| Sahyadri Conservation Series: 13 |
ENVIS Technical Report: 39, March 2012 |
 |
EXPLORING BIODIVERSITY AND ECOLOGY OF CENTRAL WESTERN GHATS |
 |
Energy and Wetlands Research Group, Centre for Ecological Sciences, Indian Institute of Science, Bangalore – 560012, India.
*Corresponding author: cestvr@ces.iisc.ac.in
|
|
AQUATIC ECOSYSTEM
FISHES
Fishes are the cold blooded aquatic vertebrates which form a major and vital part of the aquatic biodiversity. They are an integral and crucial part in the ecosystem food chain as well as form an important livelihood for many people living around the water bodies. They can be found in a wide range of habitat ranging from fresh water (ponds, lakes, rivers) to marine water (seas, oceans, estuaries) ecosystems. The fresh water fishes form a major endemic vertebrate group in the Western Ghats after amphibians and reptiles (Gururaja, 2002).
At present, nearly 22,000 species of fishes have been reported so far, of which about 40% are inhabitants of fresh and inland waters. In India, it is estimated that about 2500 fish species of which around 930 species are freshwater fishes. The freshwater fishes are distributed amongst approximately 20 orders, 100 families and 300 genera (Daniels, 2000). Fresh water fishes form a major endemic vertebrate group in the Western Ghats after amphibians and reptiles. Of the 288 fish species found in the Western Ghats, 118 species are endemic to the Western Ghats and Sri Lankan region (Dahanukar et al, 2004).
The Western Ghats forms an important watershed for the entire peninsular India, being the source of 37 west flowing rivers and three major east-flowing rivers and their numerous tributaries. The 289 freshwater fish species (41% endemic) reported from the Western Ghats belong to 12 orders, 41 families and 109 genera (Babu & Nayar, 2004; Dahanukar et al, 2004). Notable among these are 33 species from Aralam Wildlife Sanctuary (Shaji et al, 1995), 35 from Periyar River (Zacharias et al, 1996), 98 from Chalakudy River (Ajithkumar et al, 1999), 33 from the Kalakad–Mundanthurai Tiger Reserve (Johnsingh, 2001), 92 from Nilgiri Biosphere Reserve (Easa & Shaji, 1997) and 102 from Pune District (Kharat et al, 2003). Yadav (2003) reported 135 species of fish from the part of the Western Ghats covering southern Gujarat, Maharashtra and Karnataka. The four major rivers (Kali, Bedthi, Aghanashini and Sharavathi) in Uttara Kannada District, Karnataka altogether account for 92 fish species (Bhat, 2003). Arunachalam (2000) and Bhat (2003) showed that fish species diversity and abundance are linked to diversity of aquatic habitats. The studies carried out so far, however, lack landscape ecological approach and have practically little information about the nature of terrestrial landscape elements in the watershed.
The rivers of central Western Ghats are also rich in fish diversity with many rare and endemic species. Specimens of Horabagrus brachysoma Jayaram, a rare catfish of family Bagridae which had been only reported from the backwaters of Kerela was also reported by Bhat (2001) from two rivers in the Uttara Kannada district. Field surveys and fish collections were made from four main rivers in Uttara Kannada – Shravathi, Aghnashini, Bedti and Kali from 1997-1999. The presence of this rare catfish has been reported from the downstream as well as midstream reaches of the Kali and Aghnashini rivers. Bhat and Jayaram (2004)also reported the presence of a new bagrid catfish Batasio sharavatiensis from river Shravathi in Uttara Kannada district. This species is different from all the other known eight species of Batasio as it has a plain, colourless body, without any bars or bands, spots or stripes and a long adipose dorsal fin. Its distribution was thought to be confined to the Sharavathi river. Arunachalam & Muralidharan (2007) reported the same fish species from Tunga river basin showing the range extension of its distribution further south from the type locality to the other river basin.
Arunachalam et al (1999) reported a new record of marine puffer fish, Chelonodon patoca (Hamilton-Buchanon) from the Aghanishini river in the Western Ghats of Karnataka. Ali et al (2005) reported the presence of a small-sized, endangered and endemic fresh water fish, Schistura nilgiriensis (Menon) from a perennial stream of Sharavathi river in Thirthahalli taluk of Shimoga district in Karnataka.Sreekantha et al (2006) discovered two new fish species – Schistura nagodiensis and Schistura sharavathiensis from the Sharavathi River in central Western Ghats.

Figure 1: Schistura nagodiensis

Figure 2: Schistura sharavthiensis
Arunachalam et al (1997) studied the fish species richness and fish habitats in three rivers of Uttara Kannada district and found the presence of twenty species of fishes belonging to five orders, eight families and fifteen genera with three new records. Their study also pointed out that habitat heterogeneity was the main attribute for the abundance and diversity of the fishes.
