Frothing in Bellandur and Varthur Lake: Causes and Remedial Measures
A sustained inflow of sewage (~500 MLD) into Bellandur and Varthur lakes brings in a variety of natural and synthetic dissolved organic compounds, along with excrements. The frothing happens due to the activity if the surface-active agents or surfactants that reduce the surface tension of water, allowing air bubbles to persist at the water’s surface (figure 6.1). These detergents essentially consists of phosphates (~30 % STPP), and a portion of which is up-taken by aquatic plants while the balance gets trapped in the sediments.


Pre-monsoon showers coupled with gusty winds leads to the churning of lake water with upwelling of sediments. Vigorous mixing of surface water coupled with high flow across narrow channels, leading to bubble formation that persist and build up as foam (Fig. 6.1: a-d). In the lakes, foam /froth gets accumulated along windward shores. Continuous sewage fed in Bellandur and Varthur lakes, has been witnessing foam at downstream in chocked channels or below fall/discharge point since one decade (Mahapatra et al., 2013a).
Sources of these surfactants: Macrophytes and algae inhabiting the lake waters produce many organic compounds (Ramachandra et al., 2009; Mahapatra and Ramachandra, 2013, Mahapatra et al., 2013a,b,c, Ramachandra et al., 2013; Mahapatra et al., 2014), which have surfactant properties. Natural surfactants include carboxylic fatty acids derived from lipids from macrophytes/weeds etc. These are released into water and contribute to a large variety of soluble organic material known as dissolved organic carbon (DOC). Though DOC is produced within lake waters, the major source is the sustained inflow of sewage from the vicinity of the lakes and the watershed. Higher DOC concentrations in lakes, generally impart a brown colour to the water and foam. This highlights that the foam is caused by synthetically produced surfactants released through sewage to surface waters. Synthetic surfactants are widely used in household cleaning products (detergents/soaps), cosmetics and personal care products (shampoo, toothpaste etc.). Common detergents also contains branch-chained alkyl benzene sulfonate surfactants, which are non-biodegradable and results in extremely persistent foam accumulating below the fall levels in the lake and other wastewater outfalls.
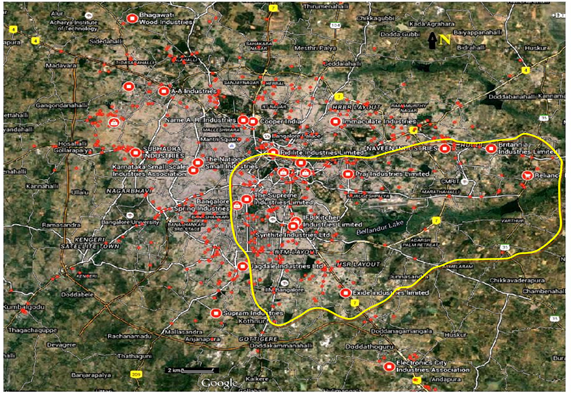
Fig. 6.2: Distribution of industries in the vicinity of Bellandur and Varthur lake and also industries scattered in the city (overlaid on Google earth image http://earth.google.com)
Detergents and soaps mostly contain phosphate (P) softeners to enhance the effectiveness of surfactants through the reduction of water hardness. P loading in lakes has contributed to nutrient enrichment with the proliferation of cyano-bacterial blooms and macrophytes (aquatic plants). There are set of advanced detergents that exclude phosphates but contain biodegradable linear alkyl benzene sulfonate surfactants, such as sodium or ammonium lauret or lauryl sulfate. Surfactants are also used by many industries (Fig. 6.2) as wetting agents, dispersants, defoamers, de-inkers, antistatic agents, and in paint and protective coatings, pesticides, leather processing, plastics and elastomer manufacturing, and oil extraction and production.
Many industries that are present (Fig. 6.2 in the upstream of Bellandur and Varthur lakes (Ramachandra and Solanki, 2007) have also contributed to high levels of surfactants in the waters due to the release of untreated effluents in addition to the domestic sewage. These surfactants are very persistent in the environment, bio accumulate in organisms and humans with various biological consequences. Alkyl phenol ethoxylates for example, which continue to be widely used by industry, have been shown to have estrogenic properties eliciting reproductive effects in fish and other organisms. Similarly, per-fluoro octanoic acid and per-fluoro octane sulfonate, which were commonly used in the production of stain resistant and non stick coatings including Scotch guard and Teflon, also have estrogenic and carcinogenic properties. In contrast to natural foam, fresh detergent based foam is of white colour with noticeable odour. Bellandur and Varthur lake have been receiving a mix of untreated and partially treated wastewaters (~500 million litres per day, MLD), from major residential areas and some industries, both synthetic and natural compounds that are present have contributed to the formation of foam.
Surface tension is an important property of water. It results from cohesion – the attraction of water molecules for one another. Cohesion gives water the ability to form droplets and contributes to the formation of waves and currents, which play an important role in the distribution of temperature, dissolved gases, nutrients, micro-organisms and plankton. At the surface of the lake (i.e. the air-water interface), cohesion creates a thin ‘film’ or tension. This allows insects like water striders to ‘walk’ on water and forms a special habitat for some aquatic organisms adapted to living on this surface film (mosquito larvae for example). Surfactants are amphipathic molecules, that is, they contain both hydrophilic (water-attracting) and hydrophobic (water-repelling) components. The hydrophilic component can form bonds with water and competes with other water molecules as they attract one another.
In this manner, surfactants reduce the overall attraction between water molecules, thus diminishing surface tension. Lower surface tension causes water to become more ‘fluid’ or elastic, and when air gets in the resulting bubbles can persist for some time. Surfactants have contributed to 50% of foaming due to a reduced surface tension and balance is due to the intrusion of air into these waters to form the foam bubbles. In the studied lakes wind-induced currents and incipient waves cause turbulent mixing of air and water. Foaming often increases during runoff and rainstorms that transport the surfactants.
