Materials and Methods
Uttara Kannada, Central Western Ghats) was divided into 5' grids (Figure 3) corresponding to a grid in 1:50000 toposheets of the Survey of India for soil sampling the sampling was carried out in two phases- first during January to March 2008, and next from October 2008 to January 2009.
During the first field work, soil samples were collected from talukes like Bhatkal, Siddapur, Honnavar, Kumta, Sirsi, Ankola and small portion of Karwar. Bhatkal, Karwar, Ankola, Honnavar, Kumta are coastal talukes where, Siddapur and Sirsi is in the Ghat region. Although all these talukes come under the high rain fall region, the Coastal talukes receive more rain (>3000 mm). Soil samples were collected in 49 grids (about 50% of total grids).
During the second phase of field work, soil samples were collected from talukes like Yellapur, Mundgod, Haliyal and Joida and some parts of Sirsi and Karwar talukes. During the second phase, soil samples were collected from 68 grids.
Landscape elements sampled: Soil samples were collected in such a way that samples represent all major land uses (evergreen forest, deciduous forest, paddy field, Arecanut gardens and open grass land, teak plantations) in the respective grid, wherever it was possible by at least a motorable road.
Fig 4: Division of Uttara Kannada into grids (5'x5') for soil sampling
Method of sample collection: Samples were collected by inserting an augur of length 14.8 cm and diameter of 9 cm. Undisturbed core sample (approx 1000 gm) was then transferred to a 2 kg capacity air tighten polythene cover. A small portion of sample (approx 100 gm) is stored in 500 gm capacity double air tighten polythene cover to estimate soil moisture. The collected samples were kept in shade to avoid direct contact of sunlight. In the lab, soil moisture was estimated by gravimetric method. Soil samples were air-dried and sieved through 2 mm sieve.
Soil parameters studied: Soil parameters studied were bulk density, soil pH, soil moisture, soil texture, total carbon, total nitrogen, sodium, potassium, calcium and magnesium.
Methods used for soil analysis (listed in Table 1)
T able 1: Parameters and method used for the analysis
No: |
Parameter |
Method |
1 |
Bulk density |
Gravimetric method |
2 |
Soil moisture |
Gravimetric method |
3 |
pH |
Potentiometer method |
4 |
Texture |
Density method |
5 |
Total Carbon |
Elemental Analyzer method |
6 |
Total Nitrogen |
Elemental Analyzer method |
7 |
Available Phosphorus |
Colorimetric method |
8 |
Available potassium |
Flame photometry |
9 |
Soluble Sodium |
Flame photometry |
10 |
Calcium |
Complex metric titration method |
11 |
Magnesium |
Complex metric titration method |
Bulk Density
Bulk density or apparent density, is the ratio of the mass of the soil to that of its total volume, include any air space and organic materials in the soil volume (Allen, 1989). It is determined by the oven dry weight of a unit volume of soil, expressed in g/cm3, Bulk density is inversely proportional to the pore space of the soil, Soil with high bulk density are less in the pore space and hence compact in nature and soil with low bulk density are highly porous and loose in nature. Bulk density of soil generally increases with depth due to decrease in soil organic matter. Bulk density is also an indicator of aeration status of the soil.
The bulk density of soil is measured by taking an undisturbed block of soil with a cylindrical metal core. Determine the volume of the core and the weight of the soil.
Soil mass (g)
Bulk Density (g/cm3) = ---------------------------
Soil volume (cm3)
Soil moisture
Water is an essential component for the soil life forms. It is necessary for microbial activity and actions, nutrient mobility and aid as a lubricating agent for the penetration of roots. Changes in the soil water content and its energy status affects many soil mechanical properties namely strength, compactability, bulk density and root penetration. Many hydrological processes are fundamentally related to soil moisture condition.
Mass or gravimetric soil water content is expressed relative to the mass of oven dry soil. 10-20 gm of fresh sample is taken in the dry, weighed evaporating basin and the sample is dried in an oven at 105° C for24 hour (Baruah, 1997). Final weight is measured after cooling the over dry sample in a desiccator. Large stone and roots were removed without weighing to avoid bias.