Bhat (2003) reported the presence of 92 fish species belonging to 48 genera and 25 families from four rivers – Sharavathi, Bedthi, Aghanashini and Kali in the central Western Ghats. The analysis revealed that while the rivers showed similarity in terms of Species richness values, they differed with respect to species composition. The temporal patterns showed that 72 fishes were collected during night time out of which 29 were exclusive to night sampling.
Sreekantha and Ramachandra (2005) documented the presence of 51 species of fishes belonging to 32 genera and 16 families from the Linganamakki reservoir of the Sharavathi river basin. According to their study, the annual fish yield of the reservoir was about 200 tonnes drawing an income of about Rs 43,84,990 to the fishermen with average individual annual earnings of about Rs. 22,042.
Sreekantha et al (2007) recorded the presence of 64 different species of fishes belonging to 38 genera and 17 families from the upper catchment of Sharavathi River of which 18 were endemic to the Western Ghats and 28 were confined to the peninsular India. The family Cyprinidae was found to be dominant with 31 species followed by Balitoridae and Bagridae with 8 and 6 species respectively. The land use analysis of the study area with the help of remote sensing and ground data highlighted that 25% of the area was covered with moist deciduous forests while 16% was under evergreen to semi-evergreen forests. The study revealed the preference of endemic fish fauna to perennial streams with their catchments having evergreen to semi-evergreen forests and higher levels of plant endemism.
Ahmad (2008) sampled seven different sites in Tunga and Bhadra rivers and recorded the presence of 77 different fish species represented by 5 orders, 18 families and 44 genera out of which 8 species were in critical category, 10 species were in high risk category, 36 species were moderately risked and 28 species were in low risk category. 36 fish species were found to be endemic to the Western Ghats, 12 species were endemic to India and 26 species were endemic to the Indian sub-continent. The species richness and abundance of fishes was higher at lower altitudes and they showed a significant relationship with depth and water velocity.
Sreekantha et al (2008) studied the nestedness pattern in the fish community of Sharavathi River in central Western Ghats. The fish sampling revealed the presence of 64 species of fishes of which 39 were found in reservoir sampling sites and 33 were found in stream islands. The nestedness index ‘T’ for the stream islands considering all the species, was 8.27°C whereas ‘T’ for the species that were common to stream islands and reservoir was 0.37°C. From the analyses of the study, it was evident that the species common to both reservoir and stream island showed greater degree of nestedness compared to species of stream islands alone. The fish species common to both reservoir and stream islands showed almost packed matrix with very low T indicating that the anthropogenic activities in the last century have resulted in homogenization of pool-loving fish fauna. It was apparent from the study that selective extinction, selective migrations and selective levels of stress tolerance of the fish species determine the nestedness in a fragmented riverscape.
Table 1:Diversity and biogeographical affinity of fishes endemic to the Western Ghats as given by Menon (1999) (source – Daniels, 2001)
| |
|
Geographical Distribution |
| Genera |
No. of freshwater species in India |
Species endemic to Western Ghats |
Western Ghats + Indian sub-continent |
Western Ghats + Eastern Himalayas + SE Asia |
| Danios, barbs, carps and loaches |
| Barilius |
15 |
4 |
1 |
0 |
| Salmostoma |
10 |
4 |
1 |
0 |
| Chela |
4 |
2 |
2 |
1 |
| Danio |
12 |
2 |
3 |
1 |
| Esomus |
4 |
1 |
1 |
0 |
| Parluciosoma |
2 |
1 |
1 |
0 |
| Neolissochilus |
2 |
1 |
0 |
0 |
| Tor |
5 |
1 |
1 |
0 |
| Osteobrama |
5 |
1 |
1 |
0 |
| Barbodes |
2 |
2 |
1 |
1 |
| Hypselobarbus |
11 |
10 |
1 |
0 |
| Eechathalakenda* |
1 |
1 |
0 |
0 |
| Puntius |
30 |
11 |
11 |
1 |
| Cyprinion** |
5 |
3 |
0 |
0 |
| Cirrhinus |
5 |
2 |
0 |
0 |
| Labeo |
17 |
5 |
8 |
1 |
| Schismatorhynchus** |
1 |
1 |
0 |
0 |
| Crossocheilus*** |
2 |
1 |
1 |
0 |
| Garra*** |
13 |
5 |
2 |
0 |
| Horalabiosa* |
2 |
2 |
0 |
0 |
| Parapsilorhynchus** |
2 |
2 |
1 |
0 |
| Botia |
7 |
1 |
0 |
0 |
| Pangio |
3 |
1 |
0 |
0 |
| Noemacheilus*** |
47 |
12 |
3 |
0 |
| Homaloptera** |
2 |
2 |
0 |
0 |
| Balitora** |
2 |
1 |
0 |
0 |
| Bhavania* |
1 |
1 |
0 |
0 |
| Travancoria* |
2 |
2 |
0 |
0 |
| Catfishes |
| Mystus |
13 |
4 |
5 |
3 |
| Batasio |
3 |
1 |
0 |
0 |
| Pseudobagrus* |
2 |
2 |
0 |
0 |
| Ompok |
4 |
1 |
1 |
1 |
| Silurus |
3 |
1 |
0 |
0 |
| Pseudeutropius |
1 |
1 |
0 |
0 |
| Silonia |
2 |
1 |
0 |
0 |
| Gagata*** |
6 |
1 |
0 |
0 |
| Glyptothorax*** |
21 |
5 |
0 |
0 |
| Clarias |
2 |
1 |
1 |
1 |
| Horaglanis* |
1 |
1 |
0 |
0 |
| Others |
| Horaichthys* |
1 |
1 |
0 |
0 |
| Aplocheilus |
4 |
2 |
0 |
0 |
| Monopterus |
6 |
3 |
0 |
0 |
| Parambassis* |
2 |
2 |
0 |
0 |
| Pristolepis |
2 |
1 |
0 |
0 |
| Etroplus |
3 |
1 |
2 |
0 |
| Pseudosphromenus |
2 |
1 |
1 |
0 |
| Macrognathus |
3 |
1 |
1 |
0 |
| Tetraodon |
2 |
1 |
0 |
0 |
| |
|
|
|
|
| Total 48 |
298 |
114 |
52 |
11 |
References:
- Ajithkumar, C. R., Devi, K. R., Thomas, K. R. and Biju, C. R. (1999), Fish fauna, abundance and distribution in Chalakudy River system, Kerala. J. Bombay Nat. Hist. Soc., 96, 244–254.
