ENVIS Technical Report: 89, July 2015 |
|
|
 |
Biodiversity, Ecology, Energy, Landscape Dynamics & Hydrology of Agastya Foundation Campus, Kuppam |
|
| Ramachandra T. V.* | Harish R.Bhat | Bharath H. Aithal | Rao G. R. | Sudarshan P.Bhat |
| Vinay S. | Ganesh Hegde | Gouri Kulkarni | Vishnu D. Mukri |
*Corresponding author: cestvr@ces.iisc.ac.in, energy@ces.iisc.ac.in [080-22933099]
SUMMARY Wetlands are vital productive freshwater resources on the Earth. They help in maintaining the ecological balance of the region. They have numerous valuable functions such as recycling of nutrients, purify water, attenuate floods, maintain stream flow, maintaining microclimate of the region, recharge ground water and also serve in providing drinking water source, fish, fodder, fuel, recreation to the society The stability of wetlands depends upon the balance between production and consumption of energy and matter at different trophic levels present in the system. The present study was done to understand the status of the lake ecosystems, which are located around Agastya Foundation campus at Kuppam. The physico-chemical and biological composition of the lakes were studied using standard protocols. The water quality of the studied lakes was found to be good with less nutrient content and organic matter. Presence of filamentous algal species and floating and submerged macrophytes species further confirms of a healthy ecosystem.
Wetlands (and lakes) constitute the most productive ecosystems with a wide array of goods and services. These ecosystems serve as life support systems; serve as habitat for a variety of organisms including migratory birds for food and shelter. They aid in bioremediation and hence aptly known as ‘kidneys of the landscape’. Major services include flood control, wastewater treatment, arresting sediment load, drinking water, protein production, and more importantly recharging of aquifers apart from aiding as sinks and climate stabilizers. The wetlands provide a low cost way to treat the community’s wastewater, while simultaneously functioning as wild fauna sanctuary, with public access. These ecosystems are valuable for education and scientific endeavours due to rich biodiversity. Wetlands help in maintaining the ecological balance of the region. Wetlands, natural and manmade, freshwater or brackish, provide numerous ecological services. They provide habitat to aquatic flora and fauna, as well as numerous species of birds, including migratory species. The density of birds, in particular, is an indication of the ecological health of a particular wetland. Wetlands also provide freshwater for agriculture, animal husbandry, and domestic use, drainage services, and provide livelihoods to fisher-folk. Larger wetlands may also comprise an important resource for sustainable tourism and recreation. The stability of wetlands depends upon the balance between production and consumption of energy and matter at different trophic levels present in the system. The trophic structure includes various trophic levels as producers (algae, bacteria), primary consumers (zooplanktons and grazers), secondary consumers (small fish), tertiary (large fish, birds, etc.). Algae being the primary producers, synthesize carbohydrates during photosynthesis and releases oxygen as a major end product. The CO2 released in large amounts gets transformed into algal biomass. This reduces GHG (Greenhouse gases) in the environment and thus, helps to combat global warming. Thus, the trophic structure and material exchange in wetlands plays a major role (Ramachandra et al., 2014). The interaction of man with wetlands during the last few decades has been a concern largely due to the rapid population growth accompanied by intensified industrial, commercial and residential development, further leading to pollution of wetlands by domestic, industrial wastewaters and agricultural runoffs as fertilizers, insecticides, and feedlot wastes (Kiran and Ramachandra, 1999). The dumping of solid waste in the catchment of lakes and or sustained inflow of partially or untreated wastewater leads to enrichment of nutrients, leading to adverse health impacts and reduction in the ecological and environmental services. The study was done to understand the status of lakes, which are located in and around Agastya Foundation campus at Kuppam. OBJECTIVE: Objective of the current investigation is to assess the physico-chemical and biological composition of lakes within 2 km boundary of Agastya Foundation campus at Kuppam. MATERIAL AND METHODS Study area: Agastya Foundation campus is situated at Gudivankaat the intersection of three rapidly developing Southern states, Andhra Pradesh, Karnataka and Tamil Nadu.The present study was carried out at 4 lakes (Neradi, Chinnakere, Checkdam and Atinattam) which are situated in the buffer zone within 2 km from boundary of Agastya Foundation (Figure 1 and 2). Analysis of physico-chemical parameters: The water temperature, pH, electrical conductivity (EC) and dissolved oxygen (DO) were determined on spot at the time of sampling. Other parameters like nitrate, phosphate, total alkalinity, calcium hardness, total hardness, chlorides, COD (Chemical oxygen demand), sodium and potassium were analysed in the laboratory by using standard methods prescribed by Trivedi and Goel (1986) and APHA (1998)(Table1). Macrophyte collection and identification: Macrophyte samples were collected and washed to get rid of adhering materials. They were identified using Cook CDK (1996).