The foam collected from the Varthur outfalls were white in colour with a greasy/oily dark materials sticking on the surface of the foam bubbles (Fig 6.3.1). The foam had a pungent odour with sulphide smell unlike the natural foam that has an earthy or fishy aroma. The analysis conducted on foaming abilities showed, mean bubble size decrease with time, and finally ends up in sizes < 2 mm in diameter. The initial bubble sizes range from 2-4 cm (Fig. 6.3.2). These white foams progressively turn off-white and then settle as dark grey residue over time. Experiments conducted in laboratory shows, the persistent nature of the foam that lasts up to 6 days (Fig. 6.4).
Moreover, the foam volumes were observed to be higher during the 2ndand 3rd day that correlated with the mean bubble size. The foam diminishes after the 6th day due to low stability. Earlier reports on wastewater systems have indicated onset of foaming is because of surfactants and bio-surfactants, abundant in wastewater and sludge. They have both hydrophobic and hydrophilic properties and tend to accumulate at air–liquid interfaces increasing surface activity. When air/gas is introduced into solution, a thin liquid film is formed around the gas bubbles as they reach the air–liquid interface preventing them from bursting. The foaming persistence tests carried out in the laboratory by stirring showed the presence of surfactants indicating highest foaming abilities. The liquid phase of the foam samples contained significant amounts of surface active groupsduring the analysis period. However the foaming potential decreased after 4 days this can be attributed to the decrease in the interactions between solid particles and the surfactants and hence the stability of the foam. Studies on wastewater systems highlights that sludge, (Mahapatra et al., 2013a) containing surfactants and the foaming potential is enhanced or reduced depending on the surfactant–surfactant and particle–surfactant interactions (Glaser et al., 2007 and Eisner et al., 2007). More importantly increase of temperature in liquids containing surfactants result in increased surface activity (lower surface tension) enhancing the foaming potential (Barber, 2005) which was also observed in the present study as the foaming events are periodic and are often noticed during the summer at lake outfalls. In order to gain a better understanding of foam creation and stabilization, the liquid phase of foams generated at the outfalls of Varthur lake was analysed for carbon assays as COD, BOD and solids. The BOD and COD values were ~0.6 g/l and 1.14 g/l respectively. High total solids (TS) of ~110 g/l were observed in the liquid phase of the foam sample out of which ~92 g/l were total volatile solids (TVS). The ash content was ~16.2 g/l and the total dissolved solids (TDS) were ~7 g/l.
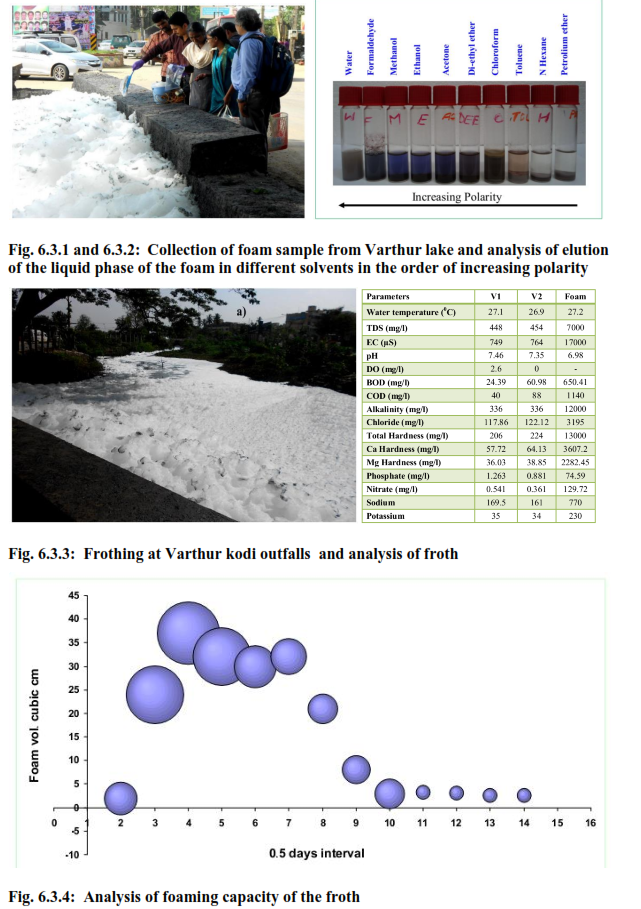
Hydrophobic compounds present in the DOC foam were confirmed by eluting the foam in non-polar and polar solvents (Mahapatra et al., 2013a-c; Mahapatra et al., 2014a,b). The solvents comprised of water, formaldehyde, methanol, ethanol, acetone, di-ethyl ether, chloroform, toluene, n-Hexane and petroleum ether that were arranged in order of decreasing polarity. The results showed the presence of amphipathic molecules as shown in Fig. 8. The analysis showed presence of both polar and non polar compounds in the liquid phase of the froth. The froth analysis showed higher values of TP >2 g/l with orthophosphate values >75 mg/l indicating higher P content in waters owing detergents and also P up-welling due to anaerobic conditions in the sediment layer of the lakes, aided my macrophyte cover over the lake surface. Laboratory analysis of the commonly used detergents as Surf Excel, Ariel, Rin etc. showed higher presence of polyphosphates with TP (~20-25 %) indicating detergents rich in P. Earlier studies on sludge sediments in Varthur lake indicated greater P influx from sediments during anaerobic conditions mostly during summer. The organic matter settled in the bottom of the lake resuspends owing to change in redox environment, that up-wells large quantities of immature sludge which imparts the dark grey colour to the lake water. Consequently, the water at the outfalls was grey in colour with higher particulate matter arising from sludge.