- Arunachalam M., Johnson J.A. and Sankaranarayanan A. (1997), Fish diversity in rivers of Northern Karnataka, Western Ghats. International Journal of Ecology and Environmental Sciences, 23(4): 327-333.
- Arunachalam M., Johnson J.A. and Shanthi N. (1999), A new record of the marine puffer fish genus, Chelonodon (Tetraodoniformes, Tetraodonitae) from fresh water habitat of Western Ghats, India. Acta Zoologica Taiwanica, 10(1): 11-14.
- Arunachalam, M. (2000), Assemblage structure of stream fishes in the Western Ghats (India). Hydrobiologia, 430, 1–30.
- Arunachalam M. and Muralidharan M. (2007), New record of Batasio sharavatiensis Bhat & Jayram from Tunga river, Karnataka.
- Babu, K. K. S. and Nayar, C. K. G. (2004), A new species of the blind fish Horaglanis Menon (Siluroifea: Claridae) from Parappukara (Trichur district) and a new report of Horaglanis krishnai Menon from Ettumanur (Kottayam district), Kerala. J. Bombay Nat. Hist. Soc., 101, 296–299.
- Bhat Anuradha (2001), New report of the species – Horabagrus brachysoma in Uttara Kannada district of Karnataka. Journal of Bombay Natural History Society, 98(2): 294-296.
- Bhat, A. (2003), Diversity and composition of freshwater fishes in four river systems of Central Western Ghats, India. Environ. Biol. Fishes, 68, 25–38.
- Bhat Anuradha (2004), Patterns in the distribution of freshwater fishes in rivers of Central Western Ghats, India and their associations with environmental gradients Hydrobiologia, 529 (1-3): 83 – 97.
- Bhat Anuradha and Jayaram K.C. (2004), A new species of the genus Batasio Blyth (Siluriformes: Bagridae) from Shravathi river, Uttara Kannada, Karnataka. Zoo’s Print Journal, 19(2): 1339-1342.
- Dahanukar, N., Raut, R. and Bhat, A. (2004). Distribution, Endemism and Threat status of fresh water fishes in Western Ghats of India. Journal of Biogeography, 31:123 – 136.
- Daniels. R.J.R.. 2000. Project Lifescape 6. Freshwater Fishes: Catfishes. Resonance 5(4): 95-107.
- Daniels R.J.R. (2001), Endemic fishes of the Western Ghats and the Satpura hypothesis. Current Science, 81(3): 240 – 244.
- Easa, P. S. and Shaji, C. P. (1997), Freshwater fish diversity in Kerala part of Nilgiri Biosphere Reserve. Curr. Sci., 73, 180–182.
- Jhingran.Y.G.. (1982), Fish and fisheries of Jndia. Hindustan Publishing Corporation (India). Delhi. pp. 3-666.
- Johnsingh, A. J. T. (2001), The Kalakad–Mundanthurai Tiger Reserve: A global heritage of biological diversity. Curr. Sci., 80, 378– 388.
- Kharat, S., Dahanukar, N., Raut, R. and Mahabaleshwarkar, M. (2003), Long-term changes in freshwater fish species composition in North Western Ghats, Pune District. Curr. Sci., 84, 816–820.
- Sameer Ali, Gururaja K.V. and Ramachandra T.V. (2005), Schistura nilgiriensis (Menon) in Sharavathi river Basin, Western Ghats, Karnataka. Zoos’ Print Journal, 20(2): 1784-1785.
- Shaji, C. P., Easa, P. S. and Basha, S. C. (1995), Freshwater fish diversity in Aralam Wildlife Sanctuary, Kerala, South India. J. Bombay Nat. Hist. Soc., 92, 360–363.