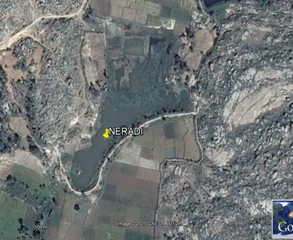
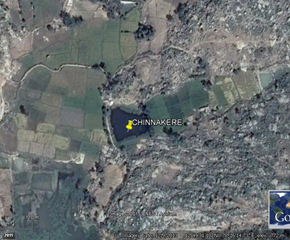
Figure 1: Google map showing the study area with studied lakes
Figure 2: Studied lakes
Table 2: Onsite Parameters
Table 3: Offsite Parameters
RESULTS AND DISCUSSION
Temperature: It is an important factor for aquatic life as it regulates the maximum dissolved oxygen concentration of the water, controls the rate of metabolic activities, reproductive activities and therefore, life cycles. The temperature of studied lakes ranges between 23.50C (Neradi) to 25.50C (Attinattam).
Dissolved Oxygen: Dissolved oxygen (DO) is the most essential feature in aquatic system that helps in aquatic respiration as well as detoxification of complex organic and inorganic mater through oxidation. The presence of organic wastes imposes a very high oxygen demand on the receiving water leading to oxygen depletion with severe impacts on the water ecosystem. The effluents also constitute heavy metals, organic toxins, oils, volatile organics, nutrients and solids. The DO of the analysed water samples varied between 5.48 to 9.84mg/l. As per IS 2296 the minimum DO for irrigation, fish culture etc. is 4 mg/l and for outdoor bathing 5 mg/l. The analysed water samples had DO higher than 5 mg/l and suitable for outdoor bathing and irrigation. Electrical Conductivity (EC): EC is the measure of the ability of an aqueous solution to convey an electric current. This ability depends upon the presence of ions, their total concentration, mobility, valence and temperature. The EC varied from 150 to 426 μS. pH: pH is a numerical expression that indicates the degree to which water is acidic or alkaline, with the lower pH value tends to make water corrosive and higher pH provides taste complaint and negative impact on skin and eyes. The pH value ranged from 7.2 to 7.6. The desirable range for pH as per IS 2296 is 6.5-8.5. The studied lakes found to be within this range. Turbidity: Clarity of water is a very important aspect for human consumption. Turbidity in water is caused by suspended and colloidal matter such as clay, silt, finely divided organic and inorganic matter, plankton and other microscopic organisms, waste discharge and sediments from erosion. The maximum permissible limit of turbidity as per BIS and World Health Organization (WHO) is 5 NTU. Turbidity values ranged from 5 to 36 NTU in most of the samples. The maximum turbidity of 36 NTU was in check dam due to presence of algae and dissolved silt matter. Total Dissolved Solids (TDS): TDS affect the water quality in myriad of ways impacting the domestic water usage for cleaning, bathing etc. as well as drinking purposes. Total dissolved solids originate from organic sources such as leaves, silt, plankton, industrial waste and sewage. Other sources come from runoff from urban areas, road salts used on street, fertilizers and pesticides used on lawns and farms [APHA, 1995]. Surface as well as groundwater with high dissolved solids are of inferior flavor and induce an unfavorable physiological reaction to the dependent population. A limit of 500 ppm TDS is desirable for drinking waters. The TDS values in the samples analysed, ranged from 66 to 217ppm across all locations. This shows that there are less contamination in these lakes. Chlorides: Chlorides are essentially potential anionic radicals that imparts chlorosity to the water. An excess of chlorides leads to the formation of potentially carcinogenic and chloro-organic compounds like chloroform, etc. Excess of chloride in inland water is usually taken as index of pollution. Chloride values in samples ranged from 17 to 79ppm. The desirable range for chlorides as per IS 2296 is 250 mg/l. In addition to the adverse taste effects, high chloride concentration levels in the water contribute to deterioration of domestic plumbing, water heaters and municipal water works equipment. Sodium: Sodium (Na) is one of the essential cations that stimulate various physiological processes and functioning of nervous system, excretory system and membrane transport in animals and humans. Increase of sodium ions has a negative impact on blood circulation, nervous coordination, thence affecting the hygiene and health of the nearby localities. According to WHO guidelines the maximum admissible limit is 200 ppm. In this study the concentration of sodium ranged from 36- 73ppm. Potassium: Potassium (K) is an essential element for both plant and animal nutrition, and occurs in ground waters as a result of mineral dissolution, decomposing of plant materials and also from agricultural runoff. Potassium ions in the plant