae |
Herb |
Paleotropics |
| Connarus wightii |
Connaraceae |
Sca.shrub |
Oriental-W. Ghats |
| Corchorus trilocularis |
Tiliaceae |
Herb |
Oriental-Tropical India |
| Costos speciosus |
Costaceae |
Herb |
Oriental-Indomalaysia |
| Cottonia peduncularis |
Orchidaceae |
Herb |
Oriental-W. Ghats, Sri Lanka |
| Crotolaria filipes |
Faboideae |
Herb |
Oriental-W. Ghats |
| Crotolaria pallida |
Faboideae |
Shrub |
Oriental,Paleotropic, Neotropic |
| Species name |
Family |
Habit |
Distribution |
| Crotolaria retusa |
Faboideae |
Shrub |
Oriental,Paleotropic, Neotropic |
| Crotolaria verrucosa |
Faboideae |
Herb |
Oriental,Paleotropic, Neotropic |
| Croton gibsonianus |
Euphorbiaceae |
Shrub |
Oriental-W. Ghats |
| Curculigo orchioides |
Liliaceae |
Herb |
India, Java |
| Curcuma neilgherrensis |
Zingiberaceae |
Herb |
Oriental-W. Ghats |
| Cyathocline purpurea |
Asteraceae |
Herb |
Oreintal-India, Myamnar |
| Cyathula prostrata |
Amaranthaceae |
Herb |
Paleotropics |
| Cyclea peltata |
Menispermaceae |
Climber |
Oriental-W. Ghats |
| Cymbidium aloifolium |
Orchidaceae |
Herb |
Oriental-Indomalaysia, Indochina |
| Cynoglossum zeylanicum |
Boraginaceae |
Herb |
Oriental-South India, Sri Lanka |
| Cyrtococcum oxyphyllum |
Poaceae |
Herb |
Oriental-Indomalaysia |
| Dalbergia Sp. |
Faboideae |
Climber |
|
| Dalbergia sympathetica |
Faboideae |
Climber |
Oriental-W. Ghats |
| Dendrobium macrostachyum |
Orchidaceae |
Herb |
Oriental-India, Sri Lanka |
| Dendrobium nanum |
Orchidaceae |
Herb |
Oriental-W. Ghats |
| Dendrobium ovatum |
Orchidaceae |
Herb |
Oriental-W. Ghats |
| Derris canarensis |
Faboideae |
Climber |
Oriental-W. Ghats |
| Desmodium laxiflorum |
Faboideae |
Shrub |
Oriental-Indomalaysia |
| Desmodium triflorum |
Faboideae |
Herb |
Tropics |
| Desmodium triquetrum |
Faboideae |
Shrub |
Indomalaysia, China |
| Desmos lawii |
Annonaceae |
Straggler |
Indomalaysia, China |
| Dichapetalum gelonioides |
Dichapetalaceae |
Shrub |
Oriental-W. Ghats |
| Dictyospermum ovalifolium |
Commelinaceae |
Herb |
Oriental-W. Ghats |
| Dillenia pentagyana |
Dilleniaceae |
Tree |
Oriental- China to Indomalaysia |
| Dimocarpus longan |
Sapindaceae |
Tree |
Oriental-Tropics |
| Dimorphocalyx beddomei |
Euphorbiaceae |
Tree |
Oriental-W. Ghats |
| Dioscorea bulbifera |
Dioscoreaceae |
Climber |
Oriental-India, Sri Lanka |
| Dioscorea oppositifolia |
Dioscoreaceae |
Climber |
Oriental-India, Sri Lanka |
| Diospyros assimilis |
Ebenaceae |
Tree |
Oriental-W. Ghats |
| Diospyros buxifolia |
Ebenaceae |
Tree |
Oriental-Indomalaysia |
| Diospyros candolleana |
Ebenaceae |
Tree |
Oriental-W. Ghats |
| Species name |
Family |
Habit |
Distribution |
| Diospyros crumenata |
Ebenaceae |
Tree |
Oriental-Western Karnataka, Sri Lanka |
| Diospyros melanoxylon |
Ebenaceae |
Tree |
Oriental-Peninsular India, |
| Diospyros montana |
Ebenaceae |
Tree |
Oriental to Tropical Australia |
| Diospyros nigrescens |
Ebenaceae |
Tree |
Oriental-W. Ghats |
| Diospyros pruriens |
Ebenaceae |
Tree |
Oriental-W. Ghats |
| Diospyros Sp. |
Ebenaceae |
Tree |
|
| Dipterocarpus indicus |
Dipterocarpaceae |
Tree |
Oriental-W. Ghats |
| Dopatrium junceum |
Scrophulariaceae |
Herb |
Oriental-Indomalaysia |
| Dracaena terniflora |
Agavaceae |
Herb |
Oriental-India, S.E. Asia |
| Drosera burmanii |
Droseraceae |
Herb |
West Africa to North east Africa |
| Drosera indica |
Droseraceae |
Herb |
Tropical Africa to Australia |
| Duranta repens |
Verbenaceae |
Shrub |
South America |
| Dysoxylum glandulosum |
Meliaceae |
Tree |
Oriental-W. Ghats |
| Ecbolium ligustrinum |
Acanthaceae |
Shrub |
Oriental-India, Sri Lanka |
| Eclipta alba |
Asteraceae |
Herb |
Pantropical |
| Elaeagnus conferta |
Elaeagnaceae |
Tree |
Oriental-Indomalaysia |
| Elaeocarpus serratus |
Elaeocarpaceae |
Tree |
Oriental-India |
| Elaeocarpus tuberculatus |
Elaeocarpaceae |
Tree |
Oriental-Indomalaysia |
| Elatostema cuneatum |
Urticaceae |
Herb |
Oriental-India, Sri Lanka |
| Elatostema lineolatum |
Urticaceae |
Herb |
Oriental-India |
| Elephantopus scaber |
Asteraceae |
Herb |
Pantropical |
| Emblica officinalis |
Euphorbiaceae |
Tree |
Palaeotropics |
| Emilia sonchifolia |
Asteraceae |
Herb |
Pantropical |
| Entada pursaetha |
Faboideae |
Climber |
Oriental-Western India, |
| Epaltes divaricata |
Asteraceae |
Herb |
W Peninsular India, China, Myanmar |
| Epipogium roseum |
Orchidaceae |
Herb |
W Africa, Indomalaysia |
| Epithema carnosum |
Gesneriaceae |
Herb |
Oriental-W. Ghats |
| Eria dalzelli |
Orchidaceae |
Herb |
Oriental-W. Ghats |
| Eriocaulon stellulatum |
Eriocaulaceae |
Herb |
Oriental-W. Ghats |
| Eriocaulon xeranthemum |
Eriocaulaceae |
Herb |
Tropical Africa, Oriental-India |
| Ervatamia heyneana |
Apocynaceae |
Tree |
Oriental-W. Ghats |
| Species name |
Family |
Habit |
Distribution |
| Euodia lunu-ankenda |
Rutaceae |
Tree |
Oriental-India to S. E. Asia |
| Euonymus indicus |
Celastraceae |
Tree |
Oriental-W. Ghats |
| Eupatorium odoratum |
Asteraceae |
Herb |
Neotropic |
| Euphorbia hirta |
Euphorbiaceae |
Herb |
Pantropical |
| Euphorbia notoptera |
Euphorbiaceae |
Herb |
Oriental-W. Ghats |
| Euphorbia thymifolia |
Euphorbiaceae |
Herb |
Tropics |
| Evolvulus alsinoides |
Convolvulaceae |
Herb |
Paleotropics |
| Evolvulus nummularius |
Convolvulaceae |
Herb |
Tropical America |
| Exacum bicolor |
Gentianaceae |
Herb |
Oriental-Peninsular India |
| Exacum carinatum |
Gentianaceae |
Herb |
Oriental-Central India, W. Ghats |
| Exacum lawii |
Gentianaceae |
Herb |
Oriental-W. Ghats, Sri Lanka? |
| Exacum pedunculatum |
Gentianaceae |
Herb |
Oriental- India |
| Ficus arnottiana |
Moraceae |
Tree |
Oriental-Deccan Peninsula, Sri Lanka |
| Ficus asperrima |
Moraceae |
Tree |
Oriental-India, Sri Lanka |
| Ficus heterophylla |
Moraceae |
Tree |
Oriental-India, Sri Lanka, |
| Ficus hispida |
Moraceae |
Tree |
Oriental-Indomalaysia |
| Ficus nervosa |
Moraceae |
Tree |
Oriental-India to Vietnam |
| Ficus Sp. |
Moraceae |
Tree |
|
| Fimbristylis camplanata |
Cyperaceae |
Herb |
Pantropical |
| Flacourtia montana |
Flacourtiaceae |
Tree |
Oriental-W. Ghats |
| Flemingia strobilifera |
Faboideae |
Shrub |
Oriental-Indomalaysia |
| Garcinia gummi-gutta |
Clusiaceae |
Tree |
Oriental-W. Ghats, Sri Lanka |
| Garcinia indica |
Clusiaceae |
Tree |
Oriental-W. Ghats, Sri Lanka |
| Garcinia morella |
Clusiaceae |
Tree |
Oriental-Indomalaysia |
| Garcinia talbotii |
Clusiaceae |
Tree |
Oriental-W. Ghats |
| Geissaspis cristata |
Faboideae |
Herb |
Oriental-W. Ghats |
| Geophila reniformis |
Rubiaceae |
Herb |
Oriental-India, Sri Lanka |
| Globba marantina |
Zingiberaceae |
Herb |
Oriental- India, Sri Lanka, Malaya |
| Glochidion sp. |
Euphorbiaceae |
Tree |
|
| Glochidion zeylanicum |
Euphorbiaceae |
Tree |
Oriental-Indomalaysia |
| Gloriosa superba |
Liliaceae |
Climber |
Paleotropics |
| Species name |
Family |
Habit |
Distribution |
| Glycosmis pentaphylla |
Rutaceae |
Shrub |
Oriental-S. India, Sri Lanka |
| Gnetum ula |
Gnetaceae |
Climber |
Oriental-South India |
| Gnidia glauca |
Thymelaeaceae |
Shrub |
Palaeotropics |
| Goniothalamus cardiopetalus |
Annonaceae |
Tree |
Oriental-W. Ghats |
| Gordonia obtusa |
Theaceae |
Tree |
Oriental-W. Ghats |
| Grangea maderaspatana |
Asteraceae |
Herb |
Paleotropics |
| Grewia disperma |
Tiliaceae |
Tree |
Paleotropics, Oriental-India, Myanmar |
| Grewia microcos |
Tiliaceae |
Tree |
Oriental-Asia |
| Grewia tiliifolia |
Tiliaceae |
Tree |
Tropical Africa, Tropical |
| Grewia umbellifera |
Tiliaceae |
Sca.shrub |
Oriental-Central and Peninsular India |
| Grewilia robusta |
Gymnosperm |
Tree |
|
| Gymnema sylvestre |
Asclepiadaceae |
Climber |
Paleotropics, Oriental |
| Gymnosporia rothiana |
Celastraceae |
Shrub |
Oriental-W. Ghats |
| Gymnostachyum latifolium |
Acanthaceae |
Shrub |
Oriental-W. Ghats |
| Habenaria crinifera |
Orchidaceae |
Herb |
Oriental- W. Ghats, Sri Lanka |
| Habenaria grandifloriformis |
Orchidaceae |
Herb |
Oriental-Deccan, W Peninsular India |
| Habenaria longicorniculata |
Orchidaceae |
Herb |
Oriental- W. Ghats |
| Harpullia imbricata |
Sapindaceae |
Tree |
Oriental-Indomalaysia |
| Hedyotis caerulea |
Rubiaceae |
Herb |
Oriental- South India |
| Hedyotis corymbosa |
Rubiaceae |
Herb |
|
| Hedyotis herbacea |
Rubiaceae |
Herb |
Paleotropic |
| Hedyotis nitida |
Rubiaceae |
Herb |
Oriental- W. Ghats, Sri Lanka |
| Helicteres isora |
Sterculiaceae |
Shrub |
Oriental- Indomalaysia |
| Heliotropium indicum |
Boraginaceae |
Herb |
Pantropical |
| Heliotropium marifolium |
Boraginaceae |
Herb |
Indomalaysia |
| Hemidesmus indicus |
Asclepiadaceae |
Climber |
Oriental-India, Sri Lanka |
| Hibiscus furcatus |
Malvaceae |
Sca.shrub |
Tropical Africa, Tropical Asia |
| Hippocratea indica |
Hippocrataceae |
Climber |
Oriental-Indomalaysia |
| Holarrhena antidysenterica |
Apocynaceae |
Tree |
Oriental-India, Malaya |
| Holigarna arnottiana |
Anacardiaceae |
Tree |
Oriental-W. Ghats |
| Holigarna beddomii |
Anacardiaceae |
Tree |
Oriental-W. Ghats |
| Species name |
Family |
Habit |
Distribution |
| Holigarna ferruginea |
Anacardiaceae |
Tree |
Oriental-W. Ghats |
| Holigarna grahamii |
Anacardiaceae |
Tree |
Oriental-W. Ghats |
| Hopea parviflora |
Dipterocarpaceae |
Tree |
Oriental-W. Ghats |
| Hopea wightiana |
Dipterocarpaceae |
Tree |
Oriental-W. Ghats |
| Hoya ovalifolia |
Asclepiadaceae |
Herb |
Oriental- Peninsular India, Sri Lanka |
| Hoya retusa |
Asclepiadaceae |
Herb |
Oriental- W. Ghats |
| Hybanthus enneaspermus |
Violaceae |
Herb |
Africa to Australia |
| Hydnocarpus laurifolia |
Flacourtiaceae |
Tree |
Oriental-W. Ghats |
| Hydrocotyl javanica |
Apiaceae |
Herb |
Tropical Africa-Indomalaysia |
| Hydrocotyl sibthorpioides |
Apiaceae |
Herb |
Tropical Africa-Indomalaysia |
| Hygrophila auriculata |
Acanthaceae |
Herb |
Oriental-India, Sri Lanka |
| Hypoxis aurea |
Hypoxidaceae |
Herb |
Oriental-India, S.E. Asia |
| Hyptis suaveolens |
Lamiaceae |
Herb |
Tropical America |
| Ichnocarpus frutescens |
Apocynaceae |
Climber |
Indomalaysia, Australia |
| Impatiens balsamina |
Balsaminaceae |
Herb |
Indomalaysia, China |
| Impatiens oppositifolia |
Balsaminaceae |
Herb |
Oriental- W. Ghats, Sri Lanka |
| Impatiens scapiflora |
Balsaminaceae |
Herb |
Oriental- W. Ghats |
| Impatiens trichocarpa |
Balsaminaceae |
Herb |
Oriental- W. Ghats |
| Iphigenia indica |
Liliaceae |
Herb |
Oriental-Indomalaysia |
| Ipomoea hederifolia |
Convolvulaceae |
Twiner |
Tropical America |
| Ischaemum indicum |
Poaceae |
Herb |
Oriental-South India |
| Ixora arborea |
Rubiaceae |
Tree |
Oriental-W. Ghats |
| Ixora brachiata |
Rubiaceae |
Tree |
Oriental-W. Ghats |
| Ixora coccinea |
Rubiaceae |
Shrub |
Oriental-W. Ghats, Sri Lanka |
| Ixora polyantha |
Rubiaceae |
Shrub |
Oriental- W. Ghats |
| Jasminum malabaricum |
Oleaceae |
Climber |
Oriental-W. Ghats |
| Jasminum ritchiei |
Oleaceae |
Climber |
Oriental-W. Ghats, Sri Lanka |
| Jasminum rottlerianum |
Oleaceae |
Climber |
Oriental-W. Ghats |
| Jerdonia indica |
Gesneriaceae |
Herb |
Oriental- W. Ghats |
| Justicia betonica |
Acanthaceae |
Herb |
Tropical Africa, India, Sri Lanka, Malaysia |
| Justicia simplex |
Acanthaceae |
Herb |
E Africa, India, Malaysia |
| Species name |
Family |
Habit |
Distribution |
| Knema attenuata |
Myristicaceae |
Tree |
Oriental-W. Ghats |
| Knoxia sumatrensis |
Rubiaceae |
Herb |
Oriental-Indomalaysia |
| Lagenandra meeboldii |
Araceae |
Herb |
Oriental- W. Ghats |
| Lagerstroemia microcarapa |
Lythraceae |
Tree |
Oriental-W. Ghats |
| Lagerstroemia parviflora |
Lythraceae |
Tree |
Oriental-W. Ghats, Myanmar |
| Lannea coromandelica |
Anacardiaceae |
Tree |
Oriental-India, Sri Lanka |
| Leea indica |
Leeaceae |
Shrub |
Oriental- India, China to Australia |
| Lepisanthes tetraphylla |
Sapindaceae |
Tree |
Oriental-W. Ghats, Sri Lanka, Myanmar |
| Leucas biflora |
Lamiaceae |
Herb |
Oriental-W Peninsular India, Sri Lanka |
| Leucas ciliata |
Lamiaceae |
Herb |
Oriental-India |
| Leucas hirta |
Lamiaceae |
Herb |
Oriental-South India |
| Leucas lavandulifolia |
Lamiaceae |
Herb |
Oriental-Indomalaysia |
| Leucus marrubioides |
Lamiaceae |
Herb |
Oriental-W. Ghats, Sri Lanka |
| Limnophila aromatica. |
Scrophulariaceae |
Herb |
Tropical India, N Australia |
| Limnophila indica |
Scrophulariaceae |
Herb |
Paleotropics |
| Lindernia anagallis |
Scrophulariaceae |
Herb |
Oriental-Indomalaysia |
| Lindernia antipoda |
Scrophulariaceae |
Herb |
Oriental-Indomalaysia |
| Lindernia ciliata |
Scrophulariaceae |
Herb |
Oriental-Indomalaysia |
| Lindernia hyssopoides |
Scrophulariaceae |
Herb |
Oriental-India, Sri Lanka |
| Lindernia nummulariifolia |
Scrophulariaceae |
Herb |
Oriental-India, Myanmar |
| Lindernia procumbens |
Scrophulariaceae |
Herb |
Temperate to tropical Eurasia |
| Lindernia pusilla |
Scrophulariaceae |
Herb |
Paleotropics |
| Lindernia rotundifolia |
Scrophulariaceae |
Herb |
Oriental-W and S. India, Sri Lanka, Madagascar |
| Linociera malabarica |
Oleaceae |
Tree |
Oriental-W. Ghats |
| Litsea laevigata |
Lauraceae |
Tree |
Oriental-W. Ghats |
| Litsea sp. |
Lauraceae |
Tree |
|
| Lobelia alsinoides |
Campanulaceae |
Herb |
Oriental-S and S.E. Asia |
| Lobelia nicotianifolia |
Campanulaceae |
Herb |
Oriental-Indomalaysia |
| Lophopetalum wightianum |
Celastraceae |
Tree |
Oriental-Indomalaysia |
| Ludwigia perennis |
Onagraceae |
Herb |
Indomalaysia, E Africa, Iran, Sri Lanka |
| Luvunga sarmentosa |
Rutaceae |
Shrub |
Oriental-Java, Sri Lanka |
| Species name |
Family |
Habit |
Distribution |
| Macaranga peltata |
Euphorbiaceae |
Tree |
Oriental-W. Ghats, Sri Lanka |
| Madhuca latifolia |
Sapotaceae |
Tree |
Oriental- India, Myanmar |
| Malaxis acuminata |
Orchidaceae |
Herb |
Oriental-Indomalaysia |
| Malaxis rheedii |
Orchidaceae |
Herb |
India, Thailand, China |
| Mallotus philippensis |
Euphorbiaceae |
Tree |
China, Indomalaysia to Australia |
| Mangifera indica |
Anacardiaceae |
Tree |
Oriental-W. Ghats |
| Mastixia arborea |
Cornaceae |
Tree |
Oriental-W. Ghats |
| Mecardonia procumbens |
Scrophulariaceae |
Herb |
Neotropic |
| Melastoma malabathricum |
Melastomataceae |
Shrub |
Oriental-India |
| Melochia corchorifolia |
Sterculiaceae |
Herb |
Tropics |
| Memecylon sp. |
Melastomataceae |
Shrub |
|
| Memecylon talbotianum |
Melastomataceae |
Tree |
Oriental-W. Ghats |
| Memecylon terminale |
Melastomataceae |
Shrub |
Oriental-W. Ghats |
| Memecylon umbellatum |
Melastomataceae |
Tree |
Oriental-W. Ghats, Sri Lanka |
| Memecylon wightii |
Melastomataceae |
Shrub |
Oriental-W. Ghats |
| Menispermaceae member |
Menispermaceae |
Climber |
|
| Mesua ferrea |
Clusiaceae |
Tree |
Oriental-Indomalaysia |
| Mimosa pudica |
Faboideae |
Herb |
Tropical America |
| Mimusops elengi |
Sapotaceae |
Tree |
Oriental-Indomalaysia |
| Mitraphora heyneana |
Annonaceae |
Tree |
Oriental-W. Ghats |
| Mollugo pentaphylla |
Molluginaceae |
Herb |
Paleotropics |
| Monochoria vaginalis |
Pontederiaeae |
Herb |
Paleotropics |
| Moullava spicata |
Faboideae |
Sca.shrub |
Oriental- W. Ghats |
| Murdannia pauciflora |
Commelinaceae |
Herb |
Oriental-S. India, Malaya |
| Murdannia semiteres |
Commelinaceae |
Herb |
Africa, S. India |
| Murdannia simplex |
Commelinaceae |
Herb |
Paleotropics |
| Murraya koenigii |
Rutaceae |
Tree |
Oriental-India, Sri Lanka |
| Murraya paniculata |
Rutaceae |
Shrub |
Oriental-Indomalaysia |
| Mussaenda bellila |
Rubiaceae |
Sca.shrub |
Oriental-Peninsular India |
| Myristica dactyloides |
Myristicaceae |
Tree |
Oriental-W. Ghats, Sri Lanka |
| Myristica malabarica |
Myristicaceae |
Tree |
Oriental-W. Ghats |
| Species name |
Family |
Habit |
Distribution |
| Naravelia zeylanica |
Ranunculaceae |
Climber |
Oriental-India, Sri Lanka, Java |
| Naregamia alata |
Meliaceae |
Herb |
Oriental-India, Angola |
| Neanotis foetida |
Rubiaceae |
Herb |
Oriental- W. Ghats |
| Neolitsea scrobiculata |
Lauraceae |
Tree |
Oriental-Western India |
| Nothapodytes nimmoniana |
Icacinaceae |
Tree |
Oriental-China, Indomalaysia |
| Nothopegia colebrookeana |
Anacardiaceae |
Tree |
Oriental-W. Ghats |
| Nymphaea nouchali |
Nymphaeaceae |
Herb |
Tropics |
| Nymphaea pubescens |
Nymphaeaceae |
Herb |
Tropics |
| Nymphoides aurantiaca |
Menyanthaceae |
Herb |
Oriental-S. India, Sri Lanka |
| Nymphoides indica |
Menyanthaceae |
Herb |
Oriental-Indomalaysia |
| Oberonia brunoniana |
Orchidaceae |
Herb |
Oriental- W. Ghats |
| Oberonia santapaui |
Orchidaceae |
Herb |
Oriental- W. Ghats |
| Olax wightiana |
Olacaceae |
Sca.shrub |
Oriental- W. Ghats, Sri Lanka |
| Olea dioica |
Oleaceae |
Tree |
Oriental-N E India, S W India |
| Ophiorrhiza hirsutula |
Rubiaceae |
Herb |
Oriental-W. Ghats |
| Osbeckia truncata |
Melastomataceae |
Herb |
Oriental- W. Ghats |
| Oxalis corniculata |
Oxalidaceae |
Herb |
Pantropical |
| Pajanelia longifolia |
Bignoniaceae |
Tree |
Oriental-India, Myanmar |
| Palaquium ellipticum |
Sapotaceae |
Tree |
Oriental-W. Ghats |
| Pandanus Sp. |
Pandanaceae |
Shrub |
|
| Paramignya monophylla |
Rutaceae |
Climber |
Oriental-India, Sri Lanka |
| Paspalum scrobiculatum |
Poaceae |
Herb |
Oriental- India |
| Passiflora subpeltata |
Passifloraceae |
Climber |
Native of Madagascar |
| Pavetta indica |
Rubiaceae |
Shrub |
Oriental-South India |
| Pennisetum pedicellatum |
Poaceae |
Herb |
India, Tropical Africa |
| Peperomia pellucida |
Piperaceae |
Herb |
S America |
| Peperomia portulacoides |
Piperaceae |
Herb |
Madagascar to S W India |
| Peristylus aristatus |
Orchidaceae |
Herb |
Oriental-India, Sri Lanka |
| Peristylus secundus |
Orchidaceae |
Herb |
Oriental-S. India |
| Persea macarantha |
Lauraceae |
Tree |
Oriental-South India, Sri Lanka |
| Phaulopsis imbricata |
Acanthaceae |
Herb |
India, Africa, Sri Lanka, Madagascar |
| Species name |
Family |
Habit |
Distribution |
| Phoebe cathia |
Lauraceae |
Tree |
Oriental-W. Ghats, C Himalayas to Myanmar |
| Phoenix humilis |
Arecaceae |
Palm |
Oriental-W. Ghats |
| Pholidota pallida |
Orchidaceae |
Herb |
Oriental-Indomalaysia |