The Bellandur and Varthur lake waters are moderately hard waters (~215 mg/l of total hardness), with high Ca and Mg concentrations. As a result, foaming is not usually excessive in these waters. The incidence of high foam is also associated with high Na content in the lake in comparison to Ca and Mg. When the water is soft foam may occur more frequently. Foam is usually harmless if they are only from vegetative origin where the foaming agents are primarily proteinaceous or carbonaceous matter. In this case only a small amount of fatty acids or other foaming agents are required to produce foam. Only about 1% of the foam is made up of the foaming agent, the remaining 99 % being air and water. The foams originating from the wastewaters, detergents and other industrial origin surfactants will have significant impacts to the aquatic ecosystem and human health. These foam can accumulate compounds that are repelled by water (hydrophobic), so foam can be enriched significantly with particulate organic and inorganic compounds such as nutrients (N, P, C), cations (K, Na, Ca, Mg), heavy metals (Cd, Cu, Fe, Pb, Zn, Cr, Ni, Hg, Ar etc.) and chlorinated hydrocarbons. Therefore when these foams get in direct contact with human beings, depending on the specificity, they can cause many stimulatory effects that can trigger the immune response in the body. Moreover, the organisms that inhabit the surface layer would be more exposed to these contaminants and this could form a pathway to introduce contaminants into the food web.
Fire associated with foam in Bellandur lake: Flammability is the ability of a substance to burn or ignite, causing fire or combustion. Incidence of foam catching fire (Fig. 6.5.1 and 6.5.2) are due to compounds with high flammability i.e. mostly higher hydrocarbons and organic polymers from nearby industries in the vicinity of Bellandur lake. High wind coupled with high intensity of rainfall leads to upwelling of sediments with the churning of water as it travels from higher elevation to lower elevation forming froth due to Phosphorous. Discharge of untreated effluents (rich in hydro carbon) with accidental fire (like throwing cigarettes, beedi) has led to the fire in the lake.

The foam is a very periodic event (annual) which happens mostly in the pre-monsoon period at the outfalls of Bellandur and Varthur lake. The foam built up at the dry periods can be attributed to churning and associated sediment re-suspension from the lake bottom. This phenomena is also triggered due to anaerobic environments in the sediments that leads to a reducing environment (-340 to -280 mV – oxidation reduction potential; ORP; Mahapatra et al., 2013a-c) where the sludge/sediment bound P along with the decomposed plant parts, oil and greasy materials gets resuspended in the water (Mahapatra et al., 2013a,b). This produces a solid black layer on the surface of water that comprise of macrophyte/plant derived organic acids. With high wind velocities and water flow, this black particle that is mostly soluble in oil phase (hydrophobic in nature) gets deposited on the surface of the foam or bubbles. Frequent aeration of the lake waters falling off from the outfalls via splashing, forms gas bubbles that increase the liquid interfacial area a here at times charging occurs. Apart from charge generation at the surface, continuous aeration aids in formation of persistent froth that lasts from hours – days. This foam is also the source of very fine mist as it bursts. The rate at which the bubble bursts is dependent on the static spark that helps in disruption of the foam.
P in Detergents: Alternatives to Phosphorus in Detergents: There has been a series of events that has very clearly highlighted the linkages of enhanced usage and influx of P with a phenomenal increase in P enrichment in surface and ground waters. During seventies and early eighties, 19th century such instances had brought about an increase in global consensus and the public awareness mostly in the European nations and triggered regulations on P loads from Industry and Urban sources. In India there has been a widespread use of P based detergents that has resulted in contamination of ground and surface waters rendering these water unsuitable for any use. One of the major constituents that form a bulk of the detergents is the builder material that is often made up of Sodium tripolyphosphate (STPP) that significantly contributes to P enrichment. The levels of P enrichment in urban water systems is enormous ranging from 0.5 to >10 mg/l of labile P. Abundant P in these systems have substantially contributed to increased biomass productivity and a leap in the net primary productivity of the urban aquatic systems that ahs resulted in rampant proliferation of aquatic macrophytes and weeds at the same time aided in the large scale algal blooms often seen sin the surfaces of these urban water bodies. The sludge P values in the initial reaches of the wastewater fed water bodies like Bellandur is ~1-3 %. During shifts in redox environments these P becomes bioavailable and results in increased primary productivity of the system. The sediment P levels varies from 0.1 – 0.28 %, mostly as NaOH soluble P forms indicating high fraction of mineralisable P in these lake systems. Two main solutions for cutting short rapid and high P influx into the system is a) Introduction of non-P based builders in detergents for example Zeolite, that can completely replace Sodium tripolyphosphates (STPP - amounts to ~50% bio-available P in municipal wastewaters) commonly seen in detergents and b) Augmenting the existing wastewater treatment system for nutrient removal and recovery. This requires various measures that aids in framing and implementatation of laws to completely replace P based builders to alternative non-P based household laundry detergents. Already the European Commission (EC) has implemented non-P based culture in detergents through the European Union (EU) and recommends appropriate measures to improve the present P enrichment scenario. The two main essential P sources in urban conglomerates are the municipal wastewaters and to a lesser extent agriculture. In most of the Bangalore’s catchment that has an inadequate treatment facility and treatment is mostly upto secondary levels. Municipal wastewaters represent the single largest P source in urban municipalities. In case of certain areas where people practice agriculture, horticulture and floriculture, a minute amount of P (synthetic fertilisers) escapes from these landscapes, where top soil erosion and land run off are the crucial means of entry of fertiliser P into the channels and freshwater lakes. It has been estimated that P from detergents contributes to an estimated 65% of P in municipal wastewaters and the rest are from excrements etc. Based on the conclusion of the present study, the recommendations are a) A ban on production of polyphosphate based detergents in Indian systems which will help in usage of trusted non-P based detergents, that would bring down the P loads contributed from detergents in municipal wastewaters and also significantly reduce P loads from all garment, textile and other industries that uses detergents substantially; b) Nutrient removal and recovery mechanisms to be augmented into the existing treatment systems by the help of phyto-phyco modules.