(Mass of the wet soil –mass of the oven dry soil) x 100
Moisture content % = -----------------------------------------------------------
(Mass of the oven dry soil)
pH
pH is the measure of H+ ion activity of the soil water system as it indicates whether the soil is acidic neutral or alkaline in nature and is defined as the logarithm to the base 10 of the reciprocal of H+ ion concentration. Based on the pH spectrum (French pedological reference, Bertrand, 1984) soil is classified as:
Table: 2 pH spectrum of soil
pH |
Classification |
pH < 3.5 |
Hyper acid |
3.5-5 |
very acid |
5 to 6.5- |
Acid |
6.5 to 7.5 |
Neutral |
7.5 to 8.7 |
Basic |
> 8.7 |
very basic |
On the basis of the ionization theory, acids are those substances that yield hydrogen (H+) ions on dissociation. Strong acids and bases are highly ionized substances and weak acids and bases are poorly ionized substances in aqueous solutions. For the H+ ion concentration, consider the dissociation of pure water at 250c, which yield one OH- ion for each H+ ion (Tomer, 1999), Pure water which is a very weak electrolyte, which on dissociation at 250C furnishes only 10-7 mole H+ ion per litre. since water produces one OH-- ion for each H+ ion on dissociation, the concentration of [OH] ion is equal to [H+] ions concentration, i.e. 10-7 moles/L .Thus for pure water at250C,
[H+] = [OH-] = 10-7
Therefore,
K w = 10-7 × 10-7 = 10-14
Addition of acid to water ionizes and release more H+ ions into the solution, solution concentration of H+ ions becomes more than 1 × 10-7 moles/L and the solution is said to be acidic. Similarly, if a base is added to water it furnishes OH- ions on ionization, which increases the concentration of OH- ions to more than 1 × 10-7 moles/L .This solution is said to be basic or alkaline. Thus, the ionization product of water enables the solution to be classified as acidic, neutral, and alkaline by considering the H+ or OH- ion concentration.
pH + pOH = 14
On this basis, a pH scale has been developed ranging from 0 to 14. A solution having pH equal 7 is neutral, the one having a pH value < 7 is acidic, and one having pH > 7 ≤ 14 is basic in nature.
Weigh 10 g of the soil dried at room temperature and sieved to 2mm and add 20 ml DDW. The suspension is stirred at regular intervals for 30 minutes and the pH is recorded.
Soil Texture
The amount of sand, silt, and clay ultimately makes up the class of the soil. To determine the class type of an unknown soil, the ratio of sand, silt, and clay particles in a specific volume of soil is determined. Soil particles are categorized into groups according to size.

Using comparative volumes to determine the ratio of these particles, based upon the fact that different particles of different sizes will fall out of solution at different rates.
Reagent: Calgon solution (5%): Dissolve 50 g of sodium-hexameta-phosphate in distilled water and make up the volume to one litre, Hydrogen peroxide 6%.
Calgon contains a dispersing agent called sodiumhexametaphosphate. In this process, the Na+ ions replace the polyvalent cations (usually Ca++) that form interparticle linkages; once the polyvalent cations are free; they react with phosphorus and precipitate out.
Take three centrifuge tubes A, B, and C. Take Soil which is sieved through 2 mm sieve. Add 6% H2O2 to remove all the organic matter. Take the soil which is free of organic matter and put it in a 15 ml in centrifuge tube A. Add more soil, if necessary, to the 15 ml mark. Add 1 ml of the soil dispersing agent (Calgon) to the soil, and add tap water to the level of 45 ml. Place the stopper firmly in the tube. Holding the stopper, shake the tube for two to 5 minutes. Remove the stopper and place the centrifuge tube in the stand for 30 seconds. The time is critical. If you allow more than 30 seconds to pass, shake the tube again and allow the tube to stand for another 30 seconds. Pour all the solution from the centrifuge tube A into centrifuge tube B (leaving the soil particles that settled out). Gently tap tube A on the table to level the soil left in the tube and return to the stand. Allow tube B to stand undisturbed for 30 minutes. At the end of the 30 minute standing time, carefully pour the solution from centrifuge tube B into centrifuge tube C (again leaving the particles that settled). Read the volume of soil particles, as accurately form tubes A and B.