| Phyllanthus debilis |
Euphorbiaceae |
Herb |
India, Tropical Africa |
| Phyllanthus niruri |
Euphorbiaceae |
Herb |
Tropics except Australia |
| Phyllanthus urinaria |
Euphorbiaceae |
Herb |
Tropics |
| Pinanga dicksonii |
Arecaceae |
Palm |
Oriental-W. Ghats |
| Piper nigrum |
Piperaceae |
Climber |
Oriental-E and W. Ghats |
| Plantanthera susannae |
Orchidaceae |
Herb |
Oriental-Indomalaysia |
| Plectranthus mollis |
Lamiaceae |
Herb |
Oriental-India |
| Plectranthus stocksii |
Lamiaceae |
Herb |
Oriental-Central and S. India |
| Plumbago zeylanica |
Plumbaginaceae |
Herb |
Tropics |
| Poeciloneuron indicum |
Clusiaceae |
Tree |
Oriental-W. Ghats |
| Polyalthia fragrance |
Annonaceae |
Tree |
Oriental-W. Ghats |
| Polygonum chinense |
Polygonaceae |
Herb |
Oriental-Indomalaysia |
| Polystachya flavescens |
Orchidaceae |
Herb |
Oriental-Indomalaysia |
| Porpax jerdoniana |
Orchidaceae |
Herb |
Oriental- W. Ghats |
| Porpax reticulata |
Orchidaceae |
Herb |
Oriental- W. Ghats |
| Portulaca oleracea |
Portulacaceae |
Herb |
Tropics |
| Pothos scandens |
Araceae |
Climber |
Oriental-India, Sri Lanka, |
| Pouzolzia zeylanica |
Urticaceae |
Herb |
China through Indomalaysia |
| Prunus ceylanica |
Rosaceae |
Tree |
Oriental-South India to S.E. Asia |
| Psychotria canarensis |
Rubiaceae |
Shrub |
Oriental-W. Ghats |
| Psychotria dalzellii |
Rubiaceae |
Shrub |
Oriental-W. Ghats |
| Psychotria flavida |
Rubiaceae |
Shrub |
Oriental-W. Ghats |
| Psychotria truncata |
Rubiaceae |
Shrub |
Oriental-W. Ghats |
| Pterocarpus marsupium |
Faboideae |
Tree |
Oriental-W. Ghats, Sri Lanka |
| Pterospermum acerifolium |
Sterculiaceae |
Tree |
Oriental-Indomalaysia |
| Pterospermum diversifolium |
Sterculiaceae |
Tree |
Oriental-W. Ghats, Java, Philippines, Malaysia |
| Ramphicarpa longiflora |
Scrophulariaceae |
Herb |
Oriental- W. Ghats |
| Randia rugulosa |
Rubiaceae |
Sca.shrub |
Oriental- W. Ghats, Sri Lanka |
| Species name |
Family |
Habit |
Distribution |
| Randia uliginosa |
Rubiaceae |
Shrub |
Oriental-India, Myanmar |
| Rauvolfia serpentina |
Apocynaceae |
Shrub |
India, Sri Lanka, Java |
| Rhynchoglossum notonianum |
Gesneriaceae |
Herb |
Oriental- W. Ghats, Sri Lanka |
| Rhynchospora wightiana |
Cyperaceae |
Herb |
Oriental- W. Ghats |
| Rhynchostylis retusa |
Orchidaceae |
Herb |
Oriental-Indomalaysia |
| Rotala densiflora |
Lythraceae |
Herb |
Oriental-Indomalaysia |
| Rotala macrandra |
Lythraceae |
Herb |
Oriental-S. India |
| Rubia cordifolia |
Rubiaceae |
Climber |
Palaeotropics |
| Rungia pectinata |
Acanthaceae |
Herb |
Oriental-India, Sri Lanka, Myanmar |
| Sageraea laurifolia |
Annonaceae |
Tree |
Oriental-W. Ghats |
| Salomonia ciliata |
Polygalaceae |
Herb |
India, Sri Lanka, Malaya, Australia |
| Santalum album |
Santalaceae |
Tree |
Oriental-South India |
| Saraca asoca |
Faboideae |
Tree |
Oriental-Indomalaysia |
| Sarcostigma kleinii |
Euphorbiaceae |
Climber |
Oriental-Eastern & W. Ghats |
| Schefflera venulosa |
Araliaceae |
Tree |
Oriental-India, Myanmar, |
| Schleichera oleosa |
Sapindaceae |
Tree |
Oriental-Indomalaysia |
| Scoparia dulcis |
Scrophulariaceae |
Herb |
Neotropic |
| Scutia myrtina |
Rhamnaceae |
Shrub |
Oriental-Myanmar, India |
| Sebastania chamaela |
Euphorbiaceae |
Herb |
Paleotropics |
| Sida acuta |
Malvaceae |
Herb |
Pantropical |
| Sida cordifolia |
Malvaceae |
Herb |
Pantropical |
| Sida rhombifolia |
Malvaceae |
Herb |
Oriental-Indomalaysia |
| Smilax zeylanica |
Smilacaceae |
Climber |
Oriental-South E. Asia to |
| Smithia conferta |
Faboideae |
Herb |
Oriental-Indomalaysia |
| Smithia hirsuta |
Faboideae |
Herb |
Oriental- W. Ghats |
| Solanum americanum |
Solanaceae |
Herb |
Temperate and Tropical |
| Solanum surattense |
Solanaceae |
Herb |
Indomalaya, Tropical Australia, Polynesia |
| Solanum violaceum |
Solanaceae |
Herb |
Tropical Africa, Indian subcontinent |
| Sophubia delphinifolia |
Scrophulariaceae |
Herb |
Oriental-S. India, Sri Lanka |
| Spermacoce articularis |
Rubiaceae |
Herb |
Oriental-Indomalaysia |
| Spermacoce mauritiana |
Rubiaceae |
Herb |
Pantropical |
| Species name |
Family |
Habit |
Distribution |
| Spermacoce pusilla |
Rubiaceae |
Herb |
Paleotropics |
| Spermacoce verticillata |
Rubiaceae |
Herb |
Pantropical |
| Stachytarpheta indica |
Verbenaceae |
Herb |
Tropical America |
| Sterculia guttata |
Sterculiaceae |
Tree |
Oriental-W. Ghats, Sri Lanka |
| Steriospermum personatum |
Bignoniaceae |
Tree |
Oriental-India, Myanmar, |
| Striga angustifolia |
Scrophulariaceae |
Herb |
Oriental-Indomalaysia |
| Striga asiatica |
Scrophulariaceae |
Herb |
Paleotropics |
| Striga densiflora |
Scrophulariaceae |
Herb |
Oriental-Central and S. India |
| Strobilanthus barbatus |
Acanthaceae |
Shrub |
Oriental-W. Ghats |
| Strobilanthus heyneanus |
Acanthaceae |
Shrub |
Oriental-W. Ghats |
| Strobilanthus integrifolius |
Acanthaceae |
Shrub |
Oriental-W. Ghats |
| Strombosia ceylanica |
Olacaceae |
Tree |
Oriental-W. Ghats |
| Strychnos dalzelli |
Loganiaceae |
Climber |
Oriental-Peninsular India |
| Swertia corymbosa |
Gentianaceae |
Herb |
Oriental- W. Ghats |
| Symplocos racemosa |
Symplocaceae |
Tree |
Oriental-W. Ghats |
| Syzygium caryophyllatum |
Myrtaceae |
Tree |
Oriental-W. Ghats, Sri Lanka |
| Syzygium cumini |
Myrtaceae |
Tree |
Oriental-Indomalaysia |
| Syzygium gardnerii |
Myrtaceae |
Tree |
Oriental-W. Ghats, Sri Lanka |
| Syzygium laetum |
Myrtaceae |
Tree |
Oriental-W. Ghats |
| Syzygium macrocephala |
Myrtaceae |
Tree |
Oriental-W. Ghats |
| Syzygium Sp.1 |
Myrtaceae |
Tree |
|
| Syzygium Sp.2 |
Myrtaceae |
Tree |
|
| Tarenna asiatica |
Rubiaceae |
Shrub |
Oriental-Indomalaysia |
| Tephrosia pulcherrima |
Faboideae |
Herb |
Oriental-W. Ghats, Sri Lanka |
| Terminalia alata |
Combretaceae |
Tree |
Oriental-India |
| Terminalia bellirica |
Combretaceae |
Tree |
Oriental-Indomalaysia |
| Terminalia chebula |
Combretaceae |
Tree |
Oriental-India, Myanmar |
| Terminalia paniculata |
Combretaceae |
Tree |
Oriental-Peninsular India |
| Tetrameles nudiflora |
Datiscaceae |
Tree |
Oriental-India, Sri Lanka, |
| Thunbergia mysorensis |
Acanthaceae |
Climber |
Oriental-W. Ghats |
| Toddalia asiatica |
Rutaceae |
Climber |
Oriental-South India |
| Species name |
Family |
Habit |
Distribution |
| Tolypanthus lagenifer |
Loranthaceae |
Shrub |
Oriental- W. Ghats |
| Toona ciliata |
Meliaceae |
Tree |
India to Australia |
| Torenia bicolor |
Scrophulariaceae |
Herb |
Oriental- W. Ghats |
| Tragia hispida |
Euphorbiaceae |
Twiner |
Oriental-Peninsular India |
| Trapa natans |
Trapaceae |
Herb |
Oriental-India, Sri Lanka |
| Trewia nudiflora |
Euphorbiaceae |
Tree |
Oriental-India, Sri Lanka |
| Tricalysia apiocarpa |
Rubiaceae |
Tree |
Oriental-W. Ghats |
| Tricholepis glaberrima |
Asteraceae |
Herb |
Oriental-India |
| Triumfetta rhomboidea |
Tiliaceae |
Herb |
Tropical Africa, Asia |
| Turnera ulmifolia |
Turneraceae |
Herb |
|
| Turraea villosa |
Meliaceae |
Shrub |
Western peninsular India, Java |
| Tylophora indica |
Asclepidiaceae |
Climber |
Oriental-Indomalaysia |
| Unidentified (from Chikmattur) |
|
Tree |
|
| Unidentified (from Karni) |
|
Tree |
|
| Unidentified (from Mayyalli) |
|
Tree |
|
| Unidentified (from Talgani) |
|
Tree |
|
| Urena lobata |
Malvaceae |
Herb |
Pantropical |
| Utricularia aurea |
Lentibulariaceae |
Herb |
Oriental and Australian |
| Utricularia praeterita |
Lentibulariaceae |
Herb |
Oriental-S. India |
| Utricularia reticulata |
Lentibulariaceae |
Herb |
Oriental-India, Sri Lanka |
| Utricularia striatula |
Lentibulariaceae |
Herb |
Paleotropics |
| Uvaria narum |
Annonaceae |
Climber |
Oriental-W. Ghats, Sri Lanka |
| Vangueria spinosa |
Rubiaceae |
Tree |
Oriental-Indomalaysia |
| Ventilago madraspatana |
Rhamnaceae |
Climber |
Oriental-W. Ghats, Sri Lanka, Java |
| Vepris bilocularis |
Rutaceae |
Tree |
Oriental-W. Ghats |
| Vernonia cineria |
Asteraceae |
Herb |
Paleotropics |
| Vitaceae member |
Vitaceae |
Climber |
|
| Vitex altissima |
Verbenaceae |
Tree |
Oriental- South India |
| Vitex negundo |
Verbenaceae |
Shrub |
Oriental-Asia |
| Vitis auriculata |
Vitaceae |
Climber |
Oriental- India |
| Wendlandia thyrsoidea |
Rubiaceae |
Tree |
Oriental-S. India, Sri Lanka |
| Species name |
Family |
Habit |
Distribution |
| Xantolis tomentosa |
Sapotaceae |
Tree |
Oriental-India,China |
| Xylia xylocarpa |
Faboideae |
Tree |
Oriental-Indomalaysia |
| Xyris pauciflora |
Xyridaceae |
Herb |
Oriental-Indomalaysia |
| Zanthoxylum ovalifolium |
Rutaceae |
Shrub |
Oriental-Singapur |
| Zanthoxylum rhetsa |
Rutaceae |
Tree |
Oriental-Indomalaysia |
| Zingiber cernum |
Zingiberaceae |
Herb |
Oriental-W. Ghats |
| Zingiber neesanum |
Zingiberaceae |
Herb |
Oriental-W. Ghats |
| Ziziphus oenoplia |
Rhamnaceae |
Sca.shrub |
Pantropics |
| Ziziphus rugosa |
Rhamnaceae |
Straggler |
Oriental-India, Sri Lanka |
| Zornia gibbosa |
Faboideae |
Herb |
Tropics |
| |
|
|
|
| Sca.shrub-Scandent Shrub |
|
|
|
Table 8.4: Details of different landscape elements sampled and their diversity indices, basal area, percentage evergreens and percentage endemics.
| Vegetation type |
Total transects |
Total quadrats |
Total area sampled (ha.) |
Total individuals |
Total species |
Estimated basal area/ha. |
Species richness |
Shannon’s diversity |
Simpson’s diversity |
% Evergreens |
% Endemics |
|
20 |
96 |
11.8 |
1818 |
128 |
35.3 |
17 |
3.94 |
0.96 |
95.8 |
56.7 |
| Semi-evergreen |
16 |
82 |
3.3 |
1916 |
138 |
31.1 |
18 |
4.03 |
0.97 |
77.1 |
40.9 |
| Moist deciduous |
3 |
18 |
0.7 |
318 |
58 |
19.8 |
10 |
3.44 |
0.95 |
43.6 |
19.7 |
| Scrub |
2 |
5 |
0.2 |
6 |
4 |
0.6 |
2 |
1.24 |
0.67 |
0.0 |
0.0 |
Lichens
Lichens are unique groups of plants exhibiting symbiotic association of fungi and algae, but represented as a single organism. Because of their sensitivity to microclimatic changes in environment, lichens aid as bioindicators. They require specific conditions in the environment and respond critically to any changes in it. Hence, they are widely used in air pollution, geochemical and geothermal emission, and biomonitoring studies. They play various roles as pioneers in successionaland climax ecosystems and could as well indicate the age and ecological continuity of a forest. Apart from this, they also can be used as tools in determining the age of an unknown rock surface (lichenometry) and soil formation (pedogenesis) during plant succession. Western Ghats harbour 800 species of lichens in which, 161 species are endemic to this region. The study in SVWS shows the presence of 46 species of lichens in the SVWS (in semi-evergreen forest of Holebagilu, Honnemaradu Island, Karumane, Muppane and Siganduru) representing 5% from the Western Ghats of Karnataka. (Table 8.4). However, lichen studies need to be carried out more intensively. Table 8.5 gives Simpson’s and Shannon-Weiner’s diversity indices. Holebagilu and Karumane are highly diverse, while Honnemaradu (Island) is least diverse in lichen composition.
Table 8.4: Lichen species recorded in SVWS
| Taxa |
Growth form |
Holebagilu |
Siganduru |
Muppane |
Karumane |
Honnemardu (I) |
Substrata |
| Arthoniaceae |
|
|
|
|
|
|
|
| Cryptothecialunulata (Zahlbr.) Makhija & Patwardhan |
C |
+ |
+ |
|
+ |
|
16,27,33,41 |
| C. phyctidiforme (Müll. Arg.) Awasthi & K Singh |
C |
|
|
+ |
|
|
1,4,47 |
| C. stirtonii A.L. Smith |
C |
+ |
|
|
|
+ |
5,13,34 |
| C.subnidulans Stirton |
C |
|
|
|
|
+ |
5,11,34,39,41 |
| Arthopyreniaceae |
|
|
|
|
|
|
|
| Arthopyrenia indusiata Müll. Arg. |
C |
+ |
|
|
|
|
1,12,24,27,31,35,39,51 |
| A. subnexa (Nyl.) Müll. Arg. |
C |
+ |
|
|
|
|
1,5,24,27,30 |
| A. terminata (Nyl.) Müll. Arg. |
C |
+ |
+ |
|
+ |
|
4,8,14,27, |
| Bacidiaceae |
|
|
|
|
|
|
|
| Bacidia incongruens (Stirton) Zahlbr. |
C |
|
|
|
+ |
|
2,22,39 |
| B. subletorum (Schreber) Lettau |
C |
|
|
+ |
|
|
36 |
| Taxa |
Growth form |
Holebagilu |
Siganduru |
Muppane |
Karumane |
Honnemardu (I) |
Substrata |
| Brigantiaea leucoxantha (Sprengel) Half. In Half & Bellem |
C |
+ |
|
|
|
|
15,25,48 |
| Caliciales (Order) |
|
|
|
|
|
|
|
| Heterocyphelium leucompyx (Tuck.) Vainio |
C |
+ |
|
|
|
|
1,6 |
| Catilariaceae |
|
|
|
|
|
|
|
| Catilaria pulverea (Borrer) Lattau |
C |
|
|
|
|
+ |
1,6,8,9,18,19,45,48 |
| Coccocarpiaceae |
|
|
|
|
|
|
|
| Coccocarpia erythroxyli (Sprengel) Swinsc and Krog. |
F |
|
+ |
|
+ |
+ |
2,4,5,14,20,22,23,27,32, 37,39,40,41, 44,48,50 |
| Collamataceae |
|
|
|
|
|
|
|
| Leptogium aurstro-americanum (Malme) Dodge |
F |
|
+ |
|
+ |
|
3,4,7,20,37,39,41,44,46, 47,50, |
| L. denticulatum Nyl. |
F |
|
|
+ |
+ |
|
2,3,10,41,42,44,50 |
| Graphidaceae |
|
|
|
|
|
|
|
| Graphina petricosa (Krempelh) A. Zahlbr. |
C |
|
|
|
+ |
|
2,4,39,41 |
| Graphis nakanishiana Patwardhan & Kulkarni |
C |
|
|
+ |
|
+ |
4,10,14,18,20,39,44,49 |
| Taxa |
Growth form |
Holebagilu |
Siganduru |
Muppane |
Karumane |
Honnemardu (I) |
Substrata |
| Xylographa vitiligo (Ach.) Laundon |
C |
|
|
+ |
+ |
+ |
2,4,10,14,16,20,27,28,30,
41,44 |
| Lecanoraceae |
|
|
|
|
|
|
|
| Lecidia sp1 |
C |
+ |
|
|
|
|
2,12 |
| Lecidia sp2 |
C |
|
|
+ |
|
|
2,4 |
| Lecidia sp3 |
C |
|
|
|
+ |
|
4,45 |
| Lecidia sp4 |
C |
+ |
|
+ |
|
+ |
2,13,16,27,34,45,46 |
| Letrouitiaceae |
|
|
|
|
|
|
|
| Letrouitia trangressa (Malme) Half. & Bellem |
C |
|
|
+ |
|
|
2,4,36,48 |
| Opegraphaceae |
|
|
|
|
|
|
|
| Opegrapha subvulgata Nyl. |
C |
+ |
|
|
|
|
6 |
| Pertusariaceae |
|
|
|
|
|
|
|
| Ochrolechia androgyna (Hoffm.) Arnold |
C |
|
+ |
|
|
|
23 |
| O. subviridis (Hoeg) Erichsen |
C |
+ |
|
|
|
|
6,27,40 |
| Pertusaria albescens (Huds.) M. Choisy & Werner |
C |
|
|
+ |
|
|
2,42 |
| P. multipunctata (Turner) Nyl. |
C |
|
|
+ |
|
|
2 |
| Phyllosporaceae |
|
|
|
|
|
|
|
| Phyllospora manipurensis (Müll. Arg.) Sch. |
F |
+ |
|
+ |
|
|
2,27 |
| P. parvifolia (Pers.) Müll. Arg. |
F |
+ |
+ |
+ |
|
|
2,12,23,31,37,38,43 |
| Taxa |
Growth form |
Holebagilu |
Siganduru |
Muppane |
Karumane |
Honnemardu (I) |
Substrata |
| Physciaceae |
|
|
|
|
|
|
|
| Physcia aipolia (Ehrh. in Humb.) Furnr |
F |
+ |
|
|
|
|
13,31 |
| P. dimidiata (Arn.) Nyl. |
F |
|
+ |
|
|
|
1,2,18,29 |
| Pilocarpaceae |
|
|
|
|
|
|
|
| Byssoid sp1 |
C |
+ |
+ |
|
+ |
|
1,2,4,12,14,20,21,25,27,32,38,39,41,50 |
| Pyrenulaceae |
|
|
|
|
|
|
|
| Pyenula mamillana (Ach.) Trevisan |
C |
+ |
+ |
|
|
|
2,8,5,17,27 |
| Pyrgillus sp. |
C |
|
+ |
|
|
|
41 |
| Teloschistaceae |
|
|
|
|
|
|
|
| Caloplaca ferruginea (Huds.) Th. Fr. |
C |
+ |
|
+ |
|
|
2,10,13,15,27,39,44 |
| Thelotremataceae |
|
|
|
|
|
|
|
| Ocellularia allosporoides (Nyl.) Patwardhan and Kulkarni |
C |
|
+ |
|
|
|
2,4,6,24,25,27,31,35,40,50, |
| O. groenhartii Hale |
C |
+ |
|
|
|
|
1,12,14,15,16,19,23,27,30,
36,38,39,40 |
| Thelotrema leprocarpum (Nyl.) Tuck. |
C |
|
+ |
+ |
|
+ |
5,14,18,34,44,45,48 |
| Trichotheliaceae |
|
|
|
|
|
|
|
| Porina interestes (Nyl.) Harm |
C |
+ |
+ |
|
|
|
1,8,27,31,44 |
| P. internigrans (Nyl.) Müll. Arg. |
C |
+ |
+ |
|
+ |
|
2,4,13,15,30,39,41,44 |
| Taxa |
Growth form |
Holebagilu |
Siganduru |
Muppane |
Karumane |
Honnemardu (I) |
Substrata |
| P. subcutanea Ach. |
C |
+ |
|
|
|
|
17,27,38 |
| P. subhibernica Upreti |
C |
+ |
|
+ |
|
+ |
4,5,6,10,11,12,13,16,17,20, 21, 23,24,26,27,29,31, 37,40,44,50,51,52 |
| Trypthilliaceae |
|
|
|
|
|
|
|
| Trypthelium tropicum (Ach.) Müll. Arg. |
C |
|
|
|
|
+ |
11 |
Note: C – Crustose, F – Foliose
Substratum of the Lichen Species
| No. |
Substratum |
No. |
Substatum |
No. |
Substratum |
| 1 |
Fallen twig |
2 |
Unidentified tree |
3 |
Rock |
| 4 |
Aglaia elaegnoidea |
5 |
Aporosa lindleyana |
6 |
Artocarpus integrifolius |
| 7 |
Artocarpus Sp. |
8 |
Canarium strictum |
9 |
Careya arborea |
| 10 |
Cinnamomum Sp. |
11 |
Diospyros condollena |
12 |
Diospyros crumenata |
| 13 |
Diospyros microphylla. |
14 |
Diospyrus Sp. |
15 |
Dysoxylun Sp. |
| 16 |
Elaeagnus latifolia. |
17 |
Elaeocarpus serratus |
18 |
Ervatamia heyneana |
| 19 |
Ficus asperrima. |
20 |
Ficus Sp. |
21 |
Garcinia morella |
| 22 |
Garcinia talbotii |
23 |
Holigarna arnottiana |
24 |
Holigarna grahamii |
| 25 |
Holigarna Sp. |
26 |
Homalium zeylanicum |
27 |
Hopea wightiana |
| 28 |
Ixora brachiata. |
29 |
Ixora parviflora |
30 |
Ixora Sp. |
| 31 |
Knema attenuata |
32 |
Lagestroemia lanceolata |
33 |
Linociera malabarica |
| 34 |
Litsea laevigata |
35 |
Mangifera indica |
36 |
Memecylon terminale |
| 37 |
Mimusops elengi |
38 |
Myristica malabarica |
39 |
Olea dioica |
| 40 |
Polyalthia Sp. |
41 |
Pterospermum reticulatum |
42 |
Salle (Kannada name) |
| 43 |
Strychnos nux-vomica |
44 |
Syzygium Sp. |
45 |
Terminalia arjuna |
| 46 |
Terminalia chebula |
47 |
Terminalia Sp. |
48 |
Terminalia tomentosa |
| 49 |
Toddalia asiatica |
50 |
Ventalago maderaspatana |
51 |
Vepris Sp. |
| 52 |
Ziziphus jujuba |
|
|
|
|
Table 8.5 Simpson’s (D) and Shannon-Weiner’s (H’) indices for the lichens of studied localities
Locality |
D |
H’ |
Honnemaradu (Island) |
1.26 |
1.397 |
Muppane |
1.21 |
1.548 |
Karumane |
1.14 |
1.675 |
Holebagilu |
1.11 |
1.969 |
Siganduru |
1.08 |
1.502 |
RANGE OF WILDLIFE, STATUS, DISTRIBUTION AND HABITAT
Forest type and cover: The biotic facing and edaphic variations have played a dominant role in determining the nature of the forests growing in the sanctuary. This sanctuary consists of multitiered vegetation that belongs to tropical evergreen type to moist deciduous type with lower, middle, top canopies, under growth and climbers. There are few areas where human interference is very low. On the fringes of villages the forest area has been degraded due to human interference as well as cattle pressure.Two types of forests are mainly found in sanctuary are:
- Southern Tropical evergreen type: This type is seen in Nagavalli, Kannor Kote, part of Biligar and Kattinkar areas. The top canopy consists of Depterocarpus indicus, Calophyllum tomentosum, Machilus mecarantha, Acrocarpus, fraxinopolius, Bischfia Javanica, Syzigium Species, Alstonia scholaris, Mangifera indica.Second canopy consists of caryota urens, Aporasa lindleyanaetc.