P is limiting and crucial: Phosphorus is the most crucial nutrient in living systems and is a key component of the genetic makeup of living organisms known as gene that essentially comprises of DNA and RNA. The phosphates in the bound forms as reducing powers are also the only energy currency to the cells in the form of ADP/ATP, that helps in production of metabolic energy and there by sustaining various coupled and biochemical processes in the cells. Belonging to the group V of elements, Phosphorus with its unique capacities of delivering and storing energy in pyrophosphates bonds is irreplaceable. The P acts as a limiting nutrient in agricultural and aquatic processes, and is thus indispensable as a source of food source and essential nutrients depended mostly on mineral inputs as phosphatic fertilisers. ~85 % of the mineral phosphates mined from various regions across the globe have been used for manufacturing fertiliser. At such unprecedented rate of mining P for meeting the global food demands and ensuring the food security in future, the natural lithological/terrestrial P pools in the system is diminishing at an alarming rate. The fact that P resources are non renewable and the world ‘P’ reserves are scant, it becomes highly imperative to identify potential P pools in nature and use sustainability concepts to pool back P reuse and recycle from the P enriched sub-systems. The P distribution in nature in unlike other essential nutrients as N and C, where the P is mostly in mineral origin, where as the major nutrient pools for N and C are the atmosphere. This makes P very unique and critical in terms of limitation in availability and as rare sources. Globally ~26-34 % i.e. 11-15 % of P by weight are found in P rock minerals (Steen, 1998)where P2O5 content is ~31 %, which means ~ 13.5 % P on a weight basis (Kratz et al, 2007). The global mining of P has been reported to be at a rate of ~160 million tonnes/annum and the total P deposits in these areas are ~16 billion tonnes (USGS, 2010) which is going to last for another 120 years at the present rate of exploitation and has been well documented and predicted by a number of scientific studies (Wagner, 2005; Rosmarin, 2004). During the mining process, numerous environmental externalities are witnessed a) with large open pit mines, continuous operations results in huge dust emissions and the generation a large quantities of mining wastes and ore tailings; b) during the production of H3PO4 from the P rich rocks, extensive acidification through sulphuric acid is undergone that produces voluminous phosphogypsum (5 ton/ton of phosphates) which is often disposed into large water systems as sea and oceans; c) the by-products produced during the mining and processing operation have squat utility due to the presence of hazardous substances as heavy metals like Cd and other naturally occurring radioactive elements as Ur and Th (Villabla et al., 2008).
During mineral processing phosphoric acid is formed by treating phosphate ore (apatite) with sulfuric acid that produces phosphogypsum a by-product
Ca5(PO4)3X + 5 H2SO4 + 10 H2O → 3 H3PO4 + 5 CaSO4.2 H2O + HX
where X includes OH, F, Cl, or Br
The mining and extraction of P are being practised only at a few locations that are known to be the global reserves of P i.e. China, Morocco, parts of Western Sahara, South Africa, Russia and the U.S. The major producers being China, U.S, Morocco and Russia (USGS, 2010). The geographical distribution of P reserves is highly uneven like oil wells and can be the reasons for instabilities across the major economies of the world, where western European nations and countries like India have to incur huge costs on import of P, having literally no domestic P generation. With the present extent of mining, as we go deeper into the lithological strata’s the phosphate ore quality drastically deteriorates, evident from an increase in Cd and Ur, that are highly hazardous and practically impossible to separate from these P rich minerals (Kratz & Schnugg, 2006).
Industrial processing without proper removal of these heavy metals from these minerals will result in extensive deposition of these hazardous elements in agricultural and farm lands. It has been observed that the organic matter content of the soil (fertility) has been rapidly declining with the natural denudation and erosion process coupled with anthropogenic soil utilisation. This has led to a very poor nutritional status of the soil witnessed mostly in the developing nations. In order to achieve higher food productivities and ensure global food security in future for a better quality of life and higher standard of living, a high demand for these rock based fertilisers are essential. Moreover to achieve this there is a shift from agrarian food culture to a meat and dairy based diet pattern that increase the present load on fertilisers to several folds. Reports suggest an annual growth rate of ~1 % until 2030 that would lead to >25 % more rock phosphate utilisation compared to present usage (FAO, 2000) that increases further load on energy (Fig. 6.6 and 6.7; Table 6.1).
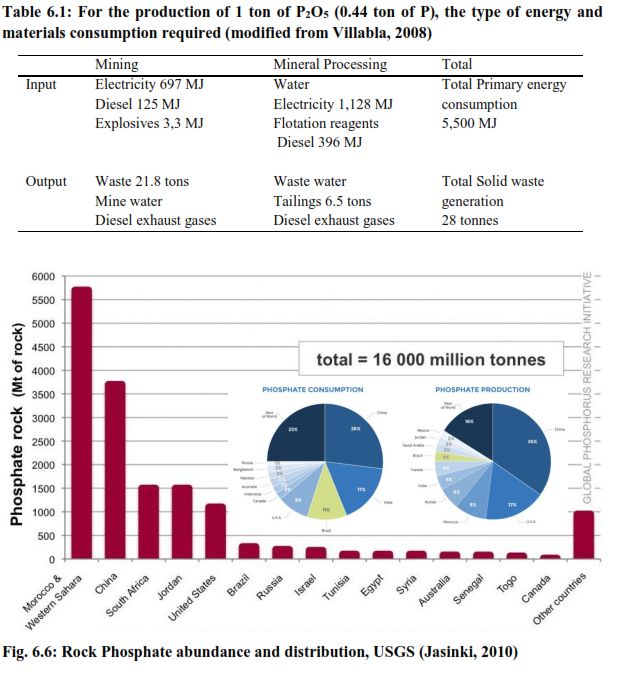
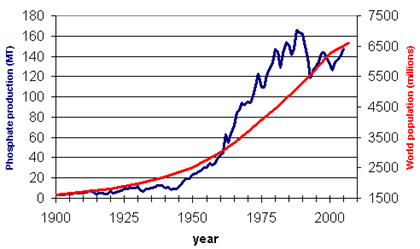
Fig. 6.7: Increase in P production with increased population growth, USGS, 2010
As apparent from the above curves, the population and the increasing demand for P goes hand in hand. However when it comes to the utilities if these P based resources, these systems are rather very inefficient. According to studies conducted on P budgeting and balance across various sub-systems (Bacinni and Brunner, 1991) only 10 % of P that is intended to be used for agriculture goes into food, there by incurring huge losses into the pedosphere and the hydrosphere, from where mining back P becomes complicated and difficult. Thus efficient P management in these spheres becomes utmost important to conserve the present day nutrient pools.