root systems helps in the cation exchange capacity to transfer essential cations like Ca and Mg from the soil systems into the vascular systems in the plants in replacement with the potassium ions. Incidence of higher potassium levels in soil system affects the solute transfer (active and passive) through the vascular conducting elements to the different parts of the plants. The potassium content in the water samples ranges between 0-6ppm. Alkalinity: Alkalinity is a measure of the buffering capacity of water contributed by the dynamic equilibrium between carbonic acid, bicarbonates and carbonates in water. Sometimes excess of hydroxyl ions, phosphate, and organic acids in water causes alkalinity. High alkalinity imparts bitter taste. The acceptable limit of alkalinity is 200 ppm. The lake water samples analysed were having lower alkalinities (120-208 mg/l) because of higher acidic environment in the soil systems. The tap water showed high alkalinity of 478 mg/l. Total hardness: Hardness is the measure of dissolved minerals that decides the utility of water for domestic purposes. Hardness is mainly due to the presence of carbonates and bicarbonates. It is also caused by variety of dissolved polyvalent metallic ions predominantly calcium and magnesium cation although, other cations like barium, iron, manganese, strontium and zinc also contribute. In the present study, the total hardness ranged between 24 to 140 mg/l.Only the tap water had highest hardness of 580mg/l. According to WHO guidelines the maximum admissible limit is 300 ppm. Calcium: Calcium (Ca) is one amongst the major macro nutrients which are needed for the growth, development and reproduction in case of both plants and animals. The presence of Ca in water is mainly due to its passage through deposits of limestone, dolomite, gypsum and other gypsi-ferous materials. Caconcentration in all samples analysed periodically ranged between 6 to 136ppm. Nutrients (nitrates and phosphates): Nutrients essentially comprise of various forms of N and P which readily dissolve in solutions that are up-taken by microbes and plant root systems in the form of inorganic mineral ions. Phosphate occurs in natural water in low quantity as many aquatic plant absorb and store phosphate many times their actual immediate needs. Accumulation of N as nitrates and P as inorganic P in aquatic ecosystems causes significant water quality problems due to higher net productivity. Together with phosphorus, nitrates in excess amounts in streams and other surface waters can accelerate aquatic plant growth causing rapid oxygen depletion or eutrophication in the water. Nitrates at high concentrations (10 mg/l or higher) in surface and groundwater used for human consumption are particularly toxic to young children affecting the oxygen carrying capacity of blood cells(RBC)causing cyanosis (methemoglobinemia). In the present study, nitrate values ranged from 0.62 to 0.73 ppm and phosphate values ranged between 0.23 to 0.32 ppm. The desirable limits as per IS 10500 for nitrates is 45 mg/l. COD and BOD: BOD and COD are important parameters that indicate the presence of organic content.
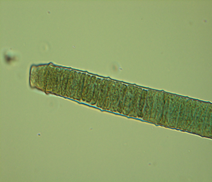
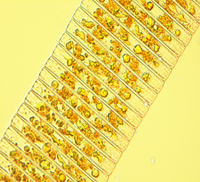
Algal species found in lakes: The dominant algal species found in the lakes were given in table. The lakes were dominated by filamentous algae like Nitella, Chara, Spirogyra, etc.
Macrophyte diversity Macrophytes, the aquatic macroscopic plants confine themselves to the shallow euphotic zone of the water bodies. In the littoral zone, macrophytes are the chief exploiters of plant nutrients from the sediments, which otherwise, are lost temporarily from the water. The nutrients so logged in the body material are released only after death, decay and subsequent mineralization, thus, they play a role in nutrient dynamics and primary productivity of shallow systems. Therefore, seasonal growth rate patterns and population dynamics of macrophytes are very important. When there is enough room for colonization and abundant availability of nutrients, macrophytes show a high growth rate. They assimilate nutrients directly into their tissues.
Figure 4: Major macrophytes found in the lakes
The physico-chemical and biological composition of the lakes were studied using standard protocols. The water quality of the studied lakes was found to be good with less nutrient content and organic matter. The lakes consisted of filamentous algal species and floating and submerged macrophytes which also indicates a healthy ecosystem.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||