Energy and Wetlands Research Group, Centre for Ecological Sciences [CES], Indian Institute of Science, Bangalore – 560012, India.
Web URL: http://ces.iisc.ac.in/energy; http://ces.iisc.ac.in/foss *Corresponding author: Ramachandra T.V Deepthi Hebbale emram.ces@courses.iisc.ac.in, deepthih@iisc.ac.in
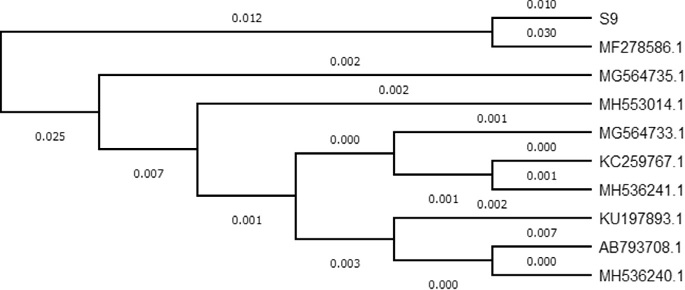
3.2. Bacterial identificationThe biochemical characterization showed the strain 9 to be gram negative, motile rods, and aerobic in nature (Table 2). The phylogenetic analysis of S9 with 16S rDNA sequence exhibited 99% homology with MF278586.1 (Vibrio parahaemoly- ticus strain ukmVp1) (Fig. 5). Vibrio belong to Vibrionaceae family, commonly in- habiting halocline aquatic environments especially brackish waters and oceans. These are associated with aquatic animals such as parasites of fish, crustaceans and molluscs. Vibrio like bacterium strain G21 was isolated from mangrove soil that produced cellulase enzyme (endo-b-1, 4-glucanase, Cel5A) (Gao et al., 2010), simi- larly Vibrio sp. LX-3 bacteria was isolated from soil for cellulase enzyme production (Li et al., 2003).
Fig. 5. Phylogenetic tree of S9 (Vibrio parahaemolyticus) associated with other members of genus
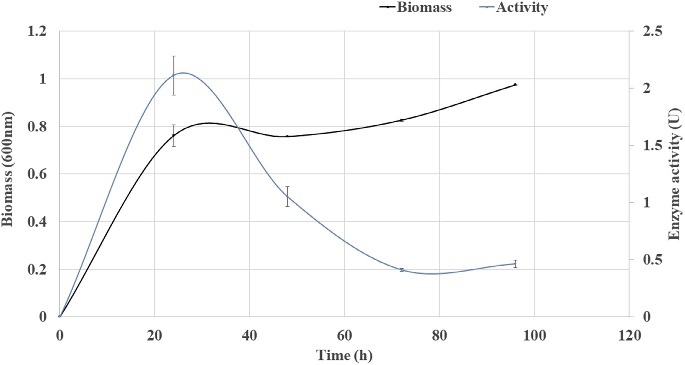
Fig. 6. Growth curve and enzyme activity of Strain 9. 3.3. Growth estimationSimultaneous growth estimation with the enzyme activity revealed maximum enzyme activity at 24 h (1 day) (Fig. 6). In a similar study, Trivedi et al. (2011) has reported the optimum time of 72 h for enzyme production through the isolation of marine bacteria Bacillus flexus from degrading Ulva lactuca for cellulase production. On CMC medium larger zone of clearance was recorded at 48 h of incubation, whereas in liquid production medium higher enzyme ac- tivity was recorded at 24 h, this is due to the presence of agar in CMC medium which provide fairly rigid matrix that slows the difusion of enzyme through the matrix (Hankin and Anagnostakis, 1977). Liquid production medium contained CMC as sole source of carbon with the higher CMCase activity of 2.11 IU/ml was recorded at 24 h. Trivedi et al. (2011) estimated Enzyme activity of 1.35 U/ml and specific activity of 4.14 U/mg from Bacillus flexus isolated from de- grading U. lactuca. Liang et al. (2014) isolated 245 bacterial strains from sugar- cane bagasse pulp supplemented with agar and 22 strains out of 245 showed hydrolyzing zone and one strain Paenibacillus terrae exhibited highest CMCase activity of 2.08 U/ml in liquid culture. Gupta et al. (2012) isolated cellulose de- grading bacteria from invertebrates such as; termites, snail, caterpillar and book- worm, endoglucanase assay ranged between 0.162 to 0.400 IU/ml for 30 mins. Vibrio harveyi and Vibrio fischeri isolated from gut of Mugil cephalus fish, ex- hibited higher CMCase activity of 0.3 U/ml and 0.35 U/ml at 30 min respectively (Ramesh and Venugopalan, 1988). CMCase activity of Vibrio LX-3 strain which was isolated from soil samples were carried out and 15.29 U/ml activity was re- corded for an incubation period of 7e8 days (Li et al., 2003). Enzyme was pro- duced from Vibrio agar-liquefaciens isolated from deteriorating wooden pilings exposed to seawater on a muddy coast, CMCase or endoglucanase activity was determined at various incubation period and highest activity of 0.09 U/ml was recorded between 8-10 days incubation (Abhaykumar and Dube, 1992). In our study, however enzyme activity of 0.13 U/ml was estimated at 30 min andhighest enzyme activity of 2.11 U/ml specific activity of 6.05 U/mg were recorded at 24 h incubation (Table 3). In comparison to the other studies, Vibrio parahaemolyticus strain showcased higher enzyme activity at lower incubation period indicating its characteristic feature to metabolize complex carbon compounds.