One of the major sources of P in wastewaters are the human excrements, household detergents and ‘P’ from other commercial and industrial sources. Urban runoff contributes to a very small amount of P loads. The P inputs from both the vegetative sources and the animal sources in our food both accounts to each ~ 0.8 – 1.2 g, while the P contributed by the detergents in around 0.2 g per day per person. An average Indian household generated ~ 1.8 – 2 g of P per capita per day, where bulk of the P in these waste are present in urine ~0.8 – 1.2 g; feces ~0.4 – 0.6 g and others utilities ~0.3 grams.
P role in surface waters: P routes into surface water bodies from various non-point sources as agricultural runoff and from point sources like municipal and industrial wastewater discharges. In Indian due to lack of norms / standards in P levels in household detergents used in laundry and dishwashers, these detergents contain bulk of phosphates as builders and interestingly ~50 % of the labile P (inorganic and soluble forms) present in municipal wastewaters are from these sources alone (Fig. 6.8).
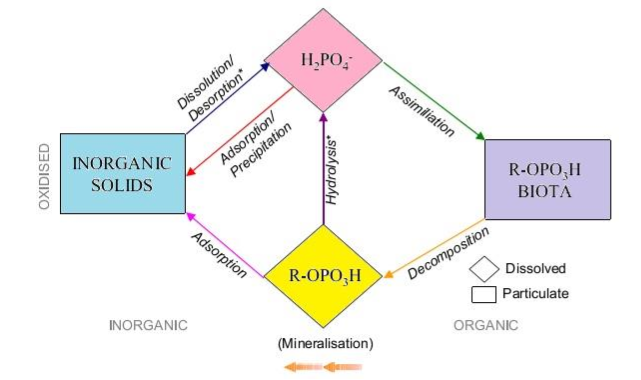
Fig. 6.8: P biochemical Cycle (terrestrial and aquatic environments)
N and P as very basic nutrients trigger aquatic plant and algal growth in surface waters and increase the primary productivity of these waters. A combination of organic nutrients with various ionic radicles and metals act as important metabolic precursors for bio-synthesis of vital energy rich molecules as fats and proteins required for the growth and development. Phosphorus is usually known as limiting nutrients that restricts the growth, and is the most crucial element that addresses nutrient enrichment in surface waters. Bulk of the ‘P’ in aquatic systems are in the form of Organic P (70 %) mostly found in the living and dead biomass and the rest comprise of the soluble and the particulate P.
Internal P cycling: In most of the lakes or surface waters, the sediments play a vital role in flux regulations and role in soluble P in waters. Annually large quantities of P are deposited in the bottom sediment owing to various physical and chemical processes; at the same time there is an ample remobilisation/resuspension of P from these sediments under certain conditions that maintains a minimum level of soluble P in these aquatic environments. Various factors affect the P exchanges across the sediment-water interface. Some of these important factors are dissolved oxygen levels, nature of the redox environment, pH, complexometic reactions, precipitations and activities of sediment microflora.
It has been observed that P concentrations in these sediments are much higher compared to those present in overlying waters. The mechanism of resuspension of P is directly linked with the dissolved oxygen concentration in these environments, where DO levels below 2 mg/l helps in the release of soluble P from these sediment pools. Under anaerobic conditions this phenomena increases manifolds. But, the P release flux is dependent on various factors as the surface properties of the particles/minerals in the sediments, acidic or basic environments, their abilities of adsorption and desorption, the oxidising/reducing nature of the overlying waters, the nature of organic C and the various biotic components in these systems.
P chemistry in very interesting with its unique abilities of forming bonds with various metal oxides, as Fe, Mn, Zn and Cu. The affinity of P to these metal oxides is governed by the prevailing redox environment at the interface between the sediment and the water layers. In aerated water systems, P readily binds with Fe oxides. However, in contrasting conditions under anaerobic environment, the Fe with the highest oxidation state i.e. +3 gets reduced to +2 ferrous, thereby releasing the soluble P into the overlaying waters. This is one of the major mechanisms of re-suspension of P from the bottom sediments under low redox conditions with high residence time.
Studies conducted in the major lakes of Bangalore city showed 11-37 mg/l TP; 1.8-16 mg/l OP; 2-4 g/kg TP in sediments; 1.2-2.8 % TP in sewage sludge; 84-111 mg/l in interstitial waters. The total P influx into Bellandur and Varthur lake systems was computed to be around ~ 18 – 10 tonnes/day. Of which ~45 % of P is trapped in the sediment pool.
Changing tropic conditions in lakes due to P accumulation: Based on the P concentrations and the prevailing trophic conditions the lake can be divided into five different categories as per OECD norms for trophic classification (Table 6.2)
Table 6.2: Trophic status of surface waters with P concentrations
Class |
Trophic Level |
Total P (µg/L) |
1 |
Oligotrophy |
<10 |
2 |
Oligo-mesotrophy |
<20 |
3 |
Mesotrophy |
<50 |
4 |
Eutrophy |
<100 |
5 |
Hypertrophy |
>100 |
The primary productivity of the surface waters is mainly dependent on the external nutrient loads. However the trophic status of the water in also to a larger extent dependent upon the internal ‘P’ loading for those water bodies that have history of nutrient enrichment. However to curtail appearance of thick algal blooms, the first step is to cut short the external loadings. However the trophic conditions in an aquatic system is influenced by the bathymetry, morphometry, mixing, flushing rates, the nature and the type of the catchment as well as the sediments, the trophic status and the history of nutrient loads.