Calculations: The mineral particles in separation tube A are sand. They are the largest and heaviest particles. Therefore, they settle out first. The particles in separation tube B are silt. Since they are lighter than sand, they take longer to settle out. The particles remaining in the final tube are clay. Clay particles swell when placed in water, and they tend to remain in water. This tube is not an accurate indication of the amount of clay in the sample. The amount of clay is more accurately determined mathematically
The Percent of Sand in the soil sample:
Volume in tube A (sand) divided by 15 ml x 100 gives the % Sand.
The Percent of Silt in the soil sample:
Volume in tube B (silt) divided by 15 ml x 100 gives the % Silt.
The Percent of Clay in the soil sample:
Add the volumes of tubes A and B then subtract that answer from 15 ml (total sample). This is the volume of clay.
Volume of clay divided by 15 x 100 gives the % Clay
Total Organic Carbon in the soil
Soil organic carbon exists both with in the active soil biomass and in the soil organic matter. Soil organic carbon is considered to be an important soil component in agronomic and natural system and has a role as a carbon reservoir in the terrestrial systems.
Total Organic carbon is determined using a Leco Truspec CHN analyzer.
Principle: The sample is oxidized at a high temperature of 950°C in a furnace. Oxygen is flushed into the furnace for the complete oxidation. The product of the combustion is again passed through a secondary furnace of 850° C for further oxidation and particulate removal. The combustion gas was then collected in a vessel called Ballast. The homogeneous combusted gas in the ballast is then purged through the CO2 and H2O infra red detector and 3cc aliquot loop. Carbon is measured as CO2 by the CO2 infra red detector.
Calculations: Directly from the instrument.
Total Nitrogen in the soil:
Nitrogen is one of the major elements required for the growth of plant. It is an integral component of many compound essential for the growth of plants include chlorophyll and many enzymes. Total nitrogen is an indicator of the soil potential for the element. Plants receiving insufficient nitrogen are stunted in growth and possess poor root system.
Principle: The sample is oxidized at a high temperature of 950°C in a furnace. Oxygen is flushed into the furnace for the complete oxidation. The product of the combustion is again passed through a secondary furnace of 850°C for further oxidation and particulate removal. The combustion gas was then collected in a vessel called Ballast. The homogeneous combusted gas in the ballast is then purged through the CO2 and H2O infra red detector and 3cc aliquot loop. The gas in the aliquot loop to the Helium carrier flow is passed through a hot copper to remove O2, CO2 and H2O. A thermal conductivity cell will determine the Nitrogen content.
Calculations: Directly from the instrument.
Available Phosphorous in the soil (Bray, 1945)
Phosphorus is a second key nutrient found in the soil. The amount of P available to plants is generally not exceeded 0.01% of the total Phosphorous. The total Phosphorous in soil found between 0.02 - 0.10 % by weight. Two forms of Phosphorous are found in the soil
Organic P (20-28%) and the rest are in organic Phosphorous. There has been no report of plant absorbing organic Phosphorous either from solid or solution phase of the soil. Inorganic Phosphorous occurs as orthophosphate (H2PO4- and HPO42-) in several forms and combinations (Baruah, 1997).
Principle: The principle of this method is that the soil is mixed thoroughly with an extraction solution of 0.03 N NH4F in 0.025 N HCl. The combination of NH4F and HCL will extract easily acid soluble forms of P largely Ca- phosphates, Al- phosphates and Fe- phosphates. The NH4F dissolve Al, Fe and Mn phosphates by forming complex ion with these metal ions in the acid solution and there by release phosphate ion into the solution. In the presence of Chloromolybdic acid in an acidic medium, the phosphate ion forms a hetropoly compound of P. On reduced by SnCl2 impart molybdenum-blue color. The intensity of blue color on reduction provides a measure of the concentration of P in the test solution. This intensity can be measured at 660 nm, using the spectrophotometer (Baruah, 1997).