- Sreekantha and Ramachandra T.V. (2005), Fish diversity in Linganamakki Reservoir, Sharavathi river. Eco. Env. & Cons. 11 (3-4): 337-348.
- Sreekantha, Gururaja K.V., Remadevi K., Indra T.J. and Ramachandra T.V. (2006), Two new fish species of the genus Schistura Mcclelland (Cypriniformes: Balitoridae) from Western Ghats, India. Zoos’ Print Journal, 21(4):2211-2216.
- Sreekantha, Chandran M.D.S., Mesta D.K., Rao G.R., Gururaja K.V., Ramachandra T.V. (2007) Fish diversity in relation to landscape and vegetation in Central Western Ghats, India. Current Science 92, 1592-1603.
- Sreekantha, Gururaja. K. V and Ramachandra. T. V. (2008), Nestedness Pattern in Freshwater Fishes of Western Ghats: An Indication of Stream Islands Along Riverscape. Current Science, 95 (12): 1707-1714.
- Yadav, B. E. (2003), Ichthyofauna of northern part of Western Ghats. Rec. Zool. Surv. India, 215, 1–40.
- Zacharias, V. J., Bharadwaj, A. K. and Jacob, P. C. (1996), Fish fauna of Periyar Tiger Reserve. J. Bombay Nat. Hist. Soc., 93, 35–43.
AMPHIBIANS
Amphibians are tetrapod vertebrates evolved from the bony fishes (Sacropterygians) and first appeared on the earth nearly 360 million years ago (in the late Devonian period). Literatures cite that early amphibian fossils were found in Madagascar Island. Amphibians are pivotal organisms both as prey and predator in many food chains and constitute a vital component of the ecosystem. In ecosystem management, they are the best biological pest controllers. Amphibians respond to the minute disturbances in their habitat or in the environment. Their relatively wide distribution, bimodal life style (aquatic tadpole and terrestrial adults), ectothermic conditions with stable environmental temperature of 20-30°C and moist permeable skin have made them highly sensitive and susceptible to the external changes. Hence amphibians are regarded as the best ecological indicators among the vertebrates. Amphibians can be seen in almost all the continents (except Antarctica) ranging from human inhabitations to deserted regions, they are present in many habitats and microhabitats. They can be found inside the water, muddy and rock crevices, burrowing deep in the soil, or bushes, high canopy trees etc. Amphibians are a plenty during rainy season, as they require water to breed and to lay eggs. Majority of the amphibians are active during night (nocturnal). Amphibians are well known for their croaking noises (vocal calls), which they generally do to attract the partner. One can easily locate and identify the amphibian species based on their calls.
India has very rich amphibian diversity with a total of about 200 species (Inger & Dutta, 1986; Chanda & Ghosh, 1988). However, the analysis of pattern of amphibian diversity by Inger & Dutta (1986) revealed that the Western Ghats are the richest in terms of amphibian diversity with about 117 species being present here out of which 76% are endemic to the Western Ghats. The Western Ghats harbours 127 species (57.73% of Indian amphibians), of which 107 (84.25%) species are endemic (Western Ghats and India). Table 1 details the species diversity of Western Ghats amphibians. Two order, viz., Salientia and Apoda with eight families and 24 genera represent the 127 species of Western Ghat amphibians. Tables 2 and 3 provide the endemic and non-endemic amphibians of the Western Ghats with IUCN criteria. There are 2 critically endangered species; 16 endangered; 36 vulnerable; 30 lower risk near threatened; and 5 with lower risk least concerned. Thirty-eight species lack sufficient data to categorize under IUCN criteria. Figures 1 and 2 depict the IUCN status of amphibians of the Western Ghats with family wise break-up.
In the last five years, 13 new species of amphibians have been discovered from the Western Ghats. Of these, nine are anurans (Dubois et al., 2001; Krishnamurthy et al., 2001; Bossuyt, 2002; Biju and Bossuyt, 2003; Kuramoto and Joshy, 2003; Biju and Bossuyt, 2005a, b; Das and Kunte, 2005) and four are caecilians (Ravichandran et al., 2003; Giri et al., 2003; Bhatta and Prashath, 2004; Bhatta and Srinivas, 2004).
Conservation of amphibians is not effective in terms of captive breeding and other ex-situ practices. It requires broad based holistic approaches focussing on habitat based conservation plans. This has to be carried out carefully considering vital components (such as humidity, temperature, vegetation etc.,) of individual species habitats. Conservation of habitats ensures the continuous availability of food, shelter and breeding grounds to amphibians and thereby ensuring their viable population in the ecosystem. Continuous monitoring and creating awareness in public and especially in the young minds will certainly help in effective conservation of amphibians.