Detergents as one of the major reasons for P enrichment in urban conglomerates: Soaps and detergents primarily comprise of surface active groups generally called as surfactants made of chemicals that aids in cleaning dirt. Soaps when used with hard water, have low cleansing action due to the presence of minerals. Thus builders that help in improving the efficiency of these surfactants have become major ingredients in soaps and detergents. These builders enhance the surface activity by counteracting minerals responsible for hardness in water, oil and grease emulsification, soil particle/dirt re-suspension and avoiding deposition. Phosphates have been by far the most extensively used builders in detergents, primarily acting on as water softener and agent for suspending dirt in aqueous systems. And has been also responsible for nutrient enrichment in surface waters (Feisthauer et al., 2004).
A looks at the utilities of detergents in India, shows tremendous use of these detergents, but facilities for recovery of detergent constituents and treatment being extremely scarce. Like other nations, India realises the implication of detergents as a potential chemical pollutant on the surface and various receiving waters through the Environment protection law (1989). Even, then these are being used relentlessly even today. Reports suggest a 1.5 fold decadal growth in the use of detergents (2.8 kg/cap/annum, 1994; >4 kg/cap/annum, 2005). Moreover, there is a high utility of the detergent bars, with annual growth of ~8 %, where ~35% of the detergents comprise of Sodium tri-polyphosphate (STPP). Such numbers highlights grave concerns on the present day deposition of such huge amount of P in aquatic systems. Most laundry detergents in India are phosphate based, as there are no norms, control or regulation of phosphates use resulting in deterioration of receiving waters.
Today, the Nations action plan towards the control of nutrient enrichment in very meagre, through the establishment of treatment plants that only treats water up to secondary levels, and the issue remains largely ignored. Though our constitution has several laws to prohibit the usage of phosphates in the day to day chemicals like the Environment Protection law (1989) and the Hazardous Waste Rules (1998) that clearly categorises the major forms of P as phosphine and phosphates as toxic chemicals. However these rules are not yet applied in the manufacturing of the household laundry soaps and detergents. Though the Bureau of Indian Standards (BIS) have set up certain grades/standards for eco-labelling with the help of Ecomark, way back in 1991. These eco-labelling indicated the brands that had surfactants that are biodegradable and packaging in recyclable and biodegradable materials.
For Indian systems where there are no norms for the use/disposal of P linked commodities, separate legislation is required to limit the P content in detergents or potential substitution of P in detergents by Zeolite are required. Lessons from European nations, the US and Canada can be take, that have attempted a ban in the sale of P-rich detergents until 2010. And have devised several strategies to minimise runoff and P input into aquatic systems.The detergents may vary depending upon utilities for example laundry detergents used in washing clothes (hand/washing machine); fabric conditioners; dishwash detergents and liquids. Generally these detergents include a set of basic compounds as the builders, the surfactants and the stain removal agents.
The builders firstly helps in providing a platforms for the water softeners imparting best water interfaces for the operation of surfactants, mostly by deactivating the freely wandering minerals in hard waters, that restricts the action of surfactants. The surfactants help in separation of phases by solubilising the dirt, by getting attached with it that renders them for mixing in water phase. These can be of various categories i.e. cationic, anionic, neutral/amphoteric. In Indian markets there is a widespread use of these anionic based surfactants in the household detergents which mostly comprise of linear alkyl benzene sulphonate (LAS) and Do-decyl benzene sulphonate (DBS). The stain removers act as very crucial agents comprising of bleaching agents and enzymes that elp in the rapid degradation/oxidation of the dirt/coloured/sticky materials ultimately removing the colour or the stain from the fabric.
Other than this various other components are used in detergent that comprise of fragrance imparting agents, enzyme activators, bleach activators, fabric conditioners, alkali etc. Builders are one of the key components of detergents, that are needed firstly for reducing water hardness that helps in enhancing surfactant efficiency by catching ca and Mg ions and encrust the surface of fabrics, secondly, these builders stabilise excessive pH conditions that are required for dirt/soil removal, thirdly they help in improving the overall solubilisation of the various components in the detergents, moreover the dirt in the fabric gets dispersed and mover out into the solution. Most importantly the builders offer a platform or skeleton for holding together the powder grains in the detergent. Present day builders are mostly made up of STPP that are environmentally harmful as they cause nutrient enrichment. Possible substitutes to these builders can be zeolite (Zeolite A) and combination of polycarboxylic acid and sodium carbonate. Zeolites are non hazardous as these are made up of alumino-silicates. Citrates can also be used as potential builders, but the cost for synthesis is pretty much high. The various builders that are used presently and can be potentially used with their possible impacts on environment are provided in Table 6.3.
There can be a lot of variations in the components of these detergents and differs across brands. While the conventional powders have similar constituents the advanced/concentrated/compact detergent powders may vary (Table 6.3). The STPP based conventional detergent powders generally have 15-30 % STPP with <5% PCA, whereas the advanced concentrated detergent forms can have many combinations of STPP i.e. 5-15 % or > 30 with 5 % PCA or 30 % STPP, with carbonates and silicates (~10 %). However in case of Zeolite based conventional powders 15-30 % Zeolites with < 5% PCA is used compared to concentrated detergents where roughly similar proportion of Zeolite i.e.e 15-30 5 is used with addition of Percarbonates (15-30 %). A comparison of the difference in various constituents in detergents conventional and advanced is provided in the Table 6.4.
Table 6.3: Available builders and alternatives to STPP for detergents
Sl No. |
Builder components |
Org/Inorg |
Abbreviation |
Actions and Impacts |
1 |
Sodium tripolyphosphate |
Inorg. |
STPP |
Contains 25% P, main cause of eutrophication
in rivers, lakes and coastal waters |
2 |
Zeolites
(A, P, X, AX) |
Inorg. |
|
No environment effect.
Increases sludge quantity.