- Southern Tropical Semi-Evergreen type:This type of forest is seen in parts of Muppane, Attigodu Satate Forests. The important species found are, Lagertroemia lanceolata,Careya arborea,Emblica officinalis, Randia species, Syzygium species, Terminalia species, Vitex altisima, Mangifera indica, Artocarpus species and Bamboos in patches.
Animals: There are several kinds of animals in the sanctuary including carnivores, herbivores, omnivores and aquatic animals. The following are the important wild animals found in the sanctuary.
- Carnivores: Tiger (Panthera tigris), Panther (Normal and Black) (Panthera pardus), Wild Dogs (Cuon alpines), Wild cats (Felis chaus), Malabar civets (Viverricule indica), Hyena (Hyena hyena)
- Herbivores: Sambar (Cervus unicolor),Barking Deer (Muntiocus muntjack), Spotted Deer (Axis axis), Zusk Deer (Moschus moschiferus), Black naped hare (Lepus nigricolis), The gaur (Bision) (Bos gaurus), Mouse Deer (Tragulus meninna).
- Scvangers:Jackal (Canis aurus)
- Reptiles: Land monitor lizard (Veranus grisens), Python (Python molurus), King Cobra (Naja naja), Tortoises (Geochelone elegars)
- Other Animals: Flying Squirrel (Refuta indica), Giant malabar squirrel (Benus hylopetus), Indian Porcupine (Hystrix indica), Common langur(Presbytis enstellus), Bonnet monkey (Macaca radiate), Lion tailed maeaque (Macaca slenus),Sloth bear (Melursus ursinus), Wild bear (Sus scrofa).
Butterflies
The Western Ghats comprises 330 species belonging to 166 genera and five families. It includes the largest butterfly, the Southern Birdwing (Troides minos) with a wingspan of about 140-190mm to the smallest, the Grass Jewel (Freyeria trochylus) and Tiny Grass Blue (Zizula hylax) with wingspan only 15-22 mm and 16-24 mm respectively. Nymphalidae and Lycaenidae are the major families that contribute to the entire Western Ghats butterfly species diversity. SVWS comprises five butterfly families with 173 species (Table 9.3). The family composition and the conservation status of the butterflies in Western Ghats are cited in Table 9.4.
Most of the Swallotails (Family; Papilionidae) show habitat preferences and hence can be used as indicators of ecosystem health. For instance, Spot Sword tail is found only in the thick evergreen forest and its larval host plants are Thottea siliquosa,etc., are found in the core and buffer zone of the sanctuary. The swallowtails are also found puddling near the streams.
The Brush-footed butterflies of the family Nymphalidae are represented by 65 species in the sanctuary. They are well distributed in the sanctuary area and some are habitat specific in nature. The Map Butterfly, Blue Nawab and Malabar Tree Nymph are found only in the moist and shady places. Blue Nawab is an endangered species encountered in Banukuli locality. Indian Sunbeam is found in moist places within the sanctuary area. Family Hesperiidae, popularly known as the family of “Skippers” comprises of tiny butterflies found throughout the sanctuary. Spotted Small Flat is endemic species found only in few localities.
Table 9.3: Butterflies species in SVWS
| Species |
Common Name |
Family: Papilionidae
Papilionidae: Papilioninae: Troidini |
| Troides minos Cramer |
Southern Birdwing (WG) |
| Pachliopta pandiyana Moore |
Malabar Rose (WG) |
| Pachliopta aristolochiae Fabricius |
Common Rose |
| Pachliopta hector L., |
Crimson Rose (PI&SL)* |
| Papilionidae: Papilioninae: Leptocircini |
| Graphium sarpedon L., |
Common Bluebottle |
| Graphium doson C&R Felder |
Common Jay |
| Graphium agamemnon L., |
Tailed Jay |
| Graphium nomius Esper |
Spot Sword Tail |
| Graphium antiphates Cramer |
Fivebar Swordtail |
| Papilionidae: Papilioninae: Papilioninii |
| Papilio clytia L., |
Common Mime |
| Papilio demoleus L., |
Lime Butterfly |
| Papilio liomedon Moore |
Malabar Banded Swallow Tail (WG)* |
| Papilio dravidarum Wood-Mason |
Malabar Raven (WG) |
| Papilio helenus L., |
Red Helen |
| Papilio polytes L., |
Common Mormon |
| Papilio polymnestor Cramer |
Blue Mormon (PI&SL) |
| Papilio paris L., |
Paris Peacock |
| Papilio buddha Westwood |
Buddha Peacock |
| Papilio crino Fabricius |
Common Banded Peacock |
Family: Pieridae
Pieridae: Coliadinae: Coliadini |
| Catopsilia pomona Fabricius |
Common Emigrant |
| Catopsilia pyranthe L., |
Mottled Emigrant |
| Eurema brigitta Cramer |
Small Grass Yellow |
| Eurema laeta Boisduval |
Spotless Grass Yellow |
| Eurema hecabe L., |
Common Grass Yellow |
| Eurema blanda Boisduval |
Three-spot Grass Yellow |
| Eurema andersoni |
One spot Grass Yellow |
| Pieridae: Pierinae: Pierini |
| Delias eucharis Drury |
Common Jezebel (PI & SL) |
| Leptosia nina Fabricius |
Psyche |
| Pieris canidia L., |
Indian Cabbage White |
| Cepora nerissa Fabricius |
Common Gull |
| Anaphaeis aurota Fabricius |
Caper White or Pioneer |
| Appias indra Moore |
Plain Puffin |
| Appias libythea Fabricius |
Striped Albatross |
| Appias albina Boisduval |
Common Albatross |
| Colotis etrida Boisduval |
Small Orange Tip |
| Colotis eucharis Fabricius |
Plain Orange Tip |
| Colotis danae Fabricius |
Crimson Tip |
| Ixias marianne Cramer |
White Orange Tip |
| Ixias pyrene L., |
Yellow Orange Tip |
| Pieridae: Pierinae: Euchlocini |
| Pareronia valeria Cramer |
Common wanderer |
| Pareronia ceylanica C&R Felder |
Dark Wanderer (PI&SL) |
| Hebomoea glaucippe L., |
Great Orange Tip |
Family: Nymphalidae
Nymphalidae: Satyrinae: Melanitini |
| Melanitis leda L., |
Common Evening Brown |
| Melanitis zitenius Herbst |
Great Evening Brown |
| Melanitis phedima Stoll |
Dark Evening Brown |
| Nymphalidae: Satyrinae: Elymniini |
| Elymnias hypermenstra L., |
Common Palmfly |
| Lethe europa |
Bamboo Tree Brown |
| Lethe rohria |
Common Tree Brown |
| Mycalesis anaxias Hewitson |
White-bar Bushbrown |
| Mycalesis mineus L., |
Dark-brand Bushbrown |
| Mycalesis perseus Fabricius |
Common Bushbrown |
| Mycalesis subditaMoore |
Tamil Bushbrown |
| Mycalesis patniaMoore |
Glad-eye Bushbrown (PI&SL) |
| Orsotrioena medus Fabricius |
The Nigger |
| Zipoetis saitis |
Tamil Catseye (WG) |
| Nymphalidae: Satyrinae: Satyrini |
| Ypthima asterope Klug |
Common Three-ring |
| Ypthima hiiebneri Kirby |
Common Four-ring |
| Ypthima baldus Fabricius |
Common Five-ring |
| Ypthima sp. |
Ring |
| Nymphalidae: Charaxinae: Charaxini |
| Polyura athamas Drury |
Common Nawab |
| Polyura schreiber |
Blue Nawab (PI&SL)* |
| Charaxes bernardus |
Tawny Rajah |
| Charaxes dolon Fabricius |
Black Rajah |
| Nymphalidae: Heliconiinae: Acraeini |
| Acraea violae Fabricius |
Tawny Coster |
| Nymphalidae: Heliconiinae: Heliconiini |
| Cethosia nietneri C&R Felder |
Tamil Lacewing (PI&SL) |
| Vindula erota Fabricius |
Cruiser |
| Nymphalidae: Heliconiinae: Argynnini |
| Cupha erymanthis Drury |
Rustic |
| Phalanta phalantha Drury |
Common Leopard |
| Cirrochroa thais Fabricius |
Tamil Yeoman (PI&SL) |
| Argyreus hyperbius L., |
Indian Fritillary |
| Nymphalidae: Aparturinae |
| Euripus consimilis |
Painted Courtesan |
| Nymphalidae: Limenitinae: Neptini |
| Neptis jumbahMoore |
Chestnut-streaked Sailer |
| Neptis hylasMoore |
Common Sailer |
| Pantoporia hordonia Stoll |
Common Lascar |
| Nymphalidae: Limenitinae: Limetini |
| Athyma perius L., |
Common Sergeant |
| Athyma nefte |
Colour Sargeant |
| Athyma ranga Moore |
Blackvein Sergeant |
| Limenitis procris Cramer |
Commander |
| Nymphalidae: Limenitinae: Parthenini |
| Parthenos sylvia Cramer |
Clipper |
| Nymphalidae: Limenitinae: Euthaliinii |
| Tanaecia lepidea Butler |
Grey Count |
| Euthalia aconthea Cramer |
Common Baron |
| Euthalia nais Forster |
Red Baron or Baronet (PI&SL) |
| Dolpha evelina Stoll |
Red-spot Duke |
| Nymphalidae: Limenitinae: Biblini |
| Byblia ilithyia |
Joker |
| Ariadne merione Cramer |
Common Castor |
| Ariadne ariadne L., |
Angled Castor |
| Nymphalidae: Limenitinae: Marpesiini |
| Cyrestis thyodamas |
Map |
| Nymphalidae: Libytheinae |
| Libythea lepitaMoore |
Common Beak |
| Nymphalidae: Nymphalinae: Nymphalini |
| Junonia hierta Fabricius |
Yellow Pansy |
| Junonia orithya L., |
Blue Pansy |
| Junonia lemonias L., |
Lemon Pansy |
| Junonia almana L., |
Peacock Pansy |
| Junonia atlites L., |
Grey Pansy |
| Junonia iphita Cramer |
Chocolate Pansy |
| Kaniska canace |
Blue Admiral |
| Cynthia cardui L., |
Painted Lady |
| Hypolimnas bolina L., |
Great Eggfly |
| Hypolimnas misippus L., |
Danaid Eggfly (PI&SL)* |
| Doleschallia bisaltide |
Autumn Leaf |
| Kallima horsfieldi Kollar |
South Indian Blue Oak Leaf (WG) |
| Nymphalidae: Danainae: Danaini |
| Parantica aglea Stoll |
Glassy Blue Tiger |
| Tirumala limniace Cramer |
Blue Tiger |
| Tirumala septentrionisButler |
Dark Blue Tiger |
| Danaus chrysippus L., |
Plain Tiger |
| Danaus genutia Cramer |
Striped Tiger |
| Nymphalidae: Danainae: Euploeini |
| Euploea core Cramer |
Common Indian Crow |
| Idea malabarica Moore |
Malabar Tree Nymph (WG) |
Family: Lycaenidae
Lycaenidae: Riodininae: Riodinini |
| Abisara echerius Stoll |
Plum Judy |
| Lycaenidae: Miletinae: Spalgini |
| Spalgis epius West Wood |
Apefly |
| Lycaenidae: Polymmatinae: Polymmatini |
| Castalius rosimon Fabricius |
Common Pierrot |
| Caleta caleta Hewitson |
Angled Pierrot |
| Discolampa ethion Doubleday & Hewitson |
Banded Blue Pierrot |
| Leptotes plinius Fabricius |
Zebra Blue |
| Azanus ubaldus |
Bright Babul Blue |
| Everes lacturnus Godart |
Indian Cupid |
| Actolepis puspa Horsfield |
Common Hedge Blue |
| Neopithecops zalmoraButler |
Quaker |
| Pseudozizeeria maha Kollar |
Pale Grass Blue |
| Zizeeria karsandraMoore |
Dark Grass Blue |
| Zizina otis Fabricius |
Lesser Grass Blue |
| Zizula hylax Fabricius |
Tiny Grass Blue |
| Chilades laius Stoll |
Lime Blue |
| Freyeria trochylus Freyer |
Grass Jewel |
| Lampides boeticus L., |
Pea Blue |
| Jamides bochus Cramer |
Dark Cerulean |
| Jamides celeno Cramer |
Common Cerulean |
| Jamides alecto Felder |
Metallic Cerulean |
| Nacaduba pactolus |
Large four line blue |
| Nacaduba hermus |
Pale-4 line Blue |
| Prosotas nora C & R Felder |
Common Lineblue |
| Prosotas dubiosa |
Tailless Lineblue |
| Talicada nyseus Guerin-Meneville |
Red Pierrot |
| Lycaenidae: Theclinae: Arhopalini |
| Arhopala amantes Hewitson |
Large Oakblue |
| Thaduka multicaudata Moore |
Many-tailed Oakblue |
| Lycaenidae: Theclinae: Amblypodiini |
| Iraota timoleon Stoll |
Silverstreak Blue |
| Amblypodia anita Hewitson |
Leaf Blue |
| Lycaenidae: Theclinae: Aphnaeini |
| Spindasis vulcanus Fabricius |
Common Silverline |
| Lycaenidae: Theclinae: Loxurini |
| Loxura atymnus |
Yamfly |
| Lycaenidae: Theclinae: Horagini |
Rathinda amor |
Monkey Puzzle |
| Lycaenidae: Theclinae: Hypolycaenini |
| Zeltus amasa |
Fluffy tit |
| Lycaenidae: Theclinae: Deudorigini |
| Deudorix epijarbas |
Cornelian |
| Deudorix isocrates |
Common Guva Blue |
| Rapala manea Hewitson |
Slate Flash |
| Rapala varuna Moore |
Indigo Flash |
| Lycaenidae: Curetinae |
| Curetis thetis |
Indian Sunbeam (PI&SL) |
Family: Hesperiidae
Hesperiidae: Coeliadinae |
| Bibasis sena Moore |
Orange Tail Awl |
| Hasora chromus Cramer |
Common Banded Awl |
| Hasora badra Moore |
Common Awl |
| Badamia exclamationis Fabricius |
Brown awl |
| Hesperiidae: Pyrginae |
| Celaenorrhinus leucocera Kollar |
Common Spotted Flat |
| Celaenorrhinus ambareesa Moore |
Malabar Spotted Flat |
| Tagiades japetus Cramer |
Common Snow Flat |
| Tagiades litigiosa Moschler |
Water Snow Flat |
| Tagiades gana Moore |
Immaculate or Suffused Snow Flat |
| Pseudocoladenia dan Fabricius |
Fulvous Pied Flat |
| Coladenia indrani Moore |
Tricolor Pied Flat |
| Sarangesa dasahara Moore |
Common Small Flat |
| Sarangesa purendra Moore |
Spotted Small Flat (WG) |
| Odontoptilum angulatum C&R Felder |
Chestnut or Banded Angle |
| Spialia galba Fabricius |
Indian Grizzled Skipper |
| Hesperiidae: Hesperiinae |
| Ampittia dioscorides |
Bush Hopper |
| Halpe porus |
Moore's ace |
| Lambrix salsala Moore |
Chestnut Bob |
| Notocrypta paralysos Wood-Mason & de Niceville |
Common Banded Demon |
| Notocrypta curvifascia C & R Felder |
Restricted Demon |
| Udaspes folus Cramer |
Grass Demon |
| Suastus gremius Fabricius |
Indian Palm Bob |
| Suastus sp. |
Bob |
| Gangara thyrsis Fabricius |
Giant Redeye |
| Taractrocera maevius Fabricius |
Common Grass Dart |
| Talicota colon Fabricius |
Pale Palm Dart |
| Borbo cinnara Wallace |
Rice Swift |
| Pelopidas mathias |
Small branded swift |
Note: * indicates Endangered species (Wildlife Protection Act, 1972)
WG – indicates Western Ghats endemic
PI&SL – indicates Endemic to Peninsular India and Sri Lanka.
Table 9.4: Butterfly family composition in SVWS.
| Family |
India |
Western Ghats |
Inside the sanctuary |
| Total species |
Endangered |
Western Ghats Endemics |
Shared Endemics (Western Ghats and Sri Lanka) |
| Papilionidae |
107 |
19 |
19 |
2 |
5 |
3 |
| Pieridae |
109 |
33 |
23 |
|
|
2 |
| Nymphalidae |
520 |
96 |
65 |
2 |
3 |
6 |
| Lycaenidae |
450 |
101 |
38 |
|
|
1 |
| Hesperiidae |
320 |
81 |
28 |
|
1 |
|
Molluscs
Molluscs are one of the most diverse groups of invertebrates - both in form and habitat. They have figured prominently in palaeobiological and biological studies, and have served as study organisms in numerous evolutionary, biomechanical, ecological, physiological, and behavioural studies. Many species of freshwater mussels and snails are threatened or endangered throughout the world. Twenty-one species belonging to 7 families were recorded from different localities. The list of species collected has been given in Table 9.5. The plight of freshwater mussels is a prime example of the decline of aquatic habitats and the species that inhabit them. If trends are not reversed and stream degradation and loss of habitat continues many of the interesting and beautiful molluscs will be lost forever.
Table 9.5: Mollusc species in SVWS
| Family |
Genera/Species |
| Cyclophoridae |
Alycaeus expatriatus |
| Cyclophoridae |
Cyclophorus jerdoni |
| Cyclophoridae |
Theobaldius Sp. |
| Cyclophoridae |
Craspedoptris Sp. |
| Cyclophoridae |
Cyathophoma Sp. |
| Diplommatinidae |
Nicida liricincta |
| Diplommatinidae |
Ophisthosoma deccanense |
| Ariophantidae |
Ariophanta immerita |
| Ariophantidae |
Ariophanta canarica |
| Ariophantidae |
Ariophanta Sp. |
| Ariophantidae |
Euplecta Sp. |
| Ariophantidae |
Macrochalmys Sp. |
| Helixarionidae |
Kaliella Sp. |
| Helixarionidae |
Kaliella sigurensis |
| Streptaxidae |
Streptaxis canarica |
| Subulinidae |
Glessula Sp. a |
| Subulinidae |
Glessula Sp. b |
| Subulinidae |
Glessula Sp. c |
| Subulinidae |
Glessula Sp. d |
| Subulinidae |
Opeas Sp. |
| Vertiginidae |
Pupisoma Sp. |
| Unidentified |
Unidentified |
Reptiles
157 species of reptiles including a crocodile Crocodulus palustris is known from the Western Ghats. Out of 157 species 97 are endemics and majority of them are snakes. Twenty-three species of reptiles were recorded in the present study (Table 10.1). Three endemic species, viz., skink, malabar pit viper and bamboo pit viper were also recorded. Tortoises are commonly found in the reservoir. Crocodile (Crocodulus palustris) was recorded from reservoir at Madenur and Muppane area during the study. Two juveniles were caught in the nets of the fishermen at Holebagilu. There was an instance of livestock death due to crocodile at Muppane. King cobra is found in shady places and in the riparian vegetation dominated by Ochlandra sp. Malabar pit viper is found in between the buttresses of the huge trees of species like, Ficus nervosa, Syzigium gardneri and on the medium height shrubs. Hump nosed pit viper is found concealing in the litter cover.