Daniels (1992) analyzed the ranges and patterns of geographical distribution of amphibians in the Western Ghats and found that the southern half of the Western Ghats and low-medium elevation hills are more diverse in the species than the northern half and higher hills. Gururaja et al (2007) identified and described a new shrub-frog taxon related to the anuran family Rhacophoridae which was named as Philautus neelanethrus sp. nov. based on morphological and morphogenetic studies. The phylogenetic analysis suggested that the new frog represented a relatively early Philautus species lineage recorded from the region whereas the distribution pattern of the species indicated its importance as a bioindicator of habitat health. Dinesh et al (2007) collected a specimen of giant wrinkled frog from Kudremukh National Park and found it to be morphologically similar to Nyctibatrachus hussaini. But since this name is invalid, they compared this specimen with other valid species of Nyctibatrachidae and hence, gave a replacement name for this species Nycibatrachus karnatakensis nom. nov. designating it as a holotype and considering N. hussaini as its objective junior synonym. Dinesh et al (2008) described a new species of Indian Nyctibatrachid frog, Nyctibatrachus dattatreyaensis sp. nov. from the montane Shola forests of Dattatreya Peeta, Bhadra Wildlife sanctuary, Karnataka. Dinesh et al. (2010) also reported the occurrence of an endangered and range restricted frog species -Rhacophorus lateralis in association with Rhacophorus malabaricus from the coffee plantations in Bhadra Tiger Reserve and Kudremukh National Park. Wilkinson et al (2007) described a new species of Indian striped Ichthyophis namely, Ichthyophis kodaguensis from the Western Ghats town of Madikeri in southern Karnataka.
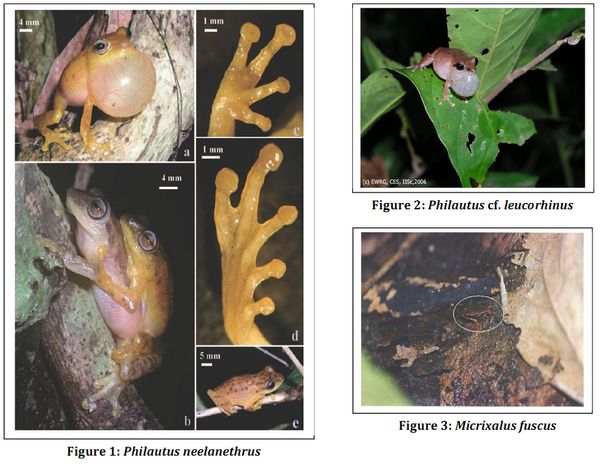
Gururaja et al (2006) assessed the diversity and distribution of amphibian species in three river basins of central Western Ghats and tried to understand their relationship with the landscape variables. Systematic sampling for recording the amphibian species was carried out in the Bedthi, Aghanashini and Sharavathi river basins while the Land-use classification was done using remote sensing data through supervised classification techniques. They found the presence of 46 amphibian species in the study region of which 59% were endemic to the Western Ghats. The endemism, species richness and abundance of endemic species was found to be more in the sub-catchments with high tree density, endemic trees, canopy cover, rainfall and lower amount of agriculture fields while the sub-catchments with lesser percentage of forest, low canopy cover, higher amount of agricultural area, low rainfall had low species richness, less endemic species and abundant non-endemic species. Gurushankara et al (2007) surveyed forest, water bodies, paddy fields and coffee plantations in the central Western Ghats and found that out of the 6303 frogs encountered, 229 showed abnormality. Out of the four different species of frogs surveyed in this study, maximum abnormality was shown by Limnonectus bravipalmata while among the four types of habitats surveyed, coffee plantations had the highest incidence of abnormality. Chemical contamination was suspected to be the cause for this abnormality, which was however, not confirmed. Girish and Krishnamurthy (2009) found a significant relationship between the habitat variables at 35 different streams of central Western Ghats with canopy cover over the streams, presence of leaf litter and high relative humidity on the occurrence of tadpoles. Their study suggested that the disturbances to forest canopy near the streams could have deleterious effects on the occurrence and distribution of tadpoles.
Savitha (2004) studied the population ecology of the anuran populations and the threats faced by them for their survival in the Western Ghats region of Bisale forests in Sakaleshpur taluk of Hassan district. A total of 28 species of anurans were recorded from the Bisale region with the endemic species like Rana curtipes being most abundant species in higher elevation forests. 8 common species representing 3 families of anurans namely – Ranidae, Bufonidae and Rhacophoridae were observed along the streams of various habitats. Significant variations were observed in the densities and abundance of anuran populations in the modified habitats like cardamom and coffee estates near the Bisale forests.
Addoor (2006) carried out a study on the amphibian communities of the central Western Ghats within Dakshina Kannada, Udupi, Shimoga and Chikmagalur districts of Karnataka by employing strip transect and time constrained search method for sampling the amphibians. A total of 29,309 species were sampled in this study representing 38 species with 27 of them being endemic to the Western Ghats. The undisturbed forests of Kudremukh National Park and Agumbe National Park were found to be having the greatest species richness followed by disturbed and most disturbed forests. The seasonal variations in the abundance of amphibian species and individuals were observed with more individuals being sampled in rainy season than in winters and summers.