Co-built with other additives, especially PCAs. |
3 |
Polycarboxylic acids |
Org. |
PCAs |
Poorly-biodegradable, adsorb to sludge.
Fate in environment – limited studies and yet to be realised; potentially used with zeolites. |
4 |
Citrates |
Org. |
|
Act as a potential chelator, more effective on Mg than Ca ions, contributes substantial BOD load at
wastewater treatment works.
Can be used especially for liquid detergents |
5 |
Nitrilotriacetic acid |
Org. |
NTA |
Increased dissolved heavy metals - Rapidly solubilises heavy metals through chelation. Is banned in EU |
6 |
Carbonates |
Inorg. |
CO32- |
Aids in water softening by precipitating free Ca ions; in hances and stabilises alkalinity |
7 |
Silicates |
Inorg. |
SiOX |
Avoids corrosion – supplying oxygen and increases alkalinity |
8 |
Phosphonates |
Org. P |
C-PO(OH)2 /C-PO(OR)2 R-alkyl, aryl |
Poorly biodegradable, metal ion chelator,
anti-redeposition agent |
9 |
Soap |
Org. salts |
RCOO-X
X-Na/K |
Inhibiting excess foaming in mechanically driven system |
10 |
Ethylenediaminotetracetic
acid |
Org. |
EDTA |
Poorly degradable. Dissolves metal ions |
11 |
Carboxymethyloxysuccinate
Carboxymethyltartronate |
Org. |
CMOS
CMT |
Weak chelator also observed with STPP.
Poor biodegradation, not trapped in primary solids;
not generally used in EU. |
12 |
Carboxymethyl cellulose |
Org. |
CMC |
Does not allow re-deposition, helps in repulsion of soil/dirt particles from fabric |
Table 6.4: Constituents of detergents conventional and compact (advanced)
Constituents (%) |
Detergents
(Conventional) |
Detergents
(Advanced) |
P rich |
P free |
P rich |
P free |
Sodium tripolyphosphate (STPP) |
20-25 |
0 |
50 |
0 |
Zeolite |
0 |
25 |
0 |
20-30 |
Polycarboxylates (PCAS) |
0 |
4 |
0 |
5 |
Organic phosphonates |
0 to 0.2 |
0.4 |
0 |
0.2 |
Sodium silicate |
6 |
4 |
5 |
4 |
Sodium carbonate |
5 |
15 |
4 |
15-20 |
Surfactants |
12 |
15 |
14 |
15 |
Sodium perborate** |
14 |
18 |
10 |
13 |
Activator |
0 to 2 |
2.5 |
3 |
5 |
Sodium sulphate |
1 to 24 |
9 |
4 |
5 |
Enzymes |
1 |
0.5 |
0.8 |
0.8 |
Anti-redeposition agents |
0.2 |
1 |
1 |
1 |
Optical brightening agents |
0.2 |
0.2 |
0.3 |
0.3 |
Perfume |
10 |
0.2 |
0.2 |
0.2 |
Water |
-- |
5 |
8 |
5 |
**monohydrated perborate is used in advanced detergents as high impact bleach
**tetrahydrated perborate used in conventional detergents
Compared to STPP based detergent systems, the Zeolite A based systems are environmentally friendly and does not fertilise aquatic resources. Zeolite A is inert and insoluble alumino-silicate, and can only contribute to high total suspended solids (TSS) and would lead to high quantity of sludge generation. If all the household detergent systems are substituted by Zeolite based systems, the mix of Zeolite and PCA can constitute upto 10 % of the dry solids in the sludge. The only concern for Zeolite based systems i.e. Zeolite A is its little affinity for heavy metals, however no evidences have been discovered yet. Zeolites have been known ti improve sludge settleability. In case of high heavy metal concentrations in the sludge/sediments, the hydrolysis of Zeolite A can potentially re-relaese these metals in soluble forms to the overlying waters. Similarly the PCA’a comprise of synthetic polymers, whose biodegradability in very low (~20 %) [Morse et al., 1994]. PCA’s are mostly captured in sludge/sediments with having no impact on the environment, and the detection of these compounds in the effluents is difficult as are a mixture of compounds.
Set of Solutions and Recommendations for avoiding P influx into aquatic systems: Rapid deterioration of aquatic systems due to environmental impacts of P presses the need for implementing various measures/control strategies and restrictions on the use of possible P sources as household detergents to bring down P loads into surface waters. The last two decades have witnesses a global consensus on the impacts of P on fast declining freshwaters reserves on earth. As an effort of resurrection and checking the environmental implications several nations have implemented schemes and legislations to avoid P based ingredients in detergent commodities. The growing consensus across nations and increasing studies on P based pollution in aquatic systems suggests a reduction by 80-90% to restore the trophic status in many of the aquatic systems. A forbid of utilities of ‘P’ based detergents can bring down the 40% of the P loads in aquatic systems, that would contribute significantly as attempts to safeguard our future water resources. Furthermore, improved wastewater treatment facilities with effective N capture mechanisms as Algal modules further aid in another 30 % restrictions of P influx into aquatic systems. In many of the countries a stringent law on restrictions on use of P in detergents and efficient wastewater treatment facilities has already resulted in the improved surface waters. In this regards identifying a suitable alternative to P based ingredients in detergents i.e. builders is essential. Zeolite A-PCA; Sodium citrate, ethylenediaminotetraacetic acid (EDTA) and Nitrilotriacetic acid (NTA) are some of the possible alternatives for substituting phosphorus completely from detergents. Sodium citrate is expensive and are ineffective in removal of hardness in water primarily caused by abundance if Ca and Mg cations. As builders EDTA and NTA have reduced efficiency in dispersion of particulates compared to P based detergents as STPP. In addition to this NTA have abilities to bind to cancer causing heavy metals in sewage sludge and enhance the mobility of these harzardous trace elements.