3NH4F + 3HF + AlPO4→ H3PO4 + (NH4)3AlF6
3NH4F+ 3HF + FePO4→ H3PO4+ (NH4)3FeF6
H3PO4 + 12H2MoO4 → H3P (Mo3O10)4 +12H2O
Reagents:
- Ammonium fluoride (NH4F), (1 N): Dissolve 37 g of NH4F in DDW and dilute the solution to 1 liter. Store the solution in polythene bottle.
- HCL, (0.5N): Dilute 20.2 ml conc. to a volume of 500 ml with DDW
- Extracting solution: Add 15 ml of 1 N NH4F and 25 ml of 0.5N HCL to 460 ml of DDW. This gives a solution composition of 0.03 N NH4F in 0.025N HCL
- Dickman and Bray's reagent: Dissolve 15 g of ammonium molybdate (AR) in 300 ml DDW, warm to about 60 o C, cool and filter add to it 342 ml conc. HCL and make up to 1 liter. This is 1.5% ammonium molydbate in HCL.
- Stannous chloride solution: Dissolve 10 g of SnCl2.2H2O (AR) crystals in 25 ml of conc. HCL and store in a brown bottle .This is 40% stannous chloride stock solution
- Stannous chloride working solution: Dilute 0.5 ml of the stock solution to 66 ml with DDW. Prepare the solution just before use.
- Preparation of standard Phosphorous solution: Dissolve 0.439 g of potassium dihydrogen phosphate (AR) in about half a litre of DDW .Add to it 25 ml of 7 N H2SO4 and make up to 1000 ml with DDW. This gives 100 ppm of stock solution of P. From this prepare a 2 ppm solution by 50 times dilution of the stock solution.
Procedure: For the extraction of P, weigh 5 g soil and transfer it to a 100 ml conical flask. Add to it 50 ml of extracting solution. Shake the content of the flask for exactly 5 minutes and filter through Whatt Mann No: 42 filter paper. A blank should be made by adding all reagents except the soil.
Colorimetric estimation of P: Take 5 ml of the soil extract as well as different concentration of P by pipetting out 0 (blank), 1, 2, 3, 4, 5, and 10 ml of 2ppm phosphate solution in 25 ml volumetric flask, add 5 ml of extracting solution of each of the P standard solution followed by the addition of 5 ml of Dickmans and Bray's reagents in all the flask. Mix the condense of the flask thoroughly with about 5 ml of DDW to remove the adhering ammonium molybdate. At last add 1 ml of working stannous chloride solution with immediate mixing and make up to the mark with DDW. Once again mix the solution thoroughly. The intensity of the blue color is measured at 660 nm just after 10 minutes and determines the concentration of P from the standard curve.
Calculation:
Available phosphorus in the soil = A x 50 x 2.24 kg/ ha
Where A = concentration of phosphorus read from the standard curve
Available Potassium in the soil
Exchangeable potassium is the major source of K to plants. The available potassium in the soil is the sum of water soluble and exchangeable potassium. The neutral ammonium acetate solution extracts both water soluble and exchangeable potassium. The term available potassium in cooperate both exchangeable and water soluble forms of nutrients present in the soil. The readily exchangeable plus water soluble potassium is determined in the neutral normal acetate extract of the soil.
Principle: The method is based on the principle of equilibrium of soils with an exchanging cation made of the solution of neutral normal ammonium acetate in a given soil: solution ratio. During the equilibrium, ammonium ions exchange with the exchangeable K ions of the soil. The K content in the equilibrium solution is estimated with a flame photometer. Since NH4+ holds highly charged layers together just as K, the release of the fixed K, in an exchangeable form, is retarded during ammonium acetate extraction (Baruah, 1997).
Reagents:
- Neutral NH4OAc solution (1N): Dissolve 77.09 g of NH4OAc in DDW and make up to 1 litre. Adjust the solution pH to 7 by adding either NH4OH or glacial acetic acid.