The Amphibians exhibit remarkable variations in development from egg to adult. One such extreme modification is direct development, wherein free-swimming tadpole stage is completely eliminated and eggs hatch into baby frogs, resembling the adults except for their size. Species adapted completely to terrestrial living generally exhibit direct development. The advantage of being adapted to such development includes avoidance of predation, which is prevalent in aquatic media, parental care and more importantly, dependency on water body for development and complex metamorphic processes (Shi, 2000). Direct development bypassing an aquatic, free-swimming tadpole stage in amphibians seems to be the fastest reproductive mechanism adapted in vertebrates and specifically among anamniotes (Duellman & Trueb, 1994; Patil & Kanamadi, 1997). The direct development has an evolutionary significance in adapting to non-aquatic habitats, resembling oviparous development of birds and reptiles.
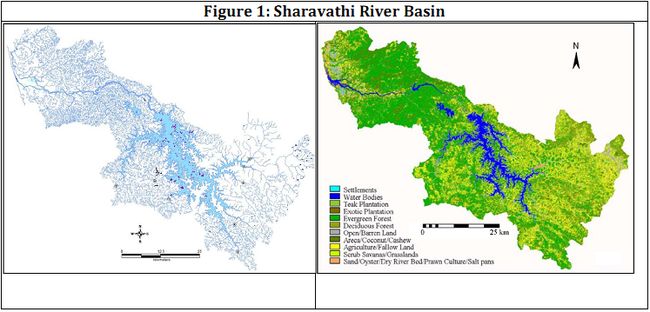
Gururaja & Ramachandra (2006) explained the direct developmental mode in the white – nosed shrub frog Philautus cf. leucorhinus. The analysis of the intra group developmental stages of P. cf. leucorhinus with P. glandulosus and P. variabilis from the Western Ghats, showed that within 144 hrs, major developmental stages like demarcation of head, mouth, eye, fore limbs and hind limbs took place and the remaining period (that varies from species to species) was utilized for differentiation of fingers, toes, mouth parts, eyes and overall morphology of the body with utilization of yolk and regression of tail, which supposedly has a respiratory function. Significant differences were observed between these three species in female sizes, number of eggs and hatching periods which might be attributed to the influence of environmental factors associated with their microhabitats.
Table 1: Amphibians of the Western Ghats
| Order |
Family |
Genera |
Species |
| Anura |
Bufonidae |
Ansonia |
3 |
| |
|
Bufo |
10 |
| |
|
Pedostibes |
1 |
| |
Microhylidae |
Kaloula |
1 |
| |
|
Melanobatrachus |
1 |
| |
|
Microhyla |
3 |
| |
|
Ramanella |
6 |
| |
|
Uperodon |
2 |
| |
Ranidae |
Euphlyctis |
2 |
| |
|
Hoplobatrachus |
2 |
| |
|
Indirana |
8 |
| |
|
Limnonectus |
8 |
| |
|
Micrixalus |
7 |
| |
|
Nyctibatrachus |
11 |
| |
|
Rana |
6 |
| |
|
Tomopterna |
6 |
| |
Rhacophoridae |
Philautus |
26 |
| |
|
Polypedates |
4 |
| |
|
Rhacophorus |
5 |
| |
Nasikabatrachidae* |
Nasikabatrachus |
1 |
| Gymnophiona |
Caecilidae |
Gegeneophis |
2 |
| |
|
Indothyphlus |
1 |
| |
Ichthyophidae |
Ichthyophis |
7 |
| |
Uraeotyphlidae |
Uraeotyphlus |
4 |
Table 2: Checklist of endemic amphibians of Western Ghats with IUCN criteria
| Sr. No. |
Scientific name |
Conservation status |
| Family: Bufonidae |
| 1. |
Ansonia kamblei |
Data Deficient |
| 2. |
Ansonia ornata |
Endangered |
| 3. |
Ansonia rubigina |
Endangered |
| 4. |
Bufo beddomii |
Lower Risk - Least concerned |
| 5. |
Bufo brevirostris |
Data deficient |
| 6. |
Bufo hololius |
Lower Risk - Near threatened |
| 7. |
Bufo koynayensis |
Endangered |
| 8. |
Bufo parietalis |
Lower Risk - Near threatened |
| 9. |
Bufo silentvalleyensis |
Vulnerable |
| 10. |
Pedostibes tuberculosus |
Vulnerable |
| Family: Microhylidae |
1. |
Microhyla sholigari |
Data deficient |
2. |
Melanobatrachus indicus |
Vulnerable |
3. |
Ramanella anamalaiensis |
Data deficient |
4. |
Ramanella minor |
Data deficient |
5. |
Ramanella montana |
Lower Risk - Near threatened |
6. |
Ramanella mormorata |
Vulnerable |
7. |
Ramanella triangularis |
Vulnerable |
| Family: Ranidae |
1. |
Indirana beddomii |
Vulnerable |
2. |
Indirana brachytarsus |
Vulnerable |
3. |
Indirana diplostictus |
Vulnerable |
4. |
Indirana gundia |
Data deficient |
5. |
Indirana leithii |
Lower Risk - Near threatened |
6. |
Indirana leptodactylus |
Vulnerable |
7. |
Indirana semipalmatus |
Vulnerable |
8. |
Indirana tenuilingua |
Data deficient |
9. |
Limnonectes brevipalmatas |
Lower Risk - Near threatened |
10. |
Limnonectes keralensis |
Lower Risk - Near threatened |