Table 10.1: Reptiles of SVWS
| Common Name |
Scientific Name |
IUCN Status |
| Crocodile |
Crocodylus palustris |
VU |
| Common Indian Monitor Lizard |
Varnus bengalensis |
VU |
| Flapshell turtles |
Lissemys punctata |
Lr-lc |
| Indian Chameleon |
Chameleon zeylanicus |
VU |
| House Gecko |
Hemidactylus frenatus |
Lr-lc |
| Gunther’s Supple Skink |
*Lygosoma guentheri |
Lr-nt |
| King Cobra |
Ophiophagus hannah |
Lr-nt |
| The Cobra |
Naja naja |
Lr-nt |
| The Krait |
Bungarus caeruleus |
Lr-nt |
| Russell’s Viper |
Daboia russelli |
Lr-nt |
| Hump-nosed Pit Viper |
Hypnale hypnale |
Lr-nt |
| Saw Scaled Viper |
*Echis carinatus |
Lr-nt |
| Malabar Pit Viper |
*Trimersurus malabaricus |
Lr-nt |
| Bronzebacked Tree Snake |
Dendrelaphis tristis |
Lr-lc |
| The Vine Snake |
Ahaetulla nasuta |
Lr-nt |
| Flying Snake |
Chrysopelea ornata |
Lr-nt |
| Trinket Snake |
Elaphe helena helena |
Lr-nt |
| Checkered Keelback |
Xenochrophis piscator piscator |
Lr-lc |
| Montane Keelback |
*Amphiesma monticola |
Lr-nt |
| Common Sand Boa |
Eryx conicus conicus |
Lr-nt |
| Shieldtail |
*Uropeltis sp. |
|
| The Rat Snake |
Ptyas mucosus |
Lr-nt |
| Python |
Python molurus |
Lr-nt |
Note: * Western Ghats endemic
VU – Vulnerable, LR-lc – Lower risk least concerned, LR-nt – Lower risk near threatened.
Avifauna
Birds are a unique group of vertebrates and can indicate the quality of habitat or environment. In the ecosystem studies, birds play a pivotal role as predators of lower organisms and prey to higher vertebrates. The bird diversity of an area not only indicates its health but also stability. A total of 122 bird species were sighted in the sanctuary area during the study period (Table 10.2). Inside the sanctuary, few localities are very important in bird diversity like, Muppane, Kanur and Govardhanagiri state forest. Muppane nature camp is an important area for bird watching as it is surrounded by heterogenous habitats from Scrub jungle to Semi-evergreen forest and also reservoir. The presence of some of the endemic and endangered species like, Malabar Grey Hornbill, and Great Indian Hornbill found in these areas signifies the presence of primary forest remnants. During the study, a flock of great Indian hornbill with 14 individuals were found in the Govardhanagiri forest. Apart from that, in some of the islands, malabar grey and Malabar Pied Hornbill are encountered which in turn shows the presence of endemic tree species, like, Myristica malabarica, Knema attenuata. In some of the forest enclosures the presence of green pigeons, hornbills and parakeets signifies also the presence of their foraging plant species.
Table 10.2: Birds of SVWS
| Family |
Sub-family |
Common name |
Scientific name |
| Podicipitidae |
|
Little grebe |
Podiceps ruficollis (Pallas) |
| Phalacrocoracidae |
|
Large cormorant |
Phalacrocorax carbo L., |
| |
|
Little cormorant |
Phalacrocorax niger Vieillot |
| |
|
Darter |
Anhinga rufa (Daudin) |
| Ardeidae |
|
Night heron |
Nycticorax nycticorax L., |
| |
|
Purple heron |
Ardea purpurea L., |
| Grey heron |
Ardea cinerea L., |
| Paddy bird |
Ardeola grayii (Sykes) |
| |
|
Cattle egret |
Bubulcus ibis L., |
| |
|
Median egret |
Egretta intermedia (Wagler) |
| |
|
Little egret |
Egretta garzetta L., |
| Ciconiidae |
|
White necked stork |
Ciconia episcopus (Boddaert) |
| Threskiornithidae |
|
White ibis |
Threskiornis aethiopica (Latham) |
| Accipitridae |
|
Pariah kite |
Milvus migrans (Boddaert) |
| |
|
Brahminy kite |
Haliastur indus (Boddaert) |
| |
|
Shikra |
Accipiter badius (Gmelin) |
| |
|
Tawny eagle |
Aquila vindhiana Franklin |
| |
|
Crested serpent eagle |
Spilornis cheela (Latham) |
| |
|
Indian griffon vulture |
Gyps fulvus (Hablizl) |
| Phasianidae |
|
Grey jungle fowl |
Gallus sonneratii Temminck |
| |
|
Red spurfowl |
Galloperdix spadicea (Gmelin) |
| |
|
Common pea fowl |
Pavo cristatus L., |
| Rallidae |
|
White breasted waterhen |
Amaurornis phoenicurus (Pennant) |
| Jacanidae |
|
Bronzewinged jacana |
Metopidius indicus Latham |
| Charadriidae |
Charadriinae |
Spotted sandpiper |
Tringa glareola L., |
| |
|
Little ringed plover |
Charadrius dubius Scopoli |
| |
|
Redwattled lapwing |
Vanellus indicus (Boddaert) |
| |
|
Yellowwattled lapwing |
Vanellus malabaricus (Boddaert) |
| Laridae |
|
River tern |
Sterna aurantia J.E.Gray |
| Columbidae |
|
Orangebreasted green pigeon |
Treron pompadora (Jerdon) |
| |
|
Greyfronted green pigeon |
Treron pompadora (Gmelin) |
| |
|
Green imperial pigeon |
Ducula aenea L., |
| |
|
Blue rock pigeon |
Columba livia (Gmelin) |
| |
|
Spotted dove |
Streptopelia senegalensis (Scopoli) |
| |
|
Emerald dove |
Chalcophaps indica L., |
| Psittacidae |
|
Roseringed parakeet |
Psittacula krameri (Scopoli) |
| |
|
Blossomheaded parakeet |
Psittacula cyanocephala (L.,) |
| |
|
Lorikeet |
Loriculus vernalis (Sparrman) |
| |
|
Bluewinged parakeet |
Psittacula columboides (Vigors)* |
| Cuculidae |
|
Cuckoo |
Cuculus canorus L., |
| |
|
Indian cuckoo |
Cuculus micropterus Gould |
| |
|
Koel |
Eudynamys scolopacea L., |
| |
|
Sirkeer cuckoo |
Taccocua leschenaultii Lesson |
| |
|
Crow-pheasant |
Centropus sinensis Stephens |
| Strigidae |
Striginae |
Forest eagle-owl |
Bubo nipalensis Hodgson |
| Trogonidae |
|
Southern trogon |
Harpactes fasciatus (Pennant) |
| Alcedinidae |
|
Small blue kingfisher |
Alcedo atthis L., |
| |
|
Whitebreasted kingfisher |
Halcyon smyrnensis L., |
| |
|
Small blue kingfisher |
Alcedo atthis (L.,) |
| |
|
Pied kingfisher |
Ceryle rudis L., |
| Meropidae |
|
Chestnutheaded bee-eater |
Merops leschenaulti (Vieillot) |
| |
|
Small green bee-eater |
Merops orientalis (Latham) |
| Upupidae |
|
Hoopoe |
Upupa epops L., |
| Bucerotidae |
|
Common grey hornbill |
Tockus birostris (Scopoli) |
| |
|
Malabar grey hornbill |
Tockus griseus Latham |
| |
|
Great pied hornbill |
Buceros bicornis L., * |
| |
|
Malabar pied hornbill |
Anthracoceros malabaricus Boddaert * |
| Capitonidae |
|
Crimson throated barbet |
Megalaima rubricapilla Gmelin |
| |
|
Small green barbet |
Megalaima viridis Boddaert |
| |
|
Large green barbet |
Megalaima zeylanica Gmelin |
| Picidae |
|
Indian goldenbacked threetoed woodpecker |
Dinopium javanense (Ljungh) |
| |
|
Great black woodpecker |
Dryocopus javensis (Horsfield) |
| |
|
Heartspotted woodpecker |
Hemicircus canente L., |
| |
|
Great black woodpecker |
Dryocopus javensis (Horsfield) |
| Alaudidae |
|
Crested lark |
Galerida cristata (L.,) |
| Hirundinidae |
|
Swallow |
Hirundo rustica L., |
| |
|
Wiretailed swallow |
Hirundo smithii Leach |
| Oriolidae |
|
Golden oriole |
Oriolus oriolus L., |
| |
|
Blackheaded oriole |
Oriolus xanthornus L., |
| Daniidae |
|
Rufousbacked shrike |
Lanius schach L., |
| Dicruridae |
|
Black drongo |
Dicrurus adsimilis (Bechstein) |
| |
|
Racket-tailed drongo |
Dicrurus paradiseus L., |
| Sturnidae |
|
Brahminy myna |
Sturnus pagodarum (Gmelin) |
| |
|
Rosy pastor |
Sturnus roseus L., |
| |
|
Indian myna |
Acridotheres tristis L., |
| |
|
Jungle myna |
Acridotheres fuscus (Wagler) |
| |
|
Hill myna |
Gracula religiosa L., |
| |
|
Bank myna |
Acridotheres ginginianus (Latham) |
| Corvidae |
|
House crow |
Corvus splendens Vieillot |
| |
|
Tree pie |
Dendrocitta vagabunda (Latham) |
| Campephagidae |
|
Scarlet minivet |
Pericrocotus roseus (Forster) |
| Irenidae |
|
Iora |
Aegithina tiphia L., |
| |
|
Goldmantled chloropsis |
Chloropsis cochinchinensis (Gmelin) |
| |
|
Fairy bluebird |
Irena puella (Latham) |
| Pycnonotidae |
|
Redvented bulbul |
Pycnonotus cafer L., |
| |
|
Rubythroated bulbul |
Pycnonotus melanicterus gularis Gould |
| |
|
Greyheaded bulbul |
Pycnonotus priocephalus Jerdon |
| |
|
Redwhiskered bulbul |
Pycnonotus jocosus L., |
| |
|
Yellowbrowed bulbul |
Hypsipetes indicus (Jerdon) |
| Muscicapidae |
Timaliinae |
Common babbler |
Turdoides caudatus (Dumont) |
| |
|
Rufous babbler |
Turdoides subrufus (Jerdon) |
| |
|
Blackheaded babbler |
Rhopocichla atriceps |
| |
|
Jungle babbler |
Turdoides striatus (Dumont) |
| |
|
Slatyheaded scimitar babbler |
Pomatorhinus horsfieldii Sykes |
| |
Muscicapinae |
Paradise flycatcher |
Terpsiphone paradisi L., |
| |
|
Redbreasted flycatcher |
Muscicapa ruficauda Swainson |
| |
|
Verditer flycatcher |
Muscicapa thalassina Swainson |
| |
Sylviinae |
Indian greatreed warbler |
Acrocephalus stentoreus (Hemprich & Ehrenberg) |
| |
Turdinae |
Magpie-robin |
Copsychus saularis L., |
| |
|
Indian robin |
Saxicoloides fulicata L., |
| |
|
Blue chat |
Erithacus brunneus (Hodgson) |
| |
|
Blue rock thrush |
Monticola solitarius L., |
| |
|
Malabar whistling thrush |
Myiophonus horsfieldii (Vigors) |
| |
|
Orangeheaded ground thrush |
Zoothera citrina (Latham) |
| Paridae |
Parinae |
Yellowcheeked tit |
Parus xanthogenys Vigors |
| Sittidae |
Sittinae |
Velvetfronted nuthatch |
Sitta frontalis Swainson |
| Motacillidae |
|
Forest wagtail |
Motacilla indica Gmelin |
| |
|
Yellow wagtail |
Motacilla flava L., |
| |
|
Yellowheaded wagtail |
Motacilla citreola Pallas |
| |
|
Grey wagtail |
Motacilla cinerea Tunstall |
| |
|
White wagtail |
Motacilla alba L., |
| |
|
Large pied wagtail |
Motacilla maderaspatensis |
| Dicaeidae |
|
Thickbilled flowerpecker |
Dicaeum agile (Tickell) |
| Nectariniidae |
|
Purplerumped sunbird |
Nectarinia zeylonica L., |
| |
|
Small sunbird |
Nectarinia minima (Sykes) |
| |
|
Purple sunbird |
Nectarinia asiatica (Latham) |
| Zosteropidae |
|
White-eye |
Zosterops palpebrosus (Temminck) |
| Ploceidae |
Passerinae |
House sparrow |
Passer domesticus L., |
| |
Ploceinae |
Baya weaver bird |
Ploceus philippinus L., |
| |
Estrildinae |
Spotted munia |
Lonchura punctulata L., |
| |
|
Blackheaded munia |
Lonchura malacca L., |
| |
|
Whitebacked munia |
Lonchura striata L., |
* Endemic birds of the region
Mammals
Mammals are the group of animals that have reached a pinnacle during the evolution of life. In wildlife conservation, prioritisation is mainly given to mammals, because of their direct relevance to human beings. Their presence in the wild is an indication of the health of that habitat. The study area harbours many mammalian species as listed in Table 11. Of the 43 mammals recorded from the sanctuary, Tiger and Lion-tailed Macaque are endangered, and leopard is vulnerable. Table 11 also lists the bats (flying mammals) found in this region. The endemic and endangered lion tailed macaque is sighted in the Karani area. Reports indicate their presence in Kogar, Gurta, Kodachadri and Sharavathi valley area. Now its population is on the decline due to the destruction and fragmentation of habitat and hunting for its skin and meat.
Tigers (Panthera tigris tigris) inhabit mostly in the evergreen and moist deciduous forests. According to forest department 7 leopard and 2 tigers were found in the sanctuary and its immediate surroundings (Figure 2.3). In the sanctuary area tiger was sighted near Shashichowka, Kogar, Karumane and Karani. Linganamakki catchment area has records of 4 leopards and 6 tigers.
Table 11: Mammals of SVWS
| Common Name |
Scientific Name |
IUCN Status |
| Slow Loris |
Loris tardigradus |
Lr-nt |
| Bonnet Macaque |
Macaca radiata |
Lr-lc |
| Lion-tailed Macaque |
*Macaca silenus |
EN |
| Hanuman Langur |
Semnopithecus entellus |
|
| Indian Jackal |
Canis aureus |
Lr-lc |
| Indian Fox |
Vulpes bengalensis |
Lr-nt |
| Wild Dog or Dhole |
*Cuon alpinus |
Lr-nt |
| Sloth Bear |
Melursus ursinus |
VU |
| Common Otter |
Lutra lutra |
NE |
| Jungle Cat |
Felis chaus |
Lr-nt |
| Fishing Cat |
Felis viverrina |
VU |
| Brown Palm Civet |
*Paradoxurus jerdoni |
VU |
| Small Indian Civet |
Viverricula indica |
Lr-nt |
| Common Indian Mongoose |
Herpestes edwardsi |
Lr-lc |
| Small Indian Mongoose |
Herpestes auropunctatus |
|
| Three-striped Palm Squirrel |
Funambulus palmarum |
Lr-lc |
| Jungle striped Squirrel |
*Funambulus tristriatus |
Lr-nt |
| Indian Giant Squirrel |
*Ratufa indica |
VU |
| Common Giant Flying Squirrel |
Petaurista petaurista |
Lr-nt |
| Indian Porcupine |
Hystrix indica |
Lr-lc |
| Indian Pangolin |
Manis crassicaudata |
Lr-nt |
| Black-naped Hare |
Lepus nigricollis |
Lr-lc |
| Leopard |
Panthera pardus |
VU |
| Tiger |
Panthera tigris |
EN |
| Wild Boar |
Sus scrofa cristatus |
Lr-lc |
| Indian Spotted Chevrotain or Mouse Deer |
Tragulus meminna |
Lr-nt |
| Barking deer or The Muntjac |
Muntiacus muntjak |
Lr-lc |
| Spotted Deer or Chital |
Axis axis |
Lr-lc |
| Sambar |
Cervus unicolor |
Lr-lc |
| The Gaur or Indian Bison |
Bos gaurus |
VU |
| Fulvous fruit bat |
Rousettus leschnaulti |
Lr-lc |
| Black-bearded tomb bat |
Taphozous melanopogon |
Lr-nt |
| Pouch bearing bat |
Saccolaimus saccolaimus |
DD |
| Greater false vampire |
Megaderma derma |
|
| Lesser false vampire |
Megaderma spasma |
DD |
| Blyth's horse-shoe bat |
Rhinolophus lepidus |
Lr-nt |
| Fulvous leaf-nosed bat |
Hipposideros fulvus |
Lr-nt |
| Kantor's leaf-nosed bat |
Hipposideros Sp. |
|
| Schneider's leaf-nosed bat |
Hipposideros speoris |
Lr-nt |
| Kelaart's leaf-nosed bat |
Hipposideros lankadiva |
VU |
| Burmese whiskered bat |
Myotis montivagus |
DD |
| Least pipistrelle |
Pipistrellus tenuis |
Lr-lc |
| Kelaart's pipistrelle |
Pipistrellus ceylonicus |
Lr-lc |
Note: * Western Ghats endemic
VU – Vulnerable, EN – Endangered, LR-lc – Lower risk least concerned, LR-nt – Lower risk near threatened, NE - Not evaluated.
Wildlife census data in the Kogaru and Kargal range in 1997
| Animals |
Kogaru range |
Kargal range |
| Block counting |
Line transect |
Block counting |
| Gaur |
52 |
65 |
61 |
| Spotted deer |
68 |
30 |
36 |
| Sambar |
13 |
18 |
13 |
| Wild Boar |
42 |
60 |
64 |
| Urial |
7 |
3 |
4 |
| Dhole |
|
9 |
17 |
| Langur |
122 |
40 |
57 |
| Sloth Bear |
4 |
|
|
| Monkey |
170 |
25 |
39 |
| Red giant squirrel |
64 |
14 |
7 |
| Peacock |
80 |
17 |
10 |
| Indian Hare |
|
6 |
3 |
| Jungle fowl |
|
10 |
16 |
| Barking deer |
|
5 |
3 |
| Hornbill |
|
6 |
2 |
| Owl |
|
2 |
2 |
| Monitor lizard |
|
4 |
4 |
| Tiger |
|
1 |
|
| Leopard |
4 (male – 3, female – 1) |
1 (male –1) |
|
Source: Tiger, Leopard and other wildlife census of 1997. Office of the Deputy Conservator of Forest, Shimoga Wildlife Division, Shimoga
Figure 2.3: Tiger and leopard distribution in SVWS
Aquatic Ecosystem
Aquatic ecosystems contribute to a large proportion of the planet's biotic productivity as about 30% of the world's primary productivity comes from plants living in the ocean. These ecosystems also include riverbanks, wetlands located at lakeshores, the ocean shoreline, and any habitat where the soil or vegetation is submerged for some duration. These ecosystems have been subjected to various levels of stresses, due to unplanned developmental activities in the last century. Anthropogenic activities involving changes in land use ultimately affects the receiving water in that drainage. Activities include unplanned agricultural practices, unscientific usage of inorganic fertiliser, pesticides and herbicides applied to crops, silt washed away because of vegetation removal, or even due to atmospheric deposition, or disposal of solid and liquid wastes. The Linganamakki reservoir (in eastern part) and Talakalale reservoir (northern side) form a part of the lacustrine ecosystem in SVWS, while many first and second order streams of river Sharavathi and Venkatapura forms the lotic ecosystem in the sanctuary.
Phytoplankton
Phytoplankton are the microscopic suspended algae that occur in different forms such as unicellular, colonial or filamentous, which are mainly photosynthetic in nature. They are one of the most rapid detectors of environmental change and are regarded as the primary producers in aquatic food chain. The family, genus and species composition of phytoplankton is listed in Table 12.1. Species list is given in Table 12.2
Table 12.1: Familywise composition of phytoplankton.
| Family composition |
I-collection |
II-collection |
III -collection |
| Desmidials |
50 |
51 |
48 |
| Bacillariophyceae |
12 |
25 |
22 |
| Cyanophyceae |
4 |
6 |
6 |
| Chlorococcales |
3 |
9 |
8 |
| Dinophyceae |
2 |
2 |
4 |
| Chrysophyceae |
|
1 |
4 |
| Total no. of genera |
44 |
64 |
61 |
| Total no. of species |
71 |
94 |
92 |
Qualitative dominance of the phytoplankton was in the order of Desmidials > Bacillariophyceae > Cyanophyceae >Chlorococcales > Dinophyceae in the first sampling. In this collection population of Desmidial member Staurastrum multispiniceps was highest (58,944/L) in Muppane of the reservoir. While, in the second sampling, qualitative dominance was in the order of Desmidials > Bacillariophyceae > Chlorococcales > Cyanophyceae > Dinophyceae >Chrysophyceae. Similarly in the third collection, it was in the order of Desmidials > Bacillariophyceae > Chlorococcales > Cyanophyceae > Dinophyceae = Chrysophyceae. Table 12.3 reveals diversity and diversity indices calculated in various sampling localities.