However detailed studies on its impacts in environments, economy and feasibility as a potential substitute to P based detergents have to be undertaken. Many of the European nations and US have completely substituted STPP by Zoelite and this intervention has rendered improved water quality in many of the freshwater systems in Europe and US. Taking lessons from the above mentioned success stories, the developing nations as India must also strictly restrict the use of P in detergents and parallely plan for economic and efficient nutrient removal systems during wastewater treatment to curtail any further P enrichment and resulting environmental degradation. Zeolite A (aluminium silicates) has been proved by far as the most acceptable and safe alternative to STPP, being inert, non toxic in aquatic systems. Many developed economies as US, Germany, Switzerland and other European nations have extensively adopted zeolite A as environmentally friendly substitute for STPP. Based on the studies of preponderance of phosphates in domestic wastewater, surface waters and sludge/sediments and the increasing enrichments of these urban surface waters with large quantum of nutrient loads from untreated wastewaters comprising of P inputs from detergents and human excrements, the following actions needs to be implemented
- Implementation of strict and mandatory rules and legislations to regulate/remove ‘P’ based ingredients in household laundry detergents. As almost all detergents brands available in market invariably constitutes bulk of ‘P’ based ingredients due lack of sufficient rules and laws,
- Identification of P detergent manufacturing units and inventorisation of phosphates based products in these units. Together with this a national accounting of total P imports, distribution, manufacturing into various end products and disposal of these commodities encompassing all sectors has to be prepared.
- More research and development on fate of P based ingredients in aquatic systems, from various sectors (Agricultural, Municipal etc.) has to be undertaken.
- Incorporating mandates for nutrient (N and P) removal and recovery to the existing wastewater treatment systems that only focuses on BOD/COD and TSS removal as a criterion for disposal of water into streams and other surface water bodies.
- Seeking participation from the local communities in surface and ground water management and strictly applying the polluters pay principle to the rapidly declining surface waters would ensure conservation and protection of the fresh water resources.
There are a number of cases where zeolite has been applied in river and lake systems and there have been significant improvements in the water quality. Tables 6.5 and 6.6 provide a comparative account on a case by case basis in improvement of water quality.
P enrichment in River - CASES |
Initiation
(History) |
Actions taken
/implemented |
Reduction of
P inputs achieved |
Effect on quality/
improvements |
Belgium – Wallonia
Meuse and Schelt rivers |
STPP based detergents
Poor standard of sewage treatment |
Change to Zeolite based detergents
Improvements in sewage treatment |
Not quantified |
Partial improvement |
France - Seine and Loire rivers |
STPP based detergents
Sewage treatment does not remove P
Intensive agriculture locally |
Partial change to Zeolite based detergents
Improvements in sewage treatment |
~50% for the Seine
Marginal for the Loire |
Partial improvement |
Germany - Rhine river |
STPP based detergents
Sewage treatment does not remove P |
Change to Zeolite based detergents Complete implementation of the
UWWT directive including P removal |
55-60% |
Partial improvement |
Hungary - Danube & Black Sea |
Mainly STPP based detergents
Poor standard of sewage collection& treatment |
Unknown |
Unknown |
Unknown |
Italy - Po river and N. Adriatic |
STPP based detergents
Sewage treatment does not remove P |
Change to Zeolite based detergents Improvements in sewage treatment |
30-40% |
Partial improvement in quality of the N. Adriatic |
Netherlands |
STPP based detergents
Sewage treatment does not remove P Intensive agriculture |
Change to Zeolite based detergents
Sewage treatment removes P
Measures to control agricultural P sources |
50% |
10% reduction in Chlorophyll a |
Table 6.5: Cases of improvement of river systems with Zeolite application
P enrichment in
Lakes - CASES |
Initiation
(History) |
Actions taken
/implemented |
Reduction of
P inputs achieved |
Effect on quality/
improvements |
France - Lac du Bourget |
Catchment runoff, detergents |
Regulations on use of detergents |
70% |
Eutrophic to meso/eutrophic.
Still in transition |
Germany - Lake Haussee |
Detergents |
Ring sewer. No domestic sewage
input |
90 % |
Eventual recovery of the
lake, >5 years after reducing
P inputs |
Italy - lago d’Iseo |
STPP based detergents
Sewage treatment does not remove P |
Change to Zeolite based detergents
P removal at main STW and diversion of some flow |
60% |
Lake still in transition from
eutrophic condition |
Italy - lago Endine |
Mainly STPP based detergents
Poor standard of sewage collection& treatment |
Change to Zeolite based detergents
Ring sewer |
80% |
Lake still in transition from
eutrophic to oligotrophic
conditio |
Switzerland - lake Geneva |
STPP based detergents
Sewage treatment does not remove P |
Change to Zeolite based detergents
sewage treatment works remove P |
60% |
Significant improvement |
USA - lake Erie |
STPP based detergents
Sewage treatment does not remove P |
Change to Zeolite based detergents
Major sewage treatment works
Remove P |
85% from municipal
wastewater, 50% overall |
Significant improvement,
recovery not complete |
Table 6.6: Cases of improvement of lake systems with Zeolite application
Source:
Ramachandra T V, Durga Madhab Mahapatra, Asulabha K S, Sincy Varghese, 2017. Foaming or Algal Bloom in Water bodies of India: Remedial Measures - Restrict Phosphate (P) based Detergents, ENVIS Technical Report 108, Environmental Information System, CES, Indian Institute of Science, Bangalore 560012
Ramachandra T V, Asulabha K S, Sincy V, Sudarshan Bhat and Bharath H.Aithal, 2015. Wetlands: Treasure of Bangalore, ENVIS Technical Report 101, Energy & Wetlands Research Group, CES, IISc, Bangalore, India
Ramachandra T V, Asulabha K S, Sincy V, Vinay S, Bharath H.Aithal, Sudarshan P. Bhat, and Durga M. Mahapatra, 2015.Pathetic status of wetlands in Bangalore: Epitome of inefficient and uncoordinated Governance, ENVIS Technical Report 93, CES, Indian Institute of Science, Bangalore 560012