- Stock potassium chloride solution: Dissolve 1.098 g of AR grade potassium chloride (Dried at 60° c for I hour) and make up the volume to I L using DDW. It gives a 1000 mg/L K solution and is treated as stock solution of potassium.
- Working Potassium solution: From the stick take aliquots and dilute with ammonium acetate solution to get 10-80 ppm of potassium solution.
Procedure: Weigh 5 g of the soil in a 100 ml of conical flask. Add to it 25 ml of neutral 1 N NH4OAc solution. Shake the condense of the conical flask on a electric shaker for 5 to 10 minutes and filter through a filter paper What man no. 1. Feed the filtrate into the atomizer of the flame photo meter and not down the reading.
W
Calculation
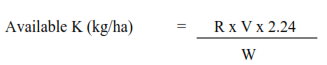
Where,
R= ppm of K in the extract
V = volume of the soil extract in ml
w = Weight of air dry samples taken for extraction in gm
Soluble Sodium in soil
Sodium (Na) can be extracted with ammonium acetate solution in the same way as K. Subsequently, Na in the extract can be determined by flame photometry.
Principle: Certain elements, including Na, have the property that, when their salts
are introduced into a flame, they emit light with a wavelength (color) specific to the element and of intensity proportional to the concentration.
This is especially true for Na emitting a sparkling yellowish-red color.
Reagents
1). Standard Stock Solution
Dry about 5 g sodium chloride (NaCl) in an oven at 110°C for 3 hours, cool it in a desiccator, and store in a tightly closed bottle.
Dissolve 2.5418 g dried sodium chloride in DI water, and bring to 1-L final volume with DI water. This solution contains 1000 ppm Na (Stock Solution). Prepare a series of Standard Solutions from the Stock Solution as follows:
Dilute 2, 4, 6, 8, 10, 15, and 20 ml Stock Solution to 100 ml final volume by adding DI water or 1 N ammonium acetate solution, Diluted Stock Solution. These solutions contain 20, 40, 60, 80,100, 150, and 200 ppm Na. Standard Solutions for measuring soluble Na should be prepared in DI water, but for measuring extractable Na the standards should be made in ammonium acetate solution.
Procedure: Operate Flame Photometer according to the instructions provided for the equipment. Run a series of suitable sodium standards. Measure Na+ in the samples, by feeding the filtrate into the flame photometer.
Calculations:
Sodium (mg/L) in soil: S/A x (Reading displayed for Na)
Where S = Sum of concentration of standard solution
A = Sum of the absorbance of the corresponding standard solution
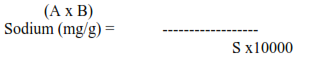
Where A = Sodium content of soil extract (mg/L)
B =Total volume of Soil extract
C = Weight of air-dry soil which is taken for the extraction
Multiply the final answer with the dilution factor.
Calcium and Magnesium in the soil
Calcium and magnesium are the two most abundance alkaline earth metals in soil. They occur as soluble ions in the soil and ions on the absorbed state (Baruah, 1997). The average calcium content of the soil is estimated to be 1.4% and it may vary from soil to soil depending upon the climate condition. The average Mg content of the soil is estimated approximately 0.5 %, where its concentration in soil water is estimated to be 10 mg/L. Quantitatively, the lowest amount of Mg occurs in podzolised and sandy soil.
Principle: The most widely used method for the determination of Ca and (Ca + Mg) is by complex metric titration, involving ethylene tetra-acetic acid (EDTA), (Schwartzenbach et at, 1946).The most widely used salt of EDTA is the disodium salt with the formula Na2H2Y.2H2O, where Y is the tetravalent anion of EDTA. When Ca+ and Mg+ are treated with H2Y-2 very stable complexes of calcium and Magnesium are formed (CaY-2 and MgY-2 respectively) in alkaline condition. For the combination determination of Ca2+ and Mg2+ Erichrome Black T (EBT) is used as an indicator. The optimum pH for the formation of Mg complex is less stable than the MgY-2 complex.