Table 12.2: Phytoplankton species in SVWS
| I. Muppane |
| Bacillariophyceae (Diatoms) |
Collections |
| I |
II |
III |
| Cymbella chandolensis Gandhi |
|
+ |
|
| Gyrosigma attenuatum (Kuetz.) Rabh. |
|
+ |
|
| Melosira islandica O. Muell v. helvetica O. Muell |
|
+ |
+ |
| M. granulata (Ehr.) Ralfs. v. mazzanensis Meister |
|
|
+ |
| Navicula pygmaea Kuetz. v. indica Skv |
+ |
|
|
| N.viridula Kuetz. V. capitata Mayer |
|
+ |
+ |
| Nitzschia philippinarum Hustedt |
+ |
|
|
| Pinnularia streptoraphe Cleve |
|
|
+ |
| Synedra ulna (Nitz.) Ehr. v. danica Kuetz. Grun. |
|
|
+ |
| Desmidials |
| Arthrodesmus curvatus Turn |
+ |
|
|
| A. psilosporus (Nodrdst. & Lofg.) De Toni Formae |
+ |
|
+ |
| Cosmarium contractum Kirchn |
|
+ |
+ |
| C. ordinatum (Borges) West & West var.borgei Scott Gronbl. |
+ |
|
|
| C. lundellii Delp var circulare (Reinch) Krieg |
+ |
|
|
| C. spinuliferum West & West |
|
|
+ |
| Desmidium baileyi (Ralfs) Nordst fa.longiprocessum |
+ |
|
|
| D. baileyi (Ralfs) Nordst fa. tetragonum Nordst |
|
+ |
|
| Euastrum gnathophorum West & West |
|
|
+ |
| Spondylosium planum (Wolle.) West & West |
+ |
|
|
| Staurastrum cerastes Scott.& Presc. |
+ |
|
|
| S. euprepes |
+ |
|
|
| S. freemanii West & West var.nudiceps Scott & Presc. |
+ |
+ |
+ |
| S. limneticum Schm. Var. burmense West & West |
+ |
+ |
+ |
| S. multispiniceps |
+ |
|
+ |
| S. peristephes |
|
+ |
+ |
| S. prionotum |
|
+ |
+ |
| S. sexangulare Lund var.productum Nordst |
+ |
|
|
| S. tauphorum West & West |
+ |
|
|
| S. thienemannii Krieg |
|
|
+ |
| S. tohopekaligense Wolle var. insigne West & West Formae |
+ |
|
+ |
| Triploceros gracile Bail fa. curvatum |
+ |
+ |
+ |
| T. gracile Bail fa. undulatum Scott & Presc. |
|
|
+ |
| Xanthedium hastiferum Turn. Var. javanicum (Nordst.) Turn. fa. Planum Turn |
|
|
|
| X. perissacanthum Scott. & Presc. Var. minus. |
+ |
+ |
|
| Chlorococcales |
| Ankistrodeimus falcatus (Corda) Ralfs |
+ |
|
+ |
| Cyanophyceae |
| Aphanocapsa rivularis (Carm) Rabenhorst |
|
|
+ |
| Chroococcus limneticus var.elegans G. M. Smith |
+ |
|
|
| Microcystis aeruginosa Kuetz, emend, Elenkin |
|
+ |
|
| Dinophyceae |
| Ceratium hirundinella (O.F. Muell) Dujardin |
+ |
|
|
| Chrysophyceae |
| Dinobryon sertularia Ehrberg. |
|
|
|
| II. Talakalale |
| Bacillariophyceae (Diatoms) |
Collections |
| I |
II |
III |
| Cymbella powaiana Gandhi |
|
|
+ |
| Gomphonema longiceps Ehr. v. subclavata Grun |
|
+ |
+ |
| Melosira islandica O. Muell v. helvetica O. Muell |
|
+ |
+ |
| Navicula cari Ehr. |
|
+ |
|
| N.viridula Kuetz. V. capitata Mayer |
|
+ |
|
| Pinnularia maharashtrensis |
|
|
+ |
| Synedra ulna (Nitz.) Ehr. v. danica Kuetz. Grun. |
|
+ |
|
| Desmidials |
| Arthodesmus psilosporus (Nodrdst. & Lofg.) De Toni Formae |
+ |
|
+ |
| Cosmarium contractum Kirchn |
|
+ |
+ |
| C. subturgidum (Turn.) Schm. Fa. minus Schm |
|
+ |
|
| Desmidium baileyi (Ralfs) Nordst |
+ |
|
|
| D. baileyi (Ralfs) Nordst fa. tetragonum Nordst |
|
+ |
|
| Euastrum gnathophorum West & West |
|
+ |
+ |
| Spondylosium planum (Wolle.) West & West |
+ |
|
|
| Staurastrum freemanii West & West var.nudiceps Scott & Presc. |
+ |
+ |
+ |
| S. limneticum Schm. Var. burmense West & West |
+ |
+ |
|
| S. longibrachiatum (Borge) Gutz. |
+ |
|
|
| S. multispiniceps. |
+ |
|
|
| S. peristephes |
+ |
+ |
+ |
| S. prionotum |
|
+ |
+ |
| S. sexangulare Lund var.productum Nordst |
+ |
|
|
| S. rosei Playf. var. stemmatum |
|
|
+ |
| S. thienemannii Krieg fa. triradiatum |
|
|
+ |
| Triploceros gracile Bail fa. curvatum |
|
+ |
|
| X. perissacanthum Scott. & Presc. Var. minus. |
+ |
|
|
| Chlorococcales |
| Ankistrodeimus falcatus (Corda) Ralfs |
|
|
+ |
| Eudorina elegans Ehrenberg |
+ |
|
|
| Scenedesmus acuminatus (Lag) Chodat |
|
|
+ |
| S. dimorphus (Turp.) Kuetzing |
|
+ |
|
| S. opoliensis var contacta Prescott |
|
|
+ |
| Cyanophyceae |
| Aphanocapsa rivularis (Carm) Rabenhorst |
|
+ |
|
| Chroococcus limneticus var.elegans G. M. Smith |
+ |
|
|
| Coelosphaerium dubium Grunow |
|
|
+ |
| Dinophyceae |
| Ceratium hirundinella (O.F. Muell) Dujardin |
+ |
+ |
+ |
| III. Reservoir centre |
| Bacillariophyceae (Diatoms) |
Collections |
| I |
II |
III |
| Cymbella ventricosa Kuetz. |
|
+ |
|
| Gomphonema lanceolatum Her |
|
+ |
|
| G. longiceps Ehr. v. subclavata Grun |
+ |
|
+ |
| Gyrosigma attenuatum (Kuetz.) Rabh. |
|
+ |
|
| Melosira islandica O. Muell v. helvetica O. Muell |
|
+ |
+ |
| Navicula cari Ehr. |
|
|
+ |
| N.viridula Kuetz. V. capitata Mayer |
|
+ |
+ |
| N. subdapaliformis Gandhi |
+ |
|
|
| Pinnularia lundii Hustedt |
|
|
+ |
| Desmidials |
| Arthodesmus psilosporus (Nodrdst. & Lofg.) De Toni Formae |
|
+ |
+ |
| Cosmarium contractum Kirchn |
|
+ |
|
| C. margaritatum (Lund) Roy & Biss var sublatum (Nordst.) Krieg |
|
+ |
|
| C. scabrum Turn |
|
+ |
|
| Desmidium baileyi (Ralfs) Nordst fa.longiprocessum |
+ |
|
|
| Euastrum ansatum Ehr. v. Triporum |
+ |
|
|
| E. gnathophorum West & West var.bulbosum |
|
+ |
+ |
| Pleurotaenium ehrenbergi (Breb.) De Bary v. undulatum Schaarschm |
|
|
+ |
| Spondylosium planum (Wolle.) West & West |
+ |
|
|
| Staurastrum cerates Lund var pulchrum Scott & Gronbl. fa |
|
+ |
|
| S. freemanii West & West var.nudiceps Scott & Presc. |
+ |
+ |
+ |
| S. limneticum Schm. Var. burmense West & West |
+ |
+ |
|
| S. longibrachiatum (Borge) Gutz. |
+ |
|
|
| S. multispiniceps |
+ |
|
|
| S. peristephes |
+ |
+ |
|
| S. prionotum |
|
+ |
+ |
| S. sexangulare Lund var.productum Nordst |
+ |
|
|
| S. thienemannii Krieg fa. triradiatum |
|
+ |
|
| X. perissacanthum Scott. & Presc. Var. minus. |
+ |
|
|
| Chlorococcales |
| Ankistrodeimus falcatus (Corda) Ralfs |
|
|
+ |
| Pediastrum simplex Meyen |
|
+ |
|
| Scenedesmus acuminatus (Lag) Chodat |
|
|
+ |
| Cyanophyceae |
| Chroococcus limneticus var.elegans G. M. Smith |
+ |
|
|
| C. turgidus (kuetz.) Naegeli |
|
+ |
|
| Microcystis aeruginosa Kuetz, emend, Elenkin |
|
+ |
+ |
| Dinophyceae |
| Ceratium hirundinella (O.F. Muell) Dujardin |
|
|
+ |
| IV. Yennehole |
| Bacillariophyceae (Diatoms) |
Collections |
| I |
II |
III |
| Eunotia praerupta Ehr. |
+ |
+ |
|
| Gomphonema gracile Ehr. v. intricatiforme Mayer |
+ |
|
|
| G.lanceolatum Her |
|
+ |
|
| Melosira islandica O. Muell v. helvetica O. Muell |
|
|
+ |
| M. granulata (Ehr.) Ralfs. v. mazzanensis Meister |
|
|
+ |
| Navicula cuspidata Kuetz.f.brevirostrata Gandhi |
+ |
|
|
| N. laeta A. Mayer |
+ |
|
|
| N. viridula Kuetz. |
+ |
|
|
| Nitzschia closterium W. Smith |
+ |
|
|
| N. obtusa W. Smith v. scalpelliformis Grun |
+ |
|
|
| Pinnularia gracioloides Hustedt |
|
|
+ |
| Synedra acus Kuetz. |
+ |
|
|
| Desmidials |
| Arthrodesmus constrictus G. M Smith var.longispinus Gronbl. |
|
+ |
+ |
| A.curvatus Turn.var.latus |
+ |
|
|
| Closterium ehrenbergii Menegh |
|
+ |
|
| C. porrectum Nordst |
+ |
|
|
| C. ralfsii Breb var.hybridrum Rab |
|
+ |
|
| Cosmarium askenasyi Schm.fa.latum Scott & Presc |
|
|
+ |
| C. contractum Kirchn |
|
+ |
|
| C. decoratum West & West |
|
|
+ |
| C. pseudoconnatum Nordst |
+ |
|
|
| C. punctulatum Breb.var.sub punctulatum (Nordst.) Borges |
+ |
|
|
| Desmidium baileyi (Ralfs) Nordst fa.longiprocessum |
|
+ |
|
| D. bengalicum Turn |
+ |
|
|
| D. quadratum Nordst |
|
+ |
|
| Euastrum gnathophorum West & West var.bulbosum |
|
+ |
|
| E. sinuosum Lenorm. var. parallelum Krieg |
+ |
|
|
| Gonatozygon aculeatum Hastings |
|
+ |
+ |
| Hyalotheca dissiliens (Smith) Breb. var. hains Wolle |
+ |
|
|
| Micrasterias foliacea Bail var. quadrinflata |
|
+ |
|
| M. mahabuleshwarensis Hobs.var.chauliodon |
+ |
|
|
| M. quadridentata (Nordst.) Gronbl.fa, indonesinsis |
|
|
+ |
| Staurastrum anceps Her. |
|
+ |
|
| S. anceps Ehr. v. hyalina Brun. et.Perag |
|
|
+ |
| S. freemanii West & West var.nudiceps Scott & Presc. |
+ |
|
+ |
| S. limneticum Schm. Var. burmense West & West |
+ |
|
+ |
| S. multispiniceps |
+ |
|
|
| S. peristephes |
|
+ |
|
| S. sebaldi Reinsch var.ornatum Nordst |
|
|
+ |
| S. tohopekaligense Wolle var. trifurcatum West & West |
+ |
|
|
| S. wildmanii Gutw. |
|
+ |
|
| Chlorococcales |
| Ankistrodeimus falcatus (Corda) Ralfs |
+ |
|
+ |
| A. spiralis (Turner) Lemmermann |
+ |
|
|
| Gomphosphaeria aponina var. delicatula virieux |
|
+ |
|
| Kirchnerilla lunaris (Krich.) Moebius |
+ |
|
|
| Muogeotia punctata Wittrock |
|
+ |
|
| Pediastrum simplex Meyen |
|
|
+ |
| Scenedesmus bijuga (Turp.) Lagerheim |
|
+ |
|
| Cyanophyceae |
| Microcystis aeruginosa Kuetz, emend, Elenkin |
+ |
+ |
+ |
| Gomphosphaeria aponina var. cordiformis Wolle |
|
|
+ |
| Dinophyceae |
| Ceratium hirundinella (O.F. Muell) Dujardin |
|
|
+ |
| Chrysophyceae |
| Dinobryon calciformis Bachmann |
|
|
+ |
| D. divergens Imhof |
|
+ |
|
| D. sertularia Ehrbg. |
|
|
+ |
| V. Madenur |
| Bacillariophyceae (Diatoms) |
Collections |
| I |
II |
III |
| Cymbella laevis Naeg |
|
+ |
|
| C. ventricosa Kuetz. |
|
+ |
|
| Gomphonema lanceolatum Her |
|
+ |
|
| G. longiceps Ehr. v. subclavata Grun |
|
+ |
+ |
| Gyrosigma attenuatum (Kuetz.) Rabh.(Nordst & Lofg.) De Toni |
|
+ |
|
| Melosira granulata (Ehr.) Ralfs. v. mazzanensis Meister |
|
|
+ |
| M. islandica O. Muell v. helvetica O. Muell |
|
+ |
+ |
| Navicula viridula Kuetz. V. capitata Mayer |
|
+ |
+ |
| Nitzschia obtusa W. Smith v. scalpelliformis Grun |
|
+ |
|
| N. radiosa Kuetz. |
|
+ |
|
| Desmidials |
| Arthodesmus psilosporus (Nodrdst. & Lofg.) De Toni Formae |
|
+ |
+ |
| Cosmarium contractum Kirchn |
|
|
+ |
| Desmidium bengalicum Turn fa.quadratum |
|
+ |
|
| Euastrum gnathophorum West & West var.bulbosum |
|
+ |
+ |
| Onychonema laeve Nordst. var. latum West & West |
|
|
+ |
| Staurastrum cerates Lund var pulchrum Scott & Gronbl. fa |
|
+ |
|
| S. emaciatum |
|
+ |
|
| S. freemanii West & West var.nudiceps Scott & Presc. |
|
+ |
+ |
| S. gralile Ralfs fa. Kriegeri |
|
|
+ |
| S. limneticum Schm. Var. burmense West & West |
|
+ |
+ |
| S. multispiniceps |
|
+ |
|
| S. prionotum |
|
+ |
+ |
| S. tohopekaligense Wolle var. insigne West & West Formae |
|
+ |
|
| S. sebaldi Reinsch var.ventriverrucosum |
|
|
+ |
| Triploceros gracile Bail fa. undulatum Scott & Presc. |
|
|
+ |
| Xanthedium perissacanthum Scott. & Presc. Var. minus. |
|
+ |
|
| Chlorococcales |
| Muogeotia punctata Wittrock |
|
+ |
|
| Spirogyra rhizobrachialis Jao |
|
+ |
+ |
| Cyanophyceae |
| Chroococcus turgidus (kuetz.) Naegeli |
|
+ |
|
| Gomphosphaeria lacustris Chodat |
|
|
+ |
| Microcystis aeruginosa Kuetz, emend, Elenkin |
|
+ |
|
| Dinophyceae |
| Ceratium hirundinella (O.F. Muell) Dujardin |
|
|
+ |
| Chrysophyceae |
| Dinobryon calciformis Brachmann |
|
|
+ |
Table 12.3: Diversity indices at various sampling localities.
| Parameter |
Collection |
Muppane |
Talakalale |
Reservoir Centre |
Yenne holé |
Madenur |
| Total individual |
1 |
10339 |
2770 |
3414 |
820 |
- |
| 2 |
49 |
96 |
33 |
437 |
74 |
| 3 |
232 |
59 |
88 |
585 |
175 |
| Total species |
1 |
21 |
13 |
14 |
23 |
- |
| 2 |
15 |
18 |
18 |
19 |
24 |
| 3 |
21 |
17 |
15 |
20 |
19 |
| Species richness |
1 |
2.16 |
1.51 |
1.59 |
3.27 |
- |
| 2 |
3.59 |
3.72 |
4.86 |
2.96 |
5.34 |
| 3 |
3.67 |
3.92 |
3.12 |
2.98 |
3.48 |
| Shannon-diversity |
1 |
1.96 |
1.85 |
2.24 |
2.69 |
- |
| 2 |
2.43 |
2.11 |
2.75 |
1.97 |
2.85 |
| 3 |
1.57 |
2.45 |
2.21 |
1.57 |
2.21 |
| Simpson-dominance |
1 |
0.2 |
0.22 |
0.12 |
0.09 |
- |
| 2 |
0.11 |
0.23 |
0.07 |
0.24 |
0.07 |
| 3 |
0.4 |
0.12 |
0.14 |
0.38 |
0.15 |
| Simpson-diversity |
1 |
0.79 |
0.77 |
0.87 |
0.9 |
- |
| 2 |
0.88 |
0.76 |
0.92 |
0.75 |
0.92 |
| 3 |
0.59 |
0.87 |
0.85 |
0.61 |
0.84 |
Abrupt variations in total number of individuals indicate that the growth and distribution patterns of phytoplankton are not uniform. High total number of individuals during I-collection compared to other two can be attributed to the rains during the month of September just prior to I-collection during October, which might have added nutrients to the waters along with run-off water from the catchment.
In order to apply biological means of determining the trophic status, Shannon and Weiner’s species diversity values were calculated. The degrees of pollution is categorized based on the ranges of Shannon and Wiener’s species diversity as ‘slight’ (species diversity range of 3.0 – 4.5), ‘light’ (2.0 – 3.0), ‘moderate’ (1.0 – 2.0) and ‘heavy’ (0.0 – 1.0).
From Table 12.3 it is clear that in general, species diversity values are in the range of moderate or light pollution level. From Shannon’s diversity indices it is clear that the waters of sanctuary area are of oligotrophic nature.
A total of 109 species are collected from the SVWS; 28 species of diatoms, 58 species of desmidials, 12 species of chlorococales, 7 species of cynophyceae, 3 species of chrysophyceae and a species of dinophyceae represent total number. The biological examination of the stream and reservoir ecosystems showed a rich and diverse phytoplankton population. Desmids predominated in reservoir waters while diatoms in streams.
Zooplankton
Zooplankton are the primary consumers of an aquatic ecosystem, which feed on phytoplankton. Rotifera, Cladocera and Copepoda are the major groups among freshwater zooplankton. A detailed knowledge about zooplankton composition and their seasonal fluctuations is essential for proper management of water bodies. To study the zooplankton diversity in aquatic systems of the SVWS water samples were collected at Muppane, Talakalale, Reservoir center and Yenneholé. Majority of rotifers inhabits freshwater but some genera also occur in brackish water and marine environment. Most species are free-living while some are epizoic or parasitic. Generally the size of the rotifera range from 400 µm to 0.2 mm. Six species belonging to two families are recorded in the present study. Number of species belonging to rotifers, cladocerons and copepoda are given in Table 12.4.
Table 12.4: Zooplankton of SVWS
| Groups |
Family |
Muppane |
Talakalale |
Reservoir
Centre |
Yenneholé |
| Rotifera |
Brachionidae |
1 |
0 |
1 |
1 |
| Lecanidae |
1 |
2 |
1 |
3 |
| Cladocera |
Sididae |
1 |
0 |
0 |
0 |
| Daphnidae |
1 |
1 |
0 |
0 |
| Moinidae |
1 |
0 |
1 |
0 |
| Macrothricidae |
0 |
0 |
0 |
1 |
| Chydoridae |
1 |
0 |
0 |
1 |
| Copepoda |
Cyclopidae |
0 |
0 |
0 |
1 |
| Diaptomidae |
1 |
1 |
1 |
0 |
Cladocerans are ubiquitous in distribution, i.e., they are found in the Arctic to Antarctic, in temperate and tropical latitudes. Recently they were also reported from ground water (Dumont, 1987; Negrea 1983). The size ranges from 0.2 mm to 18 mm. Like other Zooplankton cladocerans are excellent food for zooplanktivorous fish. Six species belonging to five families are recorded in the present study (Table 12.4). Copepods are the very ancient arthropods. In inland waters copepods are well known, up to family level, but numerous species are yet to be discovered. Of the three groups of zooplankton, Copepoda was least represented in terms of diversity with only three species (Table 12.4).
In the present study, 15 species of zooplankton were recorded from four localities along the River Sharavathi, showing a typical tropical assemblage. Table 12.5 lists locality-wise species list. Large zooplankton species were absent in this river system, probably due to high predatory pressure.
Table 12.5: Zooplankton diversity in SVWS
| Rotifers |
| Species |
Sites |
| 1 |
2 |
3 |
4 |
| Family: Brachionidae |
| Brachionus quadridentatus Hemann, 1783 |
|
|
+ |
|
| B. falcatus (Zacharias, 1898) |
+ |
|
|
|
| Keratella tropica (Apsein, 1907) |
|
|
|
+ |
| Family: Lecanidae |
| Lecane bulla (gosse, 1888). |
+ |
+ |
+ |
+ |
| L. lateralis sharma, 1978. |
|
|
|
+ |
| Lecane sp. |
|
+ |
|
+ |
| Cladocerans |
| Species |
Sites |
| 1 |
2 |
3 |
4 |
| Family:Sididae |
| Diaphanosoma sarsi Richard, 1895 |
+ |
|
|
|
| Family: Daphniidae |
| Ceriodaphnia cornuta Sars, 1885 |
+ |
+ |
|
|
| Family: Moinidae |
| Moina micrura Kurz, 1874 |
+ |
|
+ |
|
| Family: Macrothricidae |
| M. odiosa (Gurney, 1907) |
|
|
|
+ |
| Family: Chydoridae, Sub-family: Chydorinae |
| Ephimeroporus barrosi (Richard, 1894) |
+ |
|
|
|
| Sub-family: Aloninae |
| Alona verrucosa (Sars, 1901) |
|
|
|
+ |
| Copepodans |
| Species |
Sites |
| 1 |
2 |
3 |
4 |
| Family: CyclopidaeSub-family: Cyclopinae |
| Microcyclops varicans Sars, 1863 |
|
|
|
+ |
| Family: Diaptomidae |
| Heliodiptomus cinctus (Gurney, 1907) |
|
|
+ |
|
| Allodiaptomus mirabilipes (Kiefer, 1936) |
+ |
+ |
|
|
Ichthyofauna
The Western Ghats records 288 species belonging to 12 orders, 41 families and 109 genera, of which 118 species are endemic and 51 are unique. This diverse fish fauna composition aptly demonstrates the hotspots status of the Western Ghats. A major portion of the Linganamakki reservoir falls under the SVWS. The ichthyological studies gain importance, as it helps to adopt appropriate conservation strategies for sustainable management of the aquatic ecosystem. Several rivers in the Western Ghats are being exploited for fisheries and there is hardly any information available on its effect on such a pristine resource stock of the region. This necessitates a detailed investigation on fish and fisheries.
We have recored from SVWS 60 species of fishes (Table 13.1). Considering the IUCN status of these species, there are about 16.6% (10 species) endangered, 18.3% (11 species) vulnerable, 16.6% (10 species) data deficient, 33.2% (20 species) are with lower risk and the status of 3 species is unknown. There is about 16 endemic fish species in the reservoir. Compared to the Western Ghats this value is relatively low, which could be attributed to the formation of the reservoir that has lead to the flourishing of generalist species and diminishing of sensitive endemic species. Also, the introduction of the exotic species into the reservoir has increased the species richness while decreasing the endemism. About 16.6% (10 species) are restricted to peninsular India and 41.6% (25 species) have their distribution all over India.