11. |
Limnonectes murthii |
Endangered |
12. |
Limnonectes mysorensis |
Critically Endangered |
13. |
Limnonectes nilagirica |
Endangered |
14. |
Limnonectes sauriceps |
Data deficient |
15. |
Micrixalus fuscus |
Lower Risk - Near threatened |
16. |
Micrixalus gadgili |
Endangered |
17. |
Micrixalus nudis |
Vulnerable |
18. |
Micrixalus phyllophilus |
Vulnerable |
19. |
Micrixalus saxicola |
Lower Risk - Near threatened |
20. |
Micrixalus silvaticus |
Vulnerable |
21. |
Micrixalus thampii |
Endangered |
22. |
Nyctibatrachus aliciae |
Vulnerable |
23. |
Nyctibatrachus beddomii |
Lower Risk - Near threatened |
24. |
Nyctibatrachus deccanensis (pygmaeus) |
Vulnerable |
25. |
Nyctibatrachus humayuni |
Endangered |
26. |
Nyctibatrachus hussaini |
Data deficient |
27. |
Nyctibatrachus kempholeyensis |
Data deficient |
28. |
Nyctibatrachus major |
Lower Risk - Near threatened |
29. |
Nyctibatrachus minor |
Vulnerable |
30. |
Nyctibatrachus sanctipalustris |
Endangered |
31. |
Nyctibatrachus sylvaticus |
Data deficient |
32. |
Nyctibatrachus vasanthi |
Data deficient |
33. |
Rana aurantiaca |
Lower Risk - Near threatened |
34. |
Rana curtipes |
Lower Risk - Near threatened |
35. |
Rana greeni |
Data deficient |
36. |
Rana malabarica |
Lower Risk - Near threatened |
37. |
Rana temporalis |
Vulnerable |
38. |
Rana travancorica |
Data deficient |
39. |
Tomopterna dobsonii |
Data deficient |
40. |
Tomopterna leucorhyncus |
Data deficient |
41. |
Tomopterna parambikulamana |
Data deficient |
42. |
Tomopterna rufescens |
Lower Risk - Near threatened |
| Family: Nasikabatrachidae |
1. |
Nasikabatrachus sahyadrensis |
Data deficient |
| Family: Rhacophoridae |
1. |
Philautus adspersus |
Data deficient |
2. |
Philautus aurifasicatus |
Data deficient |
3. |
Philautus beddomii |
Vulnerable |
4. |
Philautus bombayensis |
Endangered |
5. |
Philautus chalzodes |
Vulnerable |
6. |
Philautus charius |
Lower Risk - Near threatened |
7. |
Philautus crnri |
Data deficient |
8. |
Philautus elegans |
Data deficient |
9. |
Philautus flaviventris |
Data deficient |
10. |
Philautus glandulosus |
Vulnerable |
11. |
Philautus hassanensis |
Data deficient |
12. |
Philautus kottigeharensis |
Data deficient |
13. |
Philautus leucorhinus |
Lower Risk - Near threatened |
14. |
Philautus melanensis |
Data deficient |
15. |
Philautus narainensis |
Data deficient |
16. |
Philautus nasutus |
Data deficient |
17. |
Philautus nobeli |
Data deficient |
18. |
Philautus parkeri |
Data deficient |
19. |
Philautus pictus |
Data deficient |
20. |
Philautus pulcherimus |
Vulnerable |
21. |
Philautus punctatus |
Data deficient |
22. |
Philautus signatus |
Vulnerable |
23. |
Philautus swamianus |
Data deficient |
24. |
Philautus temporalis |
Endangered |
25. |
Philautus travancoricus |
Data deficient |
26. |
Philautus variabilis |
Lower Risk - Near threatened |
27. |
Polypedates cruciger |
Vulnerable |
28. |
Polypedates pseudocruciger |
Data deficient |
29. |
Rhacophorus calcadensis |
Data deficient |
30. |
Rhacophorus lateralis |
Endangered |
31. |
Rhacophorus malabaricuds |
Lower Risk - Near threatened |
32. |
Rhacophorus pleurostictus |
Vulnerable |
33. |
Rhacophorus pseudomalabaricus |
Data deficient |
| Family: Caeciliidae |
1. |
Gegeneophis carnosus |
Vulnerable |
2. |
Gegeneophis ramaswamii |
Endangered |
3. |
Indotyphlus battersbyi |
Critically Endangered |
| Family: Icthyophiidae |
1. |
Ichthyophis beddomei |
Vulnerable |
2. |
Ichthyophis bombayensis |
Endangered |
3. |
Ichthyophis longicephalus |
Vulnerable |
4. |
Ichthyophis malabarensis |
Vulnerable |
5. |
Ichthyophis peninsularis |
Vulnerable |
6. |
Ichthyophis subterrestris |
Vulnerable |
7. |
Ichthyophis tricolor |
Endangered |
| Family: Uraeotyphlidae |
1. |
Uraeotyphlus malabaricus |
Endangered |
2. |
Uraeotyphlus menoni |
Vulnerable |
3. |
Uraeotyphlus narayani |
Vulnerable |
4. |
Uraeotyphlus oxyurus |
Vulnerable |
References:
- Addoor S.N.R. (2006), Study of amphibian communities in the rainforests of Karnataka Western Ghats region. PhD thesis, Department of Applied Zoology, Mangalore University.