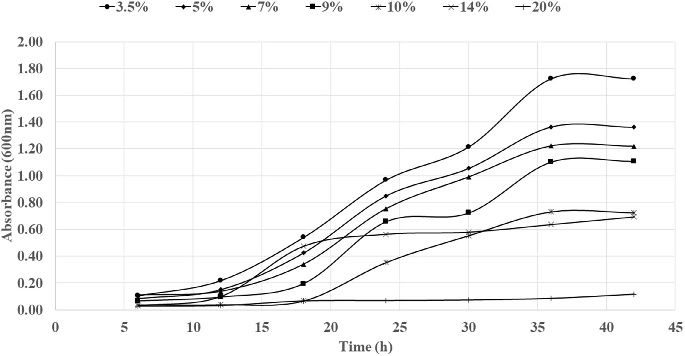
Fig. 7. Bacterial growth curve with different concentration of salt.
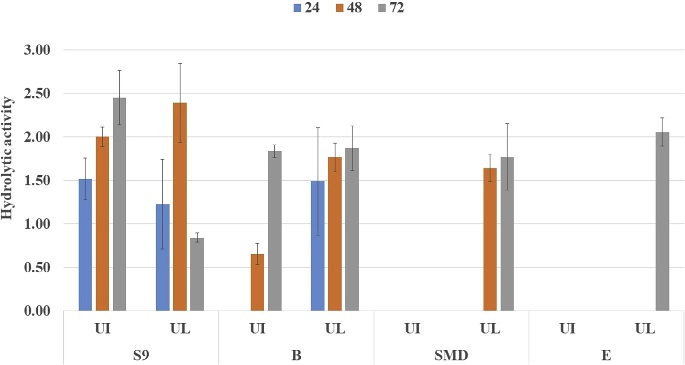
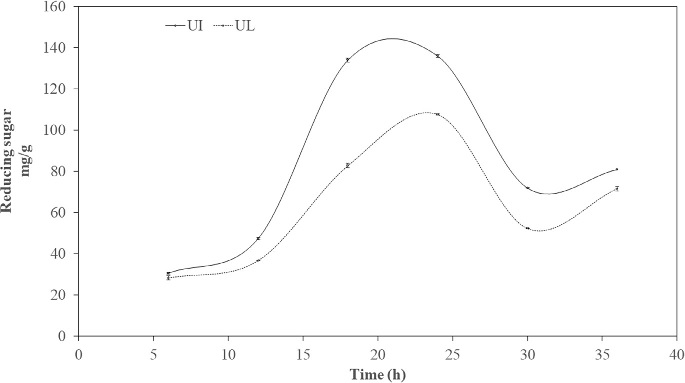
Fig. 8. Enzyme hydrolysis of pretreated U. intestinalis (UI) and U. lactuca (UL) biomass. Growth monitoring was carried out at different salt tolerance (Fig. 7) and it was seen that bacterial strain S9 exhibited optimum growth at 3.5% NaCl concentration, with extended exponential phase upto 24 h, where at different salt concentration there was decline of exponential phase. The strain 9 had tolerance level upto 10% revealing its origin from estuarine source indicating halophilic nature. Li et al. (2003) isolated Vibrio sp. LX-3 from soil, which was gram negative rod and facul- tatively anaerobic. Growth of the bacteria was supported by NaCl, bacteria pro- duce enzymes that digested both crystalline cellulose and agar. In Vibrio sp. G21, endo b-1, 4-glucanase Cel5A and Egl-AG from Bacillus agaradhaerens ac- tivity is induced in the presence of NaCl (Hirasawa et al., 2006; Gao et al., 2010; Trivedi et al., 2011). 3.4. Enzyme saccharification of macroalgal polysaccharideEnzyme hydrolysis was performed for acid pretreated U. intestinalis and U. lactuca biomass (Fig. 8). Reducing sugar was seen to increase linearly with incubation period from 12 to 24 h ranging from 47.4 mg/g to 135.9 mg/g, and decreased beyond 24 h to 71.9 mg/g in the case of U. intestinalis. Enzyme hydrolysis of U. lactuca recorded similar linear trend with reducing sugar release of 28.3 mg/g to 107.6 mg/g from 6 to 24 h. Trivedi et al (2015) isolated cellulase enzyme from Cladospo- rium sphaerospermum and subjected Ulva lactuca, green seaweed to enzyme hydro- lysis and obtained 112 mg/g of reducing sugar. However, in this study highest reducing sugar of 107.6 mg/g and was obtained from U. lactuca whereas 135.9 mg/g reducing sugar from U. intestinalis indicating enzyme ability to hydrolyze the mac- roalgal polysaccharide. Citation : Deepthi Hebbale,R. Bhargavi, T. V.Ramachandra.Saccharification of macroalgalpolysaccharides throughprioritized cellulase producingbacteria.Heliyon 5 (2019) e01372.doi: 10.1016/j.heliyon.2019.e01372
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||