Table 13.1: Fish species in SVWS
| Family |
Species |
Distribution |
IUCN status |
| Bagridae |
Batasio sharavatiensis |
Endemic to Sharavathi |
DD |
| Bagridae |
Mystus malabaricus |
Endemic to Western Ghats |
EN |
| Balitoridae |
Nemacheilus anguilla |
Endemic to Western Ghats |
LR |
| Balitoridae |
Schistura semiarmatus |
Endemic to Western Ghats |
VU |
| Cyprinidae |
Barilius bakeri |
Endemic to Western Ghats |
VU |
| Cyprinidae |
Barilius canarensis |
Endemic to Western Ghats |
DD |
| Cyprinidae |
Barilius gatensis |
Endemic to Western Ghats |
DD |
| Cyprinidae |
Garra gotyla stenorhynchus |
Endemic to Western Ghats |
EN |
| Cyprinidae |
Gonoproktopterus dubius? |
Endemic to Western Ghats |
EN |
| Cyprinidae |
Gonoproktopterus kolus |
Endemic to Western Ghats |
EN |
| Cyprinidae |
Labeo kontius |
Endemic to Western Ghats |
LR |
| Cyprinidae |
Osteocheilichthys nashii |
Endemic to Western Ghats |
VU |
| Cyprinidae |
Puntius arulius |
Endemic to Western Ghats |
EN |
| Cyprinidae |
Puntius sahyadriensis |
Endemic to Western Ghats |
DD |
| Cyprinidae |
Salmostoma boopis |
Endemic to Western Ghats |
LR |
| Sisoridae |
Glyptothorax lonah |
Endemic to Western Ghats |
LR |
| Cichlidae |
Oreochromis mossambica |
Exotic |
|
| Bagridae |
Mystus bleekeri |
India |
VU |
| Bagridae |
Mystus cavesius |
India |
LR |
| Balitoridae |
Acanthocobitis botia |
India |
LR |
| Belonidae |
Xenentodon cancilla |
India |
LR |
| Chandidae |
Chanda nama |
India |
VU |
| Chandidae |
Parambassis ranga |
India |
DD |
| Channidae |
Channa marulius |
India |
LR |
| Channidae |
Channa orientalis |
India |
VU |
| Claridae |
Clarias batrachus |
India |
VU |
| Cyprinidae |
Amblypharyngodon mola |
India |
LR |
| Cyprinidae |
Barilius bendelisis |
India |
LR |
| Cyprinidae |
Oreichthys cosuatis |
India |
DD |
| Cyprinidae |
Puntius chola |
India |
VU |
| Cyprinidae |
Puntius sophore |
India |
LR |
| Cyprinidae |
Puntius ticto |
India |
LR |
| Cyprinidae |
Rasbora daniconius |
India |
LR |
| Cyprinidae |
Tor mussullah |
India |
EN |
| Gobidae |
Glossogobius giuris |
India |
LR |
| Mastacembelidae |
Mastacembelus armatus |
India |
LR |
| Schilbeidae |
Pseudeutropius atherinoides |
India |
EN |
| Siluridae |
Ompok bimaculatus |
India |
EN |
| Siluridae |
Ompok pabo? |
India |
DD |
| Siluridae |
Wallago attu |
India |
EN |
| Cyprinidae |
Brachydanio rerio |
India |
LR |
| Claridae |
Clarias dussumieri |
India |
VU |
| Aplocheilidae |
Aplocheilus lineatus |
Southern India |
LR |
| Bagridae |
Mystus keletius |
Southern India |
DD |
| Balitoridae |
Schistura denisonii densisonii |
Southern India |
VU |
| Belontidae |
Pseudophromenus cupanus |
Southern India |
DD |
| Cobitinae |
Lepidocephalus thermalis |
Southern India |
LR |
| Cyprinidae |
Cirrhinus fulungee |
Southern India |
LR |
| Cyprinidae |
Danio aequipinnatus |
Southern India |
LR |
| Cyprinidae |
Puntius fasciatus |
Southern India |
EN |
| Cyprinidae |
Puntius filamentosus |
Southern India |
DD |
| Cyprinidae |
Tor khudree |
Southern India |
VU |
| Balitoridae |
Schistura sp. |
|
|
| Balitoridae |
Schistura sp. |
|
|
| Cyprinidae |
Catla catla |
introduced |
|
| Cyprinidae |
Cirhinus mrigala |
introduced |
|
| Cyprinidae |
Cyprinus carpio communis |
Exotic |
|
| Cyprinidae |
Cyprinus carpio |
Exotic |
|
| Cyprinidae |
Cyprinus carpio specularis |
Exotic |
|
| Cyprinidae |
Labeo rohita |
introduced |
|
Large-scale fishery began in this reservoir with the commissioning of the dam. This commercialisation of inland fishery that took place over a few decades has led to transformation of the subsistence fishing into commercial fishing in the wildlife sanctuary area of the Linganamakki reservoir. The introduction of exotic and alien species in to the Linganamakki reservoir has been practiced since 1965. Fingerlings of Catla catla, Labeo rohita, Cirhina mrigala, Cyprinus carpio and Oreochromis mossambica are introduced haphazardly into the reservoir on yearly basis.
The commercial fish catch of the reservoir is dominated by species belonging to Cyprinidae (54%). The other major families are Bagridae (23%) and the Siluridae (15%). When biomass is considered, the fast growing Indo-gangetic carps, popularly known as Indian major carps, occupy a prominent place namely Catla catla (21%), Labeo rohita (8.4%) and Cirhina mrigala (6.32%). These fishes are introduced to fulfill the commercial fish requirement along with the exotic species (Cyprinus carpio 21%). The native fishes with significant biomass are Gonoproktopterus kolus (11.5%), Ompok bimaculatus (10%) and Wallago attu (9%).
Data on fish catch of the selected five localities (Table 13.2) show that at the center of the reservoir (Holebagilu), the yield variation is very high compared to other regions. During monsoon season, the central region yields the introduced species in bulk. In the peripheral localities (Muppane, Konjavalli, Melmanji and Kogar) variation in catch during two seasons is less.
Table 13.2: Fish-catch observed at different locations during the year 2003-04
| Locality |
Catch per unit effort (Kg/boat/day) |
| Non-monsoon |
Monsoon |
| Holebagilu |
1.34 |
39.4 |
| Muppane |
7.93 |
16.5 |
| Konjavalli |
6.2 |
16.5 |
| Melmanji |
6.8 |
24.2 |
| Kogar |
8.2 |
28.5 |
The fish biomass composition (Table 13.3) in the central region is dominated by introduced species (Holebagilu - 55.8%) during monsoon season. Among the indigenous population, Ompok bimaculatus has shown significant biomass in these localities. Whereas in other localities without any introduced species, their catch includes indigenous commercial fishes like Gonoproktopterus kolus, Wallago attu, Mastacembelus armatus and Ompok bimaculatus. Apart from G. kolus, the market value for all indigenous fishes is higher than the introduced species.
Table 13.3: Percentage catch composition of various species during monsoon season
Species name |
Percentage biomass |
Holebagilu |
Muppane |
Konjavalli |
Melmanji |
Kogar |
Catla catla |
25.4 |
0.0 |
0.0 |
0.0 |
0.0 |
Cyprinus carpio |
12.7 |
0.0 |
0.0 |
0.0 |
0.0 |
Labeo rohita |
10.1 |
0.0 |
0.0 |
0.0 |
0.0 |
Cirhina mrigala |
7.6 |
0.0 |
0.0 |
0.0 |
0.0 |
Cirhinus fulungee |
0.0 |
12.7 |
5.4 |
2.5 |
3.2 |
Gonoproktopterus kolus |
0.0 |
39.0 |
43.4 |
38.9 |
43.2 |
Mastacembelus armatus |
8.1 |
9.7 |
12.1 |
4.9 |
8.4 |
Mystus bleekeri |
0.0 |
0.3 |
0.0 |
0.0 |
0.0 |
Mystus cavacius |
9.1 |
7.2 |
1.4 |
0.6 |
0.5 |
Mystus malabaricus |
1.7 |
2.7 |
1.4 |
0.6 |
2.1 |
Ompok bimaculatus |
24.0 |
23.9 |
15.9 |
8.1 |
37.1 |
Ompok pabo |
0.0 |
1.3 |
2.6 |
0.9 |
1.5 |
Osteocheilichthys nashii |
0.0 |
0.9 |
0.0 |
0.0 |
0.0 |
Pseudeutropius atherinoides |
0.0 |
0.2 |
0.3 |
0.1 |
0.2 |
Puntius filamentosus |
0.0 |
1.1 |
1.4 |
1.5 |
3.8 |
Tor khudree |
0.0 |
0.9 |
0.0 |
0.0 |
0.0 |
Tor mussullah |
0.0 |
0.0 |
0.9 |
0.6 |
0.0 |
Wallago attu |
0.0 |
0.0 |
15.1 |
41.2 |
0.0 |
Xenentodon cancilla |
1.2 |
0.0 |
0.0 |
0.0 |
0.0 |
During summer season, catch is mainly represented by Mystus cavecius, M. malabaricus and Mastacembelus armatus in almost all the localities (Table 13.4). This shows the absence of any introduced species in these localities. At the peripheral localities Gonoproktopterus kolus shows significant catch whereas its catch is negligible at the central region.
Table 13.4: Percentage catch composition of various species during non-monsoon season
| Species name |
Percentage biomass |
| Holebagilu |
Muppane |
Konjavalli |
Melmanji |
Kogar |
| Native |
|
|
|
|
|
| Gonoproktopterus kolus |
0.0 |
28.4 |
28.9 |
13.3 |
11.0 |
| Cirhina fulungee |
5.6 |
8.5 |
8.4 |
2.2 |
4.6 |
| Garra gotyla stenorhynchus |
0.0 |
1.5 |
0.6 |
0.0 |
0.0 |
| Mastacembelus armatus |
0.0 |
25.2 |
19.3 |
17.8 |
19.5 |
| Mystus cavecius |
16.8 |
3.8 |
6.0 |
8.9 |
14.6 |
| Mystus malabaricus |
22.4 |
5.7 |
7.2 |
7.8 |
10.1 |
| Ompok bimaculatus |
32.8 |
8.3 |
14.1 |
39.1 |
29.5 |
| Ompok pabo |
0.0 |
13.9 |
10.6 |
9.8 |
10.7 |
| Oreochromis mossambica |
0.0 |
1.0 |
2.4 |
1.1 |
0.0 |
| Puntius arulius |
0.1 |
0.0 |
0.0 |
0.0 |
0.0 |
| Puntius filamentosus |
17.9 |
0.0 |
0.0 |
0.0 |
0.0 |
| Tor khudree |
0.0 |
3.8 |
2.4 |
0.0 |
0.0 |
| Xenentodon cancilla |
4.5 |
0.0 |
0.0 |
0.0 |
0.0 |
The fish catch composition shows variations between different sites as the composition in the peripheral regions of the sanctuary like Holebagilu is of introduced species while Yenneholé tributary is of native species.
Fishery in the sanctuary is being practiced illegally and continued overharvesting proves to be unsustainable. Yenneholé tributary has witnessed a self-preserving fishery within the biological limits of its resource’s productivity, through a limited seasonal uptake, while ensuring future harvests. On the other hand, Holebagilu region, which supplies the fish requirements of the nearby urban centers has large number of fishermen and wider access and has led to illegal fishing activities.
The fortunes of the fishermen at the central parts of the reservoir like Holebagilu and the Hasaramakki seem to have touched bottom during recent years. During the winter and the summer seasons, the catch kg/per person/day is around one, which fetches about 25 rupees. During monsoon season, they get the introduced species. The competition for food and space between the exotic and indigenous has also led to the decline in the latter.
Variable fishing pressure: Monsoon is the peak fishing period with 3.4 times fish catch per person per day compared to non-monsoon period and accounts for 86.7% of the total fish-catch. Increased fishing pressure is noticed with migratory fishermen (accounting to 63% of the total) from various parts of peninsular India and the density of fishermen increases to 2.75 times the native fisher folk. During the initial monsoon season, the reservoir attains the minimum water spread area. It is observed that most of the fishermen get concentrated in the central regions like Holebagilu leading to overexploitation of fish resource.
Muppane, Konjavalli, Melmanji and Kogar represent the peripheral localities of the western region. The biomass composition of this region shows that in these localities, the catch is formed by the native species. These are the flood plains where majority of the fish species breed during monsoon season. Huge quantities of fish catch in these localities during monsoon season poses severe threat to their population. It is evident that the catch per unit effort increases at the periphery than the other localities.
Fish translocation from other basins: Details on the pattern of introduction clearly reveal that no scientific approach has been adopted before determining the quantity of introduction. Seeds have been introduced depending on the availability. This unscientific approach has resulted in an artificial system of fishing wherein the indigenous fishing population has to rely on an external source to increase the fishing stock. The low catches during non-monsoon season affect the permanent fishermen of the region who are completely dependent on fish resources for livelihood. The biomass composition of this region also reveals that other than catfishes, no other native species has succeeded to form a stable population. Thus the fishermen are dependent on an artificial system in the form of introduction and harvest. It is implicative that the original fauna has been changed and hardy fish species has taken advantage of the vacant niches. Thus translocation of fishes from other basins has led to changes in the species composition.
Amphibians
Amphibians are the best ecological indicators among vertebrates for the unique features like duplex life style, moist permeable skin and ectothermic nature. They are the indicators of habitat fragmentation, as they negatively respond to both qualitative and quantitative changes in the habitat, ecosystem stress, impact of anthropogenic activities like dam construction, and sedimentation in streams due to road construction.
Twenty-four species of amphibians with 178 individuals were recorded in SVWS accounting to 19% of the Western Ghats. Of the 24 species, 16 are endemic (71%) to the Western Ghats. Based on the IUCN criteria for conservation priority, 1 species endangered (Nyctibatrachus aliciae), 2 vulnerable, 2 threatened, 14 with least concern and 5 data deficient. These species belong to four families, namely bufonidae, microhylidae, ranidae and ichthyophiidae. Ranidae members predominate in the richness, abundance and endemism (20, 168, and 15). Based on the species abundance (Figure 2.4), the top six species include Euphlyctis cyanophlyctis (43)followed by Philautus cf. leucorhinus (24), Nyctibatrachus aliciae (21), Indirana semipalmata (16), Micrixalus saxicola (11) and Rana temporalis (10).Presence of endemics (16 amphibian species), endangered species Nyctibatrachus aliciae and vulnerable species Micrixalus saxicola and Nyctibatrachus major indicates the ecological importance of the region. Species list is provided in Table 13.5.
Table 13.5: Amphibians of Sharavathi Valley Wildlife Sanctuary
| Species |
Endemism |
IUCN status |
| Bufonidae |
| Bufo melanostictus |
NE |
LC |
| Bufo scaber |
NE |
LC |
| Microhylidae |
| Microhyla ornata |
NE |
LC |
| Ranidae |
| Euphlyctis cyanophlyctis |
NE |
LC |
| Fejervarya keralensis |
E |
LC |
| Fejervarya limnocharis |
NE |
LC |
| Fejervarya rufescens |
E |
LC |
| Hoplobatrachus tigerinus |
NE |
LC |
| Indirana beddomii |
E |
LC |
| Indirana semipalmata |
E |
LC |
| Micrixalus saxicola |
E |
VU |
| Nyctibatrachus aliciae |
E |
EN |
| Nyctibatrachus major |
E |
VU |
| Philautus cf. leucorhinus |
E |
DD |
| Philautus cf. luteolus |
E |
DD |
| Philautus cf. ponmudi |
E |
DD |
| Philautus tuberohumerus |
E |
DD |
| Polypedates cf. leucomystax |
NE |
LC |
| Rana curtipes |
E |
LC |
| Rana malabarica |
NE |
LC |
| Rana temporalis |
E |
NT |
| Rana sp. |
E |
DD |
| Rhacophorus malabaricus |
E |
LC |
| Ichthyophiidae |
| Ichthyophis beddomei |
E |
LC |
Note: E – Endemic to Western Ghats; NE – Non-endemic to Western Ghats; EN- Endangered; Vu – vulnerable; NT–Near threatened; LC – Least concerned; DD – data deficient.
Nair and Gadgil (1975) reported the elephants during 1960s in SVWS. Over the period the elephants have disappeared from the park. Similarly from the north of the sanctuary i.e. between Sharavathi and Aghanashini rivers also the elephants have disappeared in recent years (Kumara and Singh 2005b). The probable reasons could be developmental activities like dam, road and increased number of human enclaves, made them completely isolation from the main population, and probably resulted in biased sex ratios over a period and in turn on breeding efficiency. Further, gradual elimination of the individuals drove into local extinction.
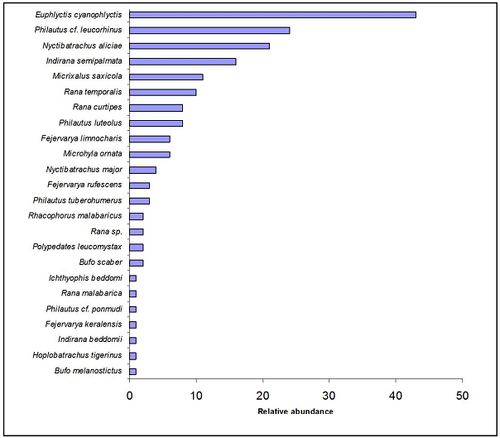
Figure 2.4: Amphibian species abundance in SVWS
An assessment of the Ecological status of Sharavathi Valley Wildlife Sanctuary (Sameer Ali et.al 2007) carried outthrough the estimation of species composition and their relative abundance with reference to space and time in a region. Faunal studies indicate the diverse groups of organisms found in the sanctuary. The data gathered both by sampling and opportunistic surveys are listed in Table 14.1.
Table 14.1: Faunal composition in Sharavathi Valley Wildlife Sanctuary
| Fauna |
Species |
| Ants |
84 |
| Coleopterans |
166 |
| Butterflies |
173 |
| Molluscs |
21 |
| Amphibians |
24 |
| Fishes |
60 |
| Birds |
122 |
| Reptiles |
23 |
| Mammals (including Bats) |
43 |
Kumara H.N. 2007 studied the mammals of Sharavathi wildlife Sanctuary. A total of 1,332 animals belongs to ten species were sighted in 550 encounters during the day walk (Table 14.2). The ten species includes four arboreal mammals and six terrestrial mammals, and the relative abundance of arboreal mammals (7.19 +0.471) was more than (z= 14.64, p< .000) the terrestrial mammals (0.24 +0.056). A total of 50 animals belonging to eight species including two unidentified small carnivores were sighted during the night walk (Table 14.3), which provides an overall relative abundance of 0.87 animals per kilometre. However the relative abundance of arboreal mammals (0.59) was higher than the small carnivores (0.22) and the other mammals include chevrotain and porcupine Hystrix indica (0.06), among arboreal mammals the slender loris (0.35) was more than the giant flying squirrel (0.23) and Travancore flying squirrel (0.01).
Table 14.2: Relative abundance of mammals in the Sharavathi Valley Wildlife Sanctuary
| Species |
No. of sightings |
Total no. ofanimals sighted during the day |
No. animals seen/km (SE) |
| Arboreal mammals |
| Hanuman langur |
243 |
835 |
4.52 (+0.380) |
| Bonnet macaque |
31 |
147 |
0.91 (+0.241) |
| Lion-tailed macaque |
7 |
23 |
0.12 (+0.057) |
| Indian giant squirrel |
238 |
287 |
1.54 (+0.117) |
| Total |
519 |
1292 |
7.19 (+0.471) |
| Terrestrial mammals |
| Gaur |
1 |
1 |
0.004 (+0.004) |
| Sambar |
9 |
13 |
0.05 (+0.020) |
| Spotted deer |
4 |
6 |
0.05 (+0.026) |
| Indian muntjac |
15 |
17 |
0.11 (+0.030) |
| Wild pig |
1 |
2 |
0.01 (+0.012) |
| Indian grey mongoose |
1 |
1 |
0.005 (+0.005) |
| Total |
|
|
|
Table 14.3: Relative abundance of mammals in Sharavathi Valley Wildlife Sanctuary
| Species |
No. animals seen during the night |
No. animals seen/km |
| Small carnivores |
| Leopard cat |
5 |
0.07 |
| Asian palm civet |
3 |
0.04 |
| Brown palm civet |
5 |
0.07 |
| Unidentified small carnivores |
2 |
0.03 |
| Total |
15 |
0.22 |
| Arboreal mammals |
| Slender loris |
24 |
0.35 |
| Giant flying squirrel |
16 |
0.23 |
| Travancore flying squirrel |
1 |
0.01 |
| Total |
41 |
0.59 |
| Other mammals |
| Indian spotted chevrotain |
3 |
0.04 |
| Indian crested porcupine |
1 |
0.01 |
| Total |
4 |
0.06 |
| Grand Total |
60 |
0.87 |
Among various anthropogenic impacts, impounding of waters for electricity generation seems to have significantly altered terrestrial as well as aquatic ecosystems and associated biota including fish fauna. In this regard, a study was conducted in Sharavathi River of central Western Ghats to understand fish species composition with respect to landscape dynamics. Of the 64 fish species recorded, 25 were exclusive to the tributary streams, 29 to the reservoir and 10 common to both. Among these, 18 species were endemic to the Western Ghats and 10 to peninsular India. The study, carried out using a combination of remote sensing data as well as field investigations, shows that the streams having their catchments covered with evergreen to semi-evergreen forests, having high levels of ever greenness and endemic tree species of Western Ghats, were also richer in fish diversity and endemism compared to those catchments with other kinds of vegetation. It also highlights that endangered and endemic fish species are precariously clinging onto the stream habitats where patches of primeval forests, though degraded substantially, are still persisting. This illustrates the composition and a distribution of fish species have strong association with the kind of terrestrial landscape elements and highlights the importance of landscape approach to conservation and management of aquatic ecosystems. Occurrence of endangered, endemic and discovery of two new species of Schistura genus re-affirms ‘hottest hotspot’ status of the Western Ghats, a repository of biological wealth of rare kind, both in its aquatic and terrestrial ecosystems. Schistura nagodiensis and S. sharavathiensis are the new fish species of Schistura described from Sharavathi River, central Western Ghats.This also reported the range extension of Schistura nilgiriensis (earlier Nemacheilus nilgiriensis, Jayaram, 1999) from Sharavathi River. (Annexure 1, 2 & 3)
Social Aspects: The sanctuary is having 40 small villages comprising usually of 1 to 10 houses in each village. These villages are situated in valleys where perennial water source and deep soil is available (figure 3.1). None of the settlements are thickly populated. The people naturally move inside the sanctuary as they are depending upon the sanctuary for their fuel, fodder, small timber and other inevitable forest produces required for normal living.
Park zonations: The sanctuary has been divided in to three zones based on the utility. Spatial extent of each zone is provided in Table 15.
- Core Area or Core Zone.
- Buffer Area or Buffer Zone.
- Tourism Area or Tourism Zone.
Table 15: Zonations ofSharavathi Valley Wildlife Sanctuary
| Zone |
Forest |
Compt |
Extent in Ha. |
| Core zone |
Karini SF |
XX -1 to 7 (17) |
5102.53 |
| Buffer zone |
Govardhanagiri SF |
XX – 1 to 34 |
13473.68 |
| Buffer zone |
Channagonda SF (P) |
XIX – 13 (P) |
701.05 |
| Tourism zone |
Attigodu SF (P) |
XIX – 1 to 3 |
763.70 |
| Tourism zone |
Muppani SF Bl. A |
XIX – 4 to 7 |
961.77 |
| Tourism zone |
Muppani SF Bl. B |
XIX – 8 to 11 |
629.16 |
| Tourism zone |
Sharavathi submerged area |
|
12363.00 |
| Tourism zone |
Islands within the submerged area |
|
507.00 |
| |
Others |
|
8621.11 |
| |
|
TOTAL:- |
43123.00 |
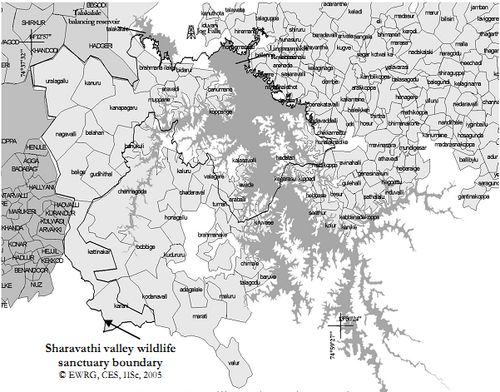
Figure 3.1: Villages in and around SVWS
Humans – Socio-Economic-Energy
Cooking and water heating are the two major end uses that require huge amount of firewood in the region. It is estimated that the average per capita firewood consumption is 1.17 tonnes/year, based on sample survey covering 25% of the villages and 20% of the households. The villagewise cooking and water heating energy consumption values are given in Table 16.1. Estimation of the total fuelwood requirement of the region amounts to 10435 tonnes for the year 1991 and it increased to 15328 during 2001.
Forest biomass availability
Spatial extent of SVWS is about 431 sq.km, with 110 sq.km under semi-evergreen to evergreen forests, 49 sq.km under deciduous forests, 66sq.km under plantations and 90 sq.km under wastelands. Considering the average secondary productivities of each type of forest (3.6 t/h/y for evergreen, 12.5 t/h/y for deciduous, 5 t/h/y for plantations and 0.6 t/h/y for scrub and waste lands), the annual availability of forest biomass as a source of fuelwood is about 189.23x103 tonnes.