- Bhatta G. and Prashanth P. (2004), Gegeneophis nadkarnii – a caecilian (Amphibia: Gymnophiona: Caeciliidae) from Bondla Wildlife Sanctuary, Western Ghats. Current Science 87: 388–392.
- Bhatta G and Srinivasa R (2004), A new species of Gegeneophis Peters (Amphibia: Gymnophiona: Caeciliidae) from the surroundings of Mookambika Wildlife Sanctuary, Karnataka, India. Zootaxa, 644: 1–8.
- Biju S.D. and Bossuyt F. (2003), New frog family from India reveals an ancient biogeographical link with Seychelles. Nature 425: 711–714.
- Biju S.D. and Bossuyt F. (2005a), A new species of frog (Ranidae, Rhacophoridae, Philautus) from the rainforest canopy in the Western Ghats, India. Current Science 88: 175–178.
- Bossuyt F (2002), A new species of Philautus (Anura: Ranidae) from the Western Ghats of India. J Herpetol 36: 656–661.
- Chanda S.K. and Ghosh A.K. (1988), Addenda to the amphibian fauna of India. J. Bomb. Nat. Hist. Soc.85: 626 – 627.
- Daniels R.J. (1992), Geographical distribution patterns of amphibians in the Western Ghats, India. Journal of Biogeography, 19(5): 521-529.
- Das I. and Kunte K. (2005), New Species of Nyctibatrachus (Anura: Ranidae) from Castle Rock, Karnataka State, Southwest India. J Herpetol 39: 465–470.
- Dinesh K.P., Radhakrishnan C., Reddy A.H.M. and Gururaja K.V. (2007), Nyctibatrachus karnatakaensis nom. nov., a replacement name for the giant wrinkled frog from the Western Ghats. Current Science, 93(2): 246 - 250.
- Dinesh K.P., Radhakrishnan C. and Bhatta G. (2008), A new species of Nyctibatrachus Boulenger (Amphibia: Anura: Nyctibatrachidae) from the surroundings of Bhadra Wildlife Sanctuary, Western Ghats, India. Zootaxa, 1914: 45-56.
- Dinesh K.P., Radhakrishnan C., Gururaja K.V. and Zacariya A. (2010), New locality records of Rhacophorus lateralis Boulenger, 1883 (Amphibia: Anura: Rhacophoridae), in Western Ghats, India. Journal of Threatened Taxa, 2(6): 986 – 989.
- Dubois A., Ohler A. and Biju S.D. (2001), A new genus and species of Ranidae (Amphibia, Anura) from south-western India. Alytes 19: 53 – 79.
- Duellman W. E. and Trueb L. (1994), The Biology of Amphibians, McGraw-Hill, New York, p. 696.
- Dutta S.K., Vasudevan K., Chaitra M.S., Shanker K., Aggarwal R.K. (2004), Jurassic frogs and the evolution of amphibian endemism in the Western Ghats. Current Science 86: 211 – 216.
- Girish K.G. and Krishnamurthy S.V.B. (2009), Distribution of tadpoles of large wrinkled frog Nyctibatrachus major in central Western Ghats: influence of variables. Acta Herpetologica, 4(2): 153-160.
- Gururaja K.V. (2002), Effect of habitat fragmentation on distribution and ecology of Anurans in some parts of central Western Ghats. PhD thesis, Department of Environment Science, Kuvempu University, Shimoga.
- Gururaja K.V., Reddy A.H.M., Keshavayya J. and Krishnamurthy S.V. (2003), Habitat occupancy and influence of abiotic factors on the occurrence of Nytibatrachus major (Boulenger) in central Western Ghats, India. Russian Journal of Herpetology, 10(2): 87 – 92.
- Gururaja, K.V. (2004), Sahyadri Mandooka, Sahyadri E-News Issue-6.
http://wgbis.ces.iisc.ac.in/biodiversity/newsletter/issue6/index.htm.
- Gururaja K.V. and Ramachandra T.V. (2005), Developmental mode in