Ca 2+ is complexed before Mg2+ and the indicator changes from red to blue, when the Mg2+ end point is reached. The reaction is as follows
MgI- + H2Y-2 → MgY-2 +HIn-2 +H+
The classical indicator employed for Ca2+ titration is ammonium purpurate (murexide). Murexide, at pH 11, is purple in colour in the absence of Ca2+ ions; it forms a pink complex, which has a dissociation constant greater than the CaY-2 complex. Thus using murexide as an indicator, Ca2+ can be titrated with EDTA even in the presence of other alkaline earth ions.
H2Y-2 + Ca2+ → CaY-2 + 2H+
Reagents:
- Buffer solution: Dissolve 67.5 g of NH4Cl in 400 ml of distilled water, then add 570 ml of conc. NH4OH and dilute up to one litre.
- Standard EDTA solution: Dissolve 1.86 g of disodium salt of EDTA in distilled water and make up to 1L to get 0.01N solution.
- Eriochrome Black-T (EBT) indicator: 0.5 g of Eriochrome Black-T indicator is dissolved in 100 ml of triethanolamine.
- Murexide indicator: Mix 0.2 g of 40 g of finely grounded potassium sulphate and grind again in agate pestle and motar
- 10% NaOH solution: Dissolve 10 g of NaOH in distilled water and make up to 100 ml.
- Buffer complex solution: Mix 50 ml of each of KCN solution , hydroxylamine hydrochloride solution, potassium ferro-cyanide solution and triethanolamine with 800 ml of buffer solution (should not be kept more than a week)
- Neutral NH4O Ac solution (1N): Dissolve 77.09 g of NH4OAc in DDW and make up to 1 liter. Adjust the solution pH to 7 by adding either NH4OH or glacial acetic acid
Procedure: 1)Calcium and Magnesium: Transfer 10 ml of ammonium acetate soil extract into a conical flask and add 5 to 10 ml buffer complex to get pH of about 10, add EBT indicator and titrate the content with standard EDTA till color changes from pink to blue.
2) Calcium: Transfer 10 ml of ammonium acetate soil extract into a conical flask and add sufficient quantity of 10% NaOH solution to attain a pH of 12 or more. Now add a pinch of Murexide indicator and titrate it against standard EDTA solution till the colour changes from pink to violet.
Calculation:

M.equ. (Mg) per 100g = M.equ. (Ca + Mg) per 100g - M.equ. Ca per 100g
Dr. T.V. Ramachandra
Centre for Sustainable Technologies,
Centre for infrastructure, Sustainable Transportation and Urban Planning (CiSTUP),
Energy & Wetlands Research Group, Centre for Ecological Sciences, Indian Institute of Science, Bangalore – 560 012, INDIA.
E-mail : cestvr@ces.iisc.ac.in
Tel: 91-080-22933099/23600985,
Fax: 91-080-23601428/23600085
Web: http://ces.iisc.ac.in/energy
Subash Chandran M.D Energy & Wetlands Research Group, Centre for Ecological Sciences, Indian Institute of Science, Bangalore – 560 012, INDIA.
E-mail:
subhashc@iisc.ac.in
Joshi N.V.Energy & Wetlands Research Group, Centre for Ecological Sciences, Indian Institute of Science, Bangalore – 560 012, INDIA.
E-mail:
nvjoshi@iisc.ac.in
Dhaval Joshi
Energy & Wetlands Research Group, Centre for Ecological Sciences, Indian Institute of Science, Bangalore – 560 012, INDIA.
Maneesh Kumar
Energy & Wetlands Research Group, Centre for Ecological Sciences, Indian Institute of Science, Bangalore – 560 012, INDIA.
Citation: Ramachandra T.V, Subash Chandran M.D, Joshi N.V, Dhaval Joshi, Maneesh Kumar, 2012. Soil Quality across Diverse Landscapes in Central Western Ghats, India , Sahyadri Conservation Series 16, ENVIS Technical Report 42, CES, Indian Institute of Science, Bangalore 560012