Population increase at 3.9% per year has resulted in increased fuel wood demand. Apart from gathering dried and fallen twigs and leaves, local people also cut young saplings, green twigs, and even whole tree. Several plant species preferred by wild animals are being cut for fuel wood as well as for mulching and fodder. Table 16.1, lists the villagewise fuelwood consumption.
Table 16.1: Villagewise per-capita fuelwood consumption (Kg/person/day)
| Village Name |
Cooking |
Water Heating |
| Monsoon |
Winter |
Summer |
| Ambargodlu |
1.5 |
1.5 |
1.2 |
1.2 |
| Chikkamathur |
1.7 |
1.5 |
1.3 |
1.3 |
| Mattikoppa |
1.5 |
1.4 |
1.2 |
1.2 |
| Hunalamadike |
1.8 |
2.8 |
1.7 |
1.7 |
| Valagere |
1.6 |
1.8 |
1.4 |
1.3 |
| Kalasavalli |
1.7 |
1.8 |
1.4 |
1.4 |
| Araballi |
1.4 |
1.8 |
1.4 |
1.4 |
| Honnemardu |
1.8 |
1.7 |
1.4 |
1.4 |
| Baliggere |
2.0 |
2.0 |
1.3 |
1.3 |
| Bannumane |
1.6 |
1.4 |
1.2 |
1.2 |
| Aravadi |
1.7 |
1.7 |
1.2 |
1.2 |
| Brahmana Ilakalale |
1.6 |
1.5 |
1.2 |
1.2 |
At present the domestic energy consumption is well within the total biomass availability from the region. However, with increasing population poses a serious threat to the sustainability of forest resources.
The average livestock holding is in the order of 3.33 buffaloes, 2.27 bullocks and 4.5 cattle per household. Most of the households opt for open grazing in forests that hampers natural regeneration. The estimated total number of livestock in the sanctuary area is 17655. Animal residue (dung) can be used for biogas production, which might minimise the fuelwood pressure on forests. Quantification shows an order of 7627 cu.m to 11122 cu.m per day of biogas generation potential in the region (assuming that a kilogram of fresh animal residue provides 0.03 – 0.042 m3 of gas), which is sufficient to meet the cooking energy needs of 27572 – 21801 persons. However the energy transition from fuelwood to biogas requires policy initiatives as most collect firewood at zero cost due to proximity of forests.
Village forest farms in the selected village would reduce the pressure of cattle on natural forests. Promotion of stall-feeding and conversion of degraded common lands to community fodder farm would bring down the pressure on forests. Village self help groups to be involved in creation and maintenance of village fodder farms in selected villages
Threats and Management
Protected areas (PAs) are established for protecting a particular area with clear management objective. Factors such as encroachment of habitats, poaching and grazing are responsible for the depletion and extinction of wildlife resources. The cases of encroachment of habitats and activities like poaching and grazing are threatening a majority of the wildlife habitats around the world. With the passage of time, human influences on habitats had an alarming impact. SVWS harbours endemics and threatened species of flora and fauna, and hence requires immediate protection and conservation measures. Already large areas of pristine forests have been cleared for hydroelectric-projects, Acacia auruculiformis plantations, and for agricultural operations, etc. The failure in the conservation of reserve forests is visible in many places with the continued process of habitat destruction. The forests need to be protected from human interference that is detrimental to the growth and regeneration of the forest. This requires improved forest security, transparency in forest product utilization, and a stronger political will.
Human and livestock inside the sanctuary
There are 121 villages inside the sanctuary, having higher human and cattle population (Table 16.2), and 59 of them are in protected area (enclosures). Increased human habitats with forest encroachments have seriously affected the wildlife population. The cultivation of Vetiveria sp. (lemon grass) extensively inside the sanctuary areas such as Meghane (located in Buffer zone of the sanctuary) poses serious threat to the wildlife population.
Table 16.2: Human and Cattle population inside the sanctuary area.
| Village name |
Cattle population |
Human population |
| Talakalale |
154 |
95 |
| B. Ilakalale |
59 |
487 |
| Karumane |
70 |
453 |
| Aralagodu |
66 |
338 |
| Bannumane |
58 |
355 |
| B.kopparige |
26 |
156 |
| Muppane |
38 |
413 |
| Arodi |
16 |
44 |
| Mandavalli |
80 |
555 |
| Ambargodlu |
41 |
192 |
| Kagarasu |
01 |
18 |
| Hedathri |
23 |
46 |
| Banukuli |
105 |
945 |
| Kanur |
45 |
501 |
| Kanapagaru |
114 |
1524 |
| Gudihithlu |
37 |
367 |
| Nagavalli |
142 |
1242 |
| Balige |
47 |
498 |
| Nelahari |
28 |
284 |
| Uralagallu |
23 |
209 |
| Chennagonda |
181 |
616 |
| Karani |
69 |
617 |
| Kattinkaru |
128 |
1058 |
| Total |
1551 |
11013 |
Source: KFD, Wildlife Division, Kargal
People depend on forests for livestock grazing, which results in soil compaction and affects natural regeneration. Apart from domestic livestock, a large population of wild cattle is trapped inside the sanctuary (due to the submersion) contributing to grazing pressure throughout the year.
Agriculture and Encroachment: Agricultural practices in the region are traditional and dependent on forests. The forests provide leaf litter, green leaves and fencing material to the farmers. The dense forest patches are the sources of water to the crops. Present study found that the forest encroachments have resulted in increased agricultural lands. It has been found that the land submergence is one of the major reasons for increased land encroachment in the forests. Migrating and migrated population, marginal farmers and economically sensitive households were major contributors of land encroachments. The widespread occurrence of encroachment is observed in the Kanur, Hebbankeri, Meghane and Nagavalli area, where slash and burn practice is prevalent for growing cash crops especially cotton, pepper, lemon grass, ginger, paddy and areca.
NTFP collection: Resource use has been restricted to the buffer zones, where it has been regulated, while core areas are completely closed. An amendment in 1991 to the Wildlife Protection Act of 1972 specifies that, in wildlife sanctuaries, the chief wildlife warden must certify that any manipulation does not harm wildlife, and that the state government approves the manipulation. The major NTFP of the area is leaves of Diospyros melanoxylon and Cinnamomum zeylanicum. Apart from these, on a minor scale, Emblica officinalis, Terminalia chebula, and various medicinal plants, cane, Bambusa sp., and honey are also collected. Destructive methods of collection of NTFP by lopping the branches of trees like, Myristica malabarica, Garcinia gummigutta, Cinnamomum zeylanicum etc. will affect the endemic tree species.
The industrial extraction of timber from the primary evergreen forests in the past has led to the depletion of valuable endemic species and loss of many special habitats such as Myristica swamps.
NTFP collection is totally banned in the core zone of the sanctuary area since it may pose a threat to the endemic tree species and their regeneration. But, in some areas, the community-based approach can be carried out instead of collections done by tenders given to non-locals by the forest department. This approach will be more appealing since each villager will become more responsible for conserving the forests, as removal of a tree would curtail the financial gains through NTFP. Destructive methods of collection of NTFP by lopping the branches of trees have to be stopped.
Timber smuggling: Timber smuggling is reported to be a major problem in the sanctuary area. It is reportedly smuggled even out of the Linganmakki islands, indicating the involvement of some organized groups. The timber smugglers take advantage of the remoteness of the islands from the human settlements for their illegal activities. We have observed timber harvesting at many places like, Karani, Banukuli, Kanur etc., within the sanctuary, calling for greater and effective vigilance from the authorities and the village forest committees (VFCs).
Monoculture Plantation : Large areas of the sanctuary (15.27%) have been planted with monoculture plantations depriving the wildlife of their habitats. Preference of single species in forest plantations is another reason responsible for depletion of fodder for animals. This could become a major drawback to any kind of habitat restoration programmes as well as energy improvement technologies. The practice of planting of acacia and casuarina is still in progress in open areas of Muppane, Aralagodu, Karani, etc. These monoculture plantations have no other advantages to the wildlife, other than aiding as hiding places for some of the small mammals and agricultural pests. Changes in microclimate and huge litter cover in plantations adjacent to the evergreen and semi evergreen forests would inhibit the growth of younger tree species of natural forests.
Grasslands have been converted to monoculture plantations in the forest enclosures like, Madenur, Muppane, and Shashichowka denying the fodder to herbivores like gaur, sambar, spotted deer, etc. The monoculture of any exotic should be strictly discouraged in the areas of high animal population and movement. Any such reforestation activity should be in accordance with the local need and with indigenous species. Gradual shifting of natural plant species in the monoculture plantation areas is to be done. Habitat improvements with fodder plants species preferred by wild animals are to be planted instead of monocultures of acacia, pinus or casuarinas.
Forest Fire: Usually in this sanctuary forest fires are associated with highly fragmented areas. This plays an important role in the distribution of ungulates and bovines. The main reasons for the fire are the dryness of the forest and the deciduous vegetation. Humans on a yearly basis to enhance the growth of grasses burn much of the forest ground vegetation. While fire generally does not kill adult trees, it will effectively destroy the seedlings and young trees, thus preventing tree regeneration, creating senescent forests and eventually leading to the disappearance of forests (Kessler, 2001). Almost every year forest department burnt the grassy blanks in some places to improve the quality of fodder for wild animals; this phenomenon also affects the habitat of burrowing small mammals. The fire has become a major factor in the degradation of forests. In order to restore the vegetation, these forests must be protected from fire, by preventing it by undertaking measures such as creation of awareness on the implication of fire among the local communities and proper maintenance of fire line.
Forest fragmentation : The humid forests, repository of diverse flora and fauna have been subjected to severe habitat fragmentation. Increase in forest fragmentation also gives rise to edge effect with respect to micro climatic changes, species invasion from surrounding vegetation, aetc. Forest fragmentation is a major problem in this sanctuary. Several roads that pass through the sanctuary and Linganmakki reservoir have dissected and cleaved the habitats. Other than these, heavy biotic pressures in terms of encroachments for human settlements, agricultural fields, etc. have contributed significantly to the fragmentation of habitats.
Past land use practices such as shifting cultivation and selective felling have influenced the present-day forest quality and biodiversity patterns, which are evident from the presence of patch and perforated forest in primary forested areas. Wide scale selective felling of tree like Poeciloneuron indicum, Callophylum tomentosum and Lopopetalum wightianum and Artocarpus hirsutus had been carried out since 1921 to 1971 for railway sleepers, match wood and plywood in places like Karani, Govardhangiri and Kanur, which comes in the core and buffer zone of the sanctuary. The study shows that the regeneration of these species especially Poeciloneuron indicum and Palaquium ellipticum in Karani and Kanur is excellent. Selection felling of industrial timbers continued almost to the mid 1980’s, causing considerable impoverishment of forests.
Human-animal conflicts: Due to fragmentation and reduction of natural habitats with the uncontrolled growth of agricultural practices in the sanctuary area over several years has resulted in repeated stress over the forest areas and acted negatively on the wildlife. Conflicts between wildlife and human have emerged as a problem in the arena of wildlife management. The conflicts, which result from the destruction of crops and damage to property, have raised both social as well as conservation issues, both in and outside the sancturary. Efforts to keep animals out of crop fields by wildlife officials have been futile and sometimes result in people perceiving the animals as being malevolent. Thus, human- animal conflict is a common scene over the entire area. Herbivore and omnivore animals like Indian gaur, Indian porcupine, sambar, wild boar, rodents, etc., inflict considerable damage to agricultural crops. Several incidences of sloth bear attack have been reported in the core and buffer zones of the wildlife sanctuary (villages like, Kattinkaru, Karani, Kanur and Kogar). To tackle this problem, fencing the crops is a common procedure, which is detrimental to both wildlife and forests. The fencing material is usually the locally available wooden log, brought from nearby forests. For supplementing the wooden logs, large number of regenerating forest trees were cut down thereby jeopardizing the forest growth itself. These fences act as enclosures for wildlife movement from one place to another.
Hunting is practised as a sport, for subsistence, for crop protection and as a part of religious tradition by many village communities. During night-times, people form groups and go for hunting. A number of communities (Nayaks, Edegaru and Namadari gowdru) in the sanctuary carry out poaching activity. They target on wild animals like mouse deer, rabbit, wild boar, etc., due to which, the wild animal population is decreasing at a rapid rate. People support hunting as it reduces the probable damage to crops. Even some of the birds like spotted dove, cattle egret, pond heron, jungle fowl, peacock etc., are being hunted for meat by the local tribes. Poaching for money is seldom indulged in and gaurs constitute the main victim. Outside people are believed to be coming to the area to carry out this kind of poaching. At least one or two episodes do occur every year. The remoteness of the area and sparsely distributed human settlements are again advantageous to these poachers. In aquatic environment high fishing activity of the local people, licensed fishermen and migratory fishermen has threatened the indigenous fish population along with the endangered tortoise population of the region.
Proposed habitat corridors: Wildlife present in the region are seasonal migrants from adjacent sanctuaries and hence, the corridors used by these animals should be given more attention. Three micro-habitat corridors have been proposed for linking fragmented habitats, so as to have continued link of populations to maintain sufficient viable reproductive groups to permit breeding. Corridors are to be developed with the native species of plants, which meet the food and fodder requirement of fauna during all seasons. Table 16.3 lists the present land-use in the proposed corridors; similarly Figure 3.2 illustrates the regions proposed for corridors.
Channagonda and Kattinkar Corridor:These corridors are proposed in the western side of the sanctuary with evergreen and semi evergreen forests. This region comes under four state forests namely Muppane, Channagonda, Karani and part of Govardhanagiri.
Table 16.3:Details of land-use pattern in the proposed corridors.
| Corridor |
LC_No. |
Village Name |
Population |
Area
(ha) |
Forest
(ha) |
Agriculture
(ha) |
CW
(ha) |
Un.Cultivated (ha) |
| 1 |
178 |
Channagonda |
861 |
6391.49 |
2353.39 |
139.04 |
2158.74 |
1740.32 |
| 2 |
200 |
Banukuli |
380 |
2250.59 |
340 |
80.68 |
1745.47 |
84.44 |
| 3 |
209 |
Mandavalli |
425 |
875.23 |
0 |
61.12 |
760.20 |
53.91 |
Note: CW – cultivable waste
Figure 3.2: Proposed wildlife corridors in the SVWS
Corridors 1 is proposed for free movement of sloth bear, sambar and gaur. It has grasslands and barren lands surrounded by a good semi evergreen and evergreen forest. It encompasses the areas like Channagonda, Kanapagaru, Muppane, Aralagodu and Bedrur. This corridor comes in Muppane state forest and Govardhanagiri state forest and has sparsely distributed semi evergreen and moist deciduous forest. This helps animals to migrate to adjoining forests of Talakalale balancing reservoir, Henni and Gerusoppa area.
Corridor 2 is proposed in Banukuli village and has grassland surrounded by semi evergreen and moist deciduous forests (Kanapagaru and Channagonda villages). Corridor 3 comes under Mandavalli village mainly for the movement of tiger, gaur and sambar. This corridor is nearer to Vatemadike village with grasslands interspersed with moist deciduous forest.
Restoration of forest: In order to restore the forest depending on the state of degradation the following list of plants has been recommended. The list of plants recommended for deciduous, semi-evergreen and evergreen degraded patches area given in Table 17.1, 17.2 and 17.3 respectively.
Table 17.1: Plant recommended for restoration of deciduous forests in the sanctuary.
| Plant species |
Common name |
Ecosystem and human value |
| Olea dioica |
Madle |
EV |
| Mimusops elengi |
Ranjalu |
EV, NTFP |
| Aporosa lindleyana |
Salle |
EV, FR |
| Dillenia pentagyna |
Kanagalu |
EV |
| Garcinia indica |
Muruga |
EV, FR, NTFP |
| Terminalia paniculata |
Hunalu |
EV |
| Flacourtia montana |
Mullu sampige |
EV, FR |
| Mangifera indica |
Mavu |
EV, FR, MD |
| Syzygium caryophyllatum |
Kunnerlu |
EV, FR |
| Syzygium cumini |
Neralu |
EV, FR |
| Artocarpus heterophylla |
Halasu |
EV, FR |
| Artocarpus gomezianus |
Wote |
EV, NTFP |
Table 17.2: Plants recommended for restoration of semi-evergreen forests in the sanctuary
| Plant species |
Common name |
Ecosystem and human value |
| Aglaia anamallayana |
Kempunola |
EV |
| Artocarpus heterophyllus |
Halasu |
NTFP, F |
| Artocarpus hirsutus |
Hebbalasu |
EV, F |
| Canarium strictum |
Kaidhupa |
EV, NTFP |
| Dimocarpus longan |
Kendal |
EV |
| Garcinia morella |
Harisina gurgi |
EV, F |
| Holigarna arnottiana |
Sannele holageru |
EV |
| Holigarna beddomei |
Doddele holageru |
EV |
| Hopea ponga |
Haiga |
EV |
| Knema attenuata |
Hedaglu |
EV |
| Mimusops elengi |
Ranjalu |
EV, NTFP |
| Vepris bilocularis |
Mangappe |
EV |
| Polyalthia sp. |
|
EV |
| Mangifera indica |
Mavu |
EV, F |
| Symplocos racemosa |
Chunga |
EV |
Table 17.3: Plants recommended for restoration of evergreen forests in the sanctuary
| Plant species |
Common name |
Ecosystem and Human value |
| Poeciloneuron indicum |
Balgi |
EV |
| Knema attenuata |
Hedaglu |
EV, F |
| Myristica malabarica |
Rampatre |
EV, NTFP |
| Myristica dactyloides |
Patre |
EV, NTFP |
| Persea macrantha |
Gulmavu |
EV |
| Calophyllum tomentosum |
Surhonne |
EV |
| Dipterocarpus indicus |
Dhuma |
EV |
| Palaquium ellipticum |
Hadasale |
EV |
| Ficus nervosa |
|
EV |
| Mastixia arborea |
Niratte |
EV |
| Vateria indica |
Saldhupa |
EV, NTFP |
| Elaeocarpus tuberculatus |
|
EV |
| Mangifera indica |
Mavu |
EV, F |
| Chrysophyllum roxburghii |
|
EV |
| Canarium strictum |
Kaidhupa |
EV |
| Calamus sp. |
Betha |
NTFP |
| Syzygium gardneri |
Nerlu |
EV, FR |
Note: EV- Ecosystem value, FR- Fruit, LM- Leaf Manure, MD- Medicinal & NTFP- Non Timber Forest Produce
Policies: In SVWS, forest enclosures play an important role in order to maintain viable wildlife population. Madenur, Muppane, Hallibyle and Shashichowka are the few forest enclosures with high density of gaur, sambar, and mouse deer. The intention of these enclosures is to provide protection to both flora and fauna of the region. These forest enclosures serve a better protection to some of the vulnerable species from the poachers. Most of these enclosures are planted with monoculture species like, Acacia, Casuarina, etc., which in turn not a suitable habitat for the above mentioned wildlife. In order to maintain the high density of these species, gradual conversion of monoculture into native species As the territories of wild animals extend beyond these enclosures, flocking of wild animals and futile attempts to cross these barriers have been noticed. This suggests the expansion of existing enclosures and creation of new enclosures, which has to be undertaken based on rigorous monitoring of wildlife movement.
Effective vigilance has to be exercised by the forest department in order to stop the further encroachments and poaching of wild animals inside the sanctuary. To avoid water scarcity, large number of water holes/percolation ponds should be constructed inside the sanctuary. The existing awareness programmes such as wildlife weeks, wildlife census, etc., have to be expanded and strengthened in the sanctuary and surrounding areas in order to educate and create awareness among local people. Interaction of forest officials with local people helps to arrive at solutions based on clear understanding of situation in the sanctuary.
In the adjoining areas like, Gerusoppa, Uttarakoppa, Aruvakki etc., Kyasanur Forest Disease (KFD) is more prevalent due to high degree of forest degradation, that has led to the extensive growth of weeds, where in ticks, the main disease vector inhabit. Therefore restoration of full-canopied natural forest in the sanctuary area is of paramount importance. The Sharavathi valley wildlife sanctuary has to be extended further so as to link to the Mookambika wildlife sanctuary to facilitate the better movement of wild animals and also conservation of endangered and endemic fauna (like Lion-tailed Macaque) and pristine forest areas (like, Kodachadri, Gurta, Malemane and Kathalekan).
3. GUDAVI BIRD SANCTUARY
Gudavi bird sanctuary is located at 14° 25' 59" to 14° 26'41" N and 75° 6' 43" to 75° 1' 28" E in Soraba Taluk of Shimoga district (Figure 4). The Gudavi Bird Sanctuary was preliminary notified vide government notification No. AHFF-262-FWL-86 Dtd 10.07.1989 of Government of Karnataka and finally notified vide Government notification no. FEE-220-FWL-99 Dtd 4.09.2000 (Karnataka Forest department, 2006). The total area of Gudavi bird Sanctuary is 73.68 Hectares of which water spread area is 33 Ha and is surrounded by moist deciduous forest, interspersed with grassy patches (Karnataka Forest department, 2006). In this sanctuary there are two ponds called as Vaddakere and Gudavi ponds. The catchment area for this sanctuary is mainly agriculture land and other wooded areas. It is surrounded by paddy fields on North, West and Southern sides and dry and on North and Eastern sides. There are three villages namely Hullemaradi, Gudavi and Kallambi with a population of about 3000. Majority of the population are agriculturists.The area is plain and soil is deep and lateritic in origin. The average rainfall of the area is 1500 mm. The maximum and minimum temperature recorded in the sanctuary is 15° C and 38º C respectively. The area surrounding the wetland is covered with dense moist deciduous forest.
Figure 4: Gudavi Bird Sanctuary |
The boundaries of the sanctuary are:
North: Tank bound and Hinduvaly S.No.199, 201, 211, 71 & 204 of Gudavi village.
East: Gudavi road and Hinduvaly S.No. 55 and 64 of Kallambi village.
South: S.No. 54 & 55 of Kallambi village.
West: Hulemardi road & Hiduvaly S.No. 182. |
Composition of vegetation
Mainly five tree species are utilized by birds for nesting – Vitex leucoxylon, Kiranganelia reticulate, Phyllanthus polyphyllus, Ficus lacur and Terminalia sp.
Bird species composition: There are about 191 species of birds, out of 63 species of birds are totally dependent on water. The number of species changes in different months. Highest number of species is observed during October and least number of species in July. (Karnataka Forest department, 2006)
Dayananda G.Y. (2009) studied the bird diversity of Gudavi Bird Sanctuary. The avifauna of Gudavi Bird Sanctuary belonged to 16 orders.Out of these members of Ciconiformes, Paliconiformes and Passeriformes contributed maximum to the avifauna of the ponds throughout the year. The members of order Anseriformes and Charadriformes are migratory specieswho utilize the ponds for foraging during winter months. However, the local migrants are widespread throughout the year and to adjacent wetlands, moving to and fro utilizing the best resources available to them. The total number of species recorded in this sanctuary was 218.Of the 218 species of birdssighted at the sanctuary, a large number of terrestrial birds (163 species) constituted 75.11% whereas 24.88% was constituted by a relatively small number of aquatic birds consisting of 54 species. In terms of species strength of families represented Muscicapidae was the largest with 35 species. (Table 18)
Table18: Species composition of birds at Gudavi Bird Sanctuary
| SN |
Common name |
Scientific Name |
Residential Status |
Abundance Status |
| 1. Family: Podicipedidae |
| 1 |
Little Grebe |
Tachybaptusruficollis |
R |
V Com |
| 2. Family: Phalacrocoracedae |
| 2 |
Great Cormorant |
Phalacrocoraxcarbo |
RM |
Com |
| 3 |
Indian Shag |
Phalacrocoraxfuscicollis |
RM |
Com |
| 4 |
Little Cormorant |
Phalacrocoraxniger |
RM |
L Com |
| 3. Family: Anhingidae |
| 5 |
Darter or Snake bird |
Anhingamelamogaster |
RM |
L Com |
| 4. Family: Ardeidae |
| 6 |
Grey Heron |
Ardeacinerea |
RM |
L Com |
| 7 |
Purple Heron |
Ardeapurpurea |
RM |
L Com |
| 8 |
Little Green Heron |
Butoridesstriatus |
RM |
Ra |
| 9 |
Pond Heron |
Ardeolagrayii |
R |
L Com |
| 10 |
Cattle Egret |
Bubulcusibis |
RM |
Com |
| 11 |
Large Egret |
Casmerodiusalbus |
RM |
Com |
| 12 |
Smaller Egret |
Mesophoyxintermedia |
RM |
L Com |
| 13 |
Little Egret |
Egrettagarzetta |
R |
L Com |
| 14 |
Night Heron |
Nycticoraxnycticorax |
R |
L Com |
| 15 |
Chestnut Bittern |
Ixobrychuscinnamoneus |
RM |
L Com |
| 5. Ciconiidae |
| 16 |
Painted Stork |
Mycterialeucocephala |
RM |
L Com |
| 17 |
Openbill Stork |
Anastomusoscitans |
R |
L Com |
| 6. Family: Threskiornithidae |
| 18 |
Black-headed Ibis |
Threskiornismelanocephalus |
R |
L Com |
| 19 |
Black Ibis |
Pseudibispapillosa |
R |
Un Com |
| 20 |
Glossy Ibis |
Plegadisfalcinellus |
RM |
Un Com |
| 21 |
Spoonbill |
Platalealeucorodia |
RM |
L Com |
| 7. Family: Anatidae |
| 22 |
Lesser whistling Teal |
Dendrocygnajavanica |
R |
L Com |
| 23 |
Pintail |
Anusacuta |
M |
Com |
| 24 |
Common Teal |
Anascrecca |
RM |
V Com |
| 25 |
Spot-billed Duck |
Anaspoecilorhyncha |
RM |
Com |
| 26 |
Garganey |
Anasquerquedula |
M |
V Com |
| 27 |
Shoveller |
Anasclypeata |
M |
Com |
| 28 |
Cotton Teal |
Nettapuscoromandelianus |
R |
L Com |
| 29 |
Nakta or Comb Duck |
Sarkidiornismelanotos |
R |
Un Com |
| 8. Accipitridae |
| 30 |
Crested Honey-Buzzard |
Pernisptilorhyncus |
R |
L Com |
| 31 |
Common P ariahkite |
Milvusmigrans |
R |
Com |
| 32 |
Brahminy kite |
Haliasturindus |
R |
L Com |
| 33 |
Shikra |
Accipiterbadius |
RM |
Com |
| 34 |
Sparrow Hawk |
Accipiternisus |
R |
L Com |
| 35 |
Besra Sparrow Hawk |
Accipitervirgatus |
R |
L Com |
| 36 |
Tawny Eagle |
Aquilarapax |
RM |
L Com |
| 37 |
Greater spotted Eagle |
Aquilaclanga |
RM |
Ra |
| 38 |
Ring tailed fishing Eagle |
Haliaeetusleucoryphus |
M |
Ra |
| 39 |
P ale Harrier |
Circusmacrourus |
RM |
L Com |
| 40 |
P aid Harrier |
Circusmelanoleucos |
M |
Ra |
| 41 |
Marsh Harrier |
Circusaeruginosus |
R |
L Com |
| 42 |
Common Kestrel |
Falcotinnunculus |
RM |
L Com |
| 9. Phasianidae |
| 43 |
Grey Partridge |
Francolinuspondicerianus |
R |
Com |
| 44 |
Jungle bush Quill |
Perdiculaasiatica |
R |
Com |
| 45 |
Red Spurfowl |
Galloperdixspadicea |
R |
L Com |
| 46 |
Grey Jungle fowl |
Gallussonneratii |
R |
Com |
| 47 |
Common Peafowl |
Pavocristatus |
R |
Com |
| 10. Turnicidae |
| 48 |
Common Bustard Quail |
Turnixsuscitator |
R |
L Com |
| 11. Rallidae |
| 49 |
IndianRail Blue-breasted Banded |
Rallusstriatus |
RM |
Un Com |
| 50 |
Slaty-legged Banded Crake |
Rallinaeurizonoides |
R |
Un Com |
| 51 |
Brown Crake |
Amaurornisakool |
R |
Com |
| 52 |
White breasted Water hen |
Amaurornisphoenicurus |
R |
Com |
| 53 |
Water Cock |
Gallicrexcinerea |
RM |
L Com |
| 54 |
Indian Moorhen |
Gallinulachloropus |
R |
Com |
| 55 |
Purple Moorhen |
Porphyrioporphyrio |
RM |
L Com |
| 56 |
Coot |
Fulicaatra |
R |
V Com |
| 12. Jacanidae |
| 57 |
P heasant tailed Jacana |
Hydrophasianuschirurgus |
R |
Un Com |
| 58 |
Bronze winged Jacana |
Metopidicusindicus |
R |
L Com |
| 13. Charadriidae |
| 59 |
Red-wattled Lapwing |
Vanellusindicus |
R |
Com |
| 60 |
Yellow-wattled Lapwing |
Vanellusmalabaricus |
M |
L Com |
| 61 |
Grey P lover |
Pluvialissquatarola |
M |
Un Com |
| 62 |
Golden Plover |
Pluvialisdominica |
RM |
Va |
| 63 |
Little ringed Plover |
Charadriusdubius |
M |
Com |
| 64 |
Marsh Sandpiper |
Tringastagnatilis |
M |
L Com |
| 65 |
Spotted Sandpiper |
Tringaglareola |
RM |
L Com |
| 14. Scolopacidae |
| 66 |
Common or Fantail Snipe |
Gallinagogallinago |
R |
Com |
| 15. Recurvirostridae |
| 67 |
Black-winged Stilt |
Himantopushimantopus |
R |
Com |
| 16. Laridae |
| 68 |
Indian River Tern |
Sternaaurantia |
RM |
L Com |
| 69 |
Common Tern |
Sternahirundo |
R |
L Com |
| 17. Culumbidae |
| 70 |
Grey fronted Green Pigeon |
Treronpompadora |
R |
L Com |
| 71 |
Common Green Pigeon |
Treronphoenicoptera |
R |
Com |
| 72 |
Green Imperial Pigeon |
Duculabadia |
R |
L Com |
| 73 |
Blue Rock Pigeon |
Columbalivia |
R |
V Com |
| 74 |
Spotted Dove |
Streptopeliachinensis |
R |
V Com |
| 18. Psittacidae |
| 75 |
Rose ringed P arakeet |
Psittaculakrameri |
R |
V Com |
| 76 |
Blossom headed P arakeet |
Psittaculacyanocephala |
RM |
L Com |
| 77 |
Lorikeet |
Loriculusvernalis |
RM |
L Com |
| 19. Cuculidae |
| 78 |
P ied crested Cuckoo |
Clamatorjacobinus |
R |
L Com |
| 79 |
Common hawk Cuckoo |
Cuculusvarius |
R |
L Com |
| 80 |
Indian Cuckoo |
Cuculusmicropterus |
R |
L Com |
| 81 |
Koel |
Eudynamysscolopacea |
R |
V Com |
| 82 |
Large Greenbilled Malkoha |
Rhopodytestristis |
R |
L Com |
| 83 |
Small Greenbilled Malkoha |
Rhopodytesviridirostris |
R |
L Com |
| 84 |
Coucal or Crow-Pheasant |
Centropussinensis |
R |
V Com |
| 85 |
Lesser Coucal |
Centropusbengalensis |
R |
V Com |
| 20. Strigidae |
| 86 |
Barred Jungle Owlet |
Glaucidiumradiatum |
R |
L Com |
| 87 |
Spotted Owlet |
Athenebrama |
R |
Com |
| 88 |
Mottled wood Owl |
Strixocellata |
R |
L Com |
| 89 |
Brown wood Owl |
Strixleptogrammica |
RM |
L Com |
| 21. Apodidae |
| 90 |
Indian edible nest Swiftlet |
Collocaliaunicolor |
R |
Com |
| 91 |
House Swift |
Apusaffinis |
RM |
Com |
| 22. Alcedinidae |
| 92 |
Pied Kingfisher |
Cerylerudis |
R |
Com |
| 93 |
Small blue Kingfisher |
Alcedoatthis |
R |
Com |
| 94 |
Stork-billed Kingfisher |
Pelargopsiscapensis |
R |
L Com |
| 95 |
White-breasted Kingfisher |
Halcyonsmyrnensis |
R |
Com |
| 23. Meropidae |
| 96 |
Chestnut headed Bee-ater |
Meropsleschenaultia |
RM |
L Com |
| 97 |
Small green Bee-eater |
Meropsorientalis |
R |
L Com |
| 98 |
Blue bearded Bee-eater |
Nyctyornisathertoni |
R |
L Com |
| 24. Coraciidae |
| 99 |
Roller or Blue jay |
Coraciasbenghalensis |
R |
Com |
| 25. Upupidae |
| 100 |
Hoopoe |
Upupaepops |
R |
V Com |
| 26. Bucerotidae |
| 101 |
Common grey Hornbill |
Tockusbirostris |
R |
Com |
| 102 |
Malabar Grey Hornbill |
Tockusgriseus |
R |
Com |
| 103 |
Malabar pied Hornbill |
Anthracoceroscoronatus |
R |
Com |
| 27. Megalaimidae |
| 104 |
Large green Barbet |
Megalaimazeylanica |
R |
Com |
| 105 |
Lineated Barbet |
Megalaimalineate |
R |
L Com |
| 106 |
Small green Barbet |
Megalaimaviridis |
R |
Com |
| 107 |
Crimson throated Barbet |
Megalaimarubricapilla |
R |
Com |
| 108 |
Crimson breasted Barbet |
Megalaimahaemacephala |
R |
Com |
| 28. Picidae |
| 109 |
Rufous Woodpecker |
Micropternusbrachyurus |
R |
L Com |
| 110 |
LesserWoodpeckergolden backed |
Dinopiumbenghalense |
R |
L Com |
| 111 |
Great black Woodpecker |
Dryocopusjavensis |
R |
L Com |
| 112 |
YellowWoodpeckerfronted pied |
Picoidesmahrattensis |
R |
L Com |
| 113 |
Pigmy Woodpecker |
Picoidesnanus |
R |
L Com |
| 29. Pittidae |
| 114 |
Indian Pitta |
Pittabrachyura |
R |
Com |
| 30. Alaudidae |
| 115 |
Bush Lark |
Mirafraassamica |
R |
Com |
| 116 |
Red winged Bush-Lark |
Mirafraerythroptera |
R |
L Com |
| 117 |
Black bellied Finch-Lark |
Eremopterixgrisea |
RM |
L Com |
| 118 |
Rufous tailed Finch-Lark |
Ammomanesphoenicurus |
R |
L Com |
| 119 |
Crested Lark |
Galeridacristata |
R |
L Com |
| 120 |
Sykes's Crested Lark |
Galeridadeva |
RM |
L Com |
| 31. Hirundinidae |
| 121 |
Swallow |
Hirundorustica |
RM |
L Com |
| 122 |
Wire tailed Swallow |
Hirundosmithii |
RM |
L Com |
| 123 |
Indian cliff Swallow |
Hirundofluvicola |
R |
L Com |
| 32. Laniidae |
| 124 |
Rufous backed Shrike |
Laniusschach |
R |
L Com |
| 33. Oriolidae |
| 125 |
Golden Oriole |
Oriolusoriolus |
RM |
Com |
| 126 |
Black naped Oriole |
Orioluschinensis |
R |
Com |
| 127 |
Black headed Oriole |
Oriolusxanthornus |
R |
Com |
| 34. Dicruridae |
| 128 |
King Crow or Black Drongo |
Dicrurusadsimilis |
R |
Com |
| 129 |
Grey or Ashy Drongo |
Dicrurusleucophaeus |
R |
Com |
| 130 |
Racket tailed Drongo |
Dicrurusparadiseus |
R |
Com |
| 35. Sturnidae |
| 131 |
Brahminy Myna |
Sturnuspagodarum |
R |
Com |
| 132 |
Indian Myna |
Acridotherestristis |
R |
Com |
| 133 |
Jungle Myna |
Acridotheresfuscus |
R |
Com |
| 36. Corvidae |
| 134 |
Tree pie |
Dendrocittavagabunda |
R |
Com |
| 135 |
White bellied Treepie |
Dendrocittaleucogastra |
R |
Com |
| 136 |
House Crow |
Couvussplendens |
R |
V Com |
| 137 |
Jungle Crow |
Corvusmacrorhynchos |
R |
V Com |
| 37. Campephagidae |
| 138 |
Pied Flycatcher-Shrike |
Hemipuspicatus |
R |
Com |
| 139 |
Large Wood Shrike |
Tephrodornisgularis |
R |
Com |
| 140 |
Common Wood Shrike |
Tephrodornispandicerianus |
R |
V Com |
| 141 |
Black headed cuckoo Shrike |
Coracinamelanoptera |
R |
Com |
| 142 |
Scarlet Minivet |
Pericrocotusflammeus |
R |
Un Com |
| 143 |
Long tailed Minivet |
Pericrocotusethologus |
R |
Un Com |
| 144 |
Small Minivet |
Pericrocotuscinnamomeus |
R |
Com |
| 145 |
White bellied Minivet |
Pericrocotuserythropygius |
R |
Com |
| 38. Irenidae |
| 146 |
Common Iora |
Aegithinatiphia |
R |
V Com |
| 147 |
Marshall's Iora |
Aegithinanigrolutea |
R |
Com |
| 148 |
Gold fronted Chloropsis |
Chloropsisaurifrons |
R |
Com |
| 39. Pycnonotidae |
| 149 |
Red whiskered Bulbul |
Pycnonotusjocosus |
R |
V Com |
| 150 |
White cheeked Bulbul |
Pycnonotusleucogenys |
R |
Com |
| 151 |
Red vented Bulbul |
Pycnonotuscafer |
R |
V Com |
| 152 |
Black Bulbul |
Hypsipetesmadagascariensis |
R |
Com |
| 40. Muscicapidae |
| Sub Family :Timalinae |
| 153 |
Spotted Babbler |
Pellorneumruficeps |
R |
Com |
| 154 |
Slaty headed scimitar Babbler |
Pomatorhinushorsfieldi |
R |
Com |
| 155 |
Common Babbler |
Turdoidescaudatus |
R |
V Com |
| 156 |
Large grey Babbler |
Turdoidesmalcolmi |
R |
V Com |
| 157 |
Jungle Babbler |
Turdoidesstriatus |
R |
V Com |
| 158 |
White headed Babbler |
Turdoidesaffinis |
R |
Com |
| 159 |
Wynaad laughing Thrush |
Garrulaxdelesserti |
R |
Un Com |
| 160 |
Jerdon’s laughing Thrush |
Garrulaxjerdoni |
R |
Un Com |
| 161 |
White headed shrike Babbler |
Gampsorhynchusrufulus |
R |
Un Com |
| 162 |
Black capped Sibia |
Heterophasiacapistrata |
R |
Com |
| Sub Family: Muscicapinae |
| 163 |
Black and Orange Flycatcher |
Muscicapanigrorufa |
RM |
Un Com |
| 164 |
Tickll's blue Flycatcher |
Muscicapatickelliae |
R |
Com |
| 165 |
Verditer Flycatcher |
Muscicapathalassina |
R |
Com |
| 166 |
Nilgiri verditer Flycatcher |
Muscicapaalbicaudata |
R |
Com |
| 167 |
White spotted fantail Flycatcher |
Rhipiduraalbicollis |
R |
Com |
| 168 |
Paradise Flycatcher |
Terpsiphoneparadise |
R |
Com |
| 169 |
Black naped blue Flycatcher |
Hypothymisazurea |
R |
Com |
| Sub Family: Sylviinae |
| 170 |
Rufous fronted Wren-Warbler |
Priniabuchanani |
RM |
Com |
| 171 |
Ashy Wren-Warbler |
Priniasocialis |
M |
Ra |
| 172 |
Jungle Wren-Warbler |
Priniasylvatica |
M |
Ra |
| 173 |
Tailor bird |
Orthotomussutorius |
RM |
V Com |
| 174 |
Striated marsh Warbler |
Megaluruspalustris |
R |
Un Com |
| 175 |
Booted Warbler |
Hippolaiscaligata |
R |
Un Com |
| 176 |
Common Chiffchaff |
Phylloscopuscollybita |
R |
Un Com |
| 177 |
Large billed leaf Warbler |
Phylloscopusoccipitalis |
R |
Un Com |
| Sub Family: Turdinae |
| 178 |
Blue Chat |
Erithacusbrunneus |
R |
V Com |
| 179 |
Magpie Robin |
Copsychussaularis |
RM |
V Com |
| 180 |
Shama |
Copsychusmalabaricus |
R |
Un Com |
| 181 |
Brown rock Chat |
Cercomelafusca |
RM |
Un Com |
| 182 |
Pied bush Chat |
Saxicolacaprata |
R |
Com |
| 183 |
Jerdon’s bush Chat |
Saxicolajerdoni |
R |
Com |
| 184 |
Indian Robin |
Saxicoloidesfulicata |
R |
V Com |
| 185 |
Blue Rock Thrush |
Monticolasolitarius |
R |
Com |
| 186 |
Orange headed Thrush |
Zootheracitrinecyanotus |
R |
Com |
| 187 |
Black Bird |
Turdusmerula |
RM |
Com |
| 41. Paridae |
| 188 |
Grey Tit |
Parusmajor |
RM |
Com |
| 189 |
White winged Black Tit |
Parusnuchalis |
R |
Com |
| 190 |
Yellow cheeked Tit |
Parusxanthogenys |
R |
Com |
| 42. Sittidae |
| 191 |
Chestnut bellied Nuthatch |
Sittacastanea |
R |
Com |
| 43. Motacillidae |
| 192 |
Paddy field Pipit |
Anthusrufulus |
R |
L Com |
| 193 |
Forest Wagtail |
Motacillaindica |
R |
L Com |
| 194 |
Yellow Wagtail |
Motacillaflava |
R |
Lcom |
| 195 |
White Wagtail |
Motacillaalba |
R |
Com |
| 196 |
Large pied Wagtail |
Motacillamaderaspatensis |
R |
Lcom |
| 44. Dicaeidae |
| 197 |
Tickell's Flowerpecker |
Dicaeumerythrorhynchos |
R |
L Com |
| 198 |
Plain coloured Flowerpecker |
Dicaeunconcolor |
R |
L Com |
| 199 |
Fire breasted Flowerpecker |
Dicaeunignipectus |
R |
L Com |
| 45. Nectariniidae |
| 200 |
Purple rumped Sunbird |
Nectariniazeylonica |
R |
Com |
| 201 |
Small Sunbird |
Nectariniaminima |
R |
Com |
| 202 |
Maroon breasted Sunbird |
Nectarinialotenia |
R |
Com |
| 203 |
Purple Sunbird |
Nectariniaasiatica |
R |
Com |
| 204 |
Little Spider hunter |
Arachnotheralongirostra |
R |
L Com |
| 46. Zosteropidae |
| 205 |
White Eye |
Zosteropspalpebrosus |
RM |
L Com |
| 47. Ploceidae |
| Sub Family : Passerinae |
| 206 |
House Sparrow |
Passerdomesticus |
R |
Com |
| Sub Family: Ploceinae |
|
|
|
| 207 |
Baya weaver bird |
Ploceusphilippinus |
R |
Com |
| 208 |
Balck breasted weaver bird |
Ploceusbenghalensis |
R |
Com |
| 209 |
Streaked weaver bird |
Ploceusmanyar |
R |
L Com |
| Sub Family: Estrildinae |
|
|
|
| 210 |
Red Munia |
Estrildaamandava |
R |
Com |
| 211 |
Green Munia |
Estrildaformosa |
R |
Un Com |
| 212 |
White throated Munia |
Lonchuramalabarica |
R |
Com |
| 213 |
White backed Munia |
Lonchurastriata |
R |
Com |
| 214 |
Spotted Munia |
Lonchurapunctulata |
R |
Com |
| 215 |
Black headed Munia |
Lonchuramalacca |
R |
Com |
| 48. Fringillidae |
|
|
| Sub Family: Carduelinae |
|
|
| 216 |
Rose Finch |
Carpodacuserythrinus |
RM |
Com |
| Sub Family: Emberizinae |
|
|
| 217 |
House Striolated |
Emberizastriolata |
R |
L Com |
| 218 |
Crested Bunting |
Emberizalathami |
R |
L Com |
| Status of the birds: WM= Winter migrant, Com= Common, R/LM= Resident with local movements, L Com=Locally common, R= Resident, Un Com= Un common, M= Migratory, V Com= Very common, R/LM/SM=Resident with local as well as summer movements. |
External threats
About 8.0 Ha of the sanctuary area has been encroached. The tank is drained by the villagers during summer for agriculture and this disturbs the water bird habitation.
REFERENCES
- Dayananda G.Y. (2009). Avifaunal diversity of Gudavi Bird Sanctuary, Sorab, Shimoga Karnataka. Our Nature 7:100-109.
- Karnataka Forest Department, Management plan for Sharavathi Wildlife Sanctuary (2001-2006) Deputy Conservator of forests Wildlife division Shimoga.
- Karnataka Forest Department, Management plan for Shettihalli Wildlife Sanctuary (2001-2006) Deputy Conservator of forests Wildlife division Shimoga.
- Karnataka Forest Department, Management plan for Shettihalli Wildlife Sanctuary (2005-06 to 2014-15). Deputy Conservator of forests Wildlife division Shimoga
- Karnataka Forest Department, Management plan for Gudavi Bird Sanctuary (2006-07 to 2015-16) Deputy Conservator of forests Wildlife division Shimoga.
- Kumara, H.N.(2007) Impact of local hunting on abundance of large mammals in three protected areas of the Western Ghats, Karnataka. Technical report submitted to Rufford Maurice Laing Foundation, U.K.
- Kumara, H.N. and Singh, M. (2005b). Disappearance of elephants in Uttara Kannada. Journal of Bombay Natural History Society, 102:337
- Nair, P.V and Gadgil, M (1978). The status and distribution of elephant population of Karnataka. Journal of the Bombay Natural History Society, 75: 1000-1016.
- Pramod Kumar M.P.M., Hosetti B.B., Poornesha H.C. and Raghavendra Gowda H.T. (2007). Butterflies of the Tiger-Lion Safari, Thyaverekoppa, Shimoga, Karnataka, Zoos print Journal 22(8):2805.
- Sameer Ali, Rao.G.R, Divakar Mesta, Sreekantha, Vishnu D.Mukri, Subhashchandran M.D., Gururaja K.V., Joshi.N.V, and Ramachandra.T.V, 2007. Ecological Status of Sharavathi Wildlife Sanctuary Sahyadri Conservation series-1, ETR-19. Centre For Ecological Sciences,Indian Institute of Sciences, Bangalore.
|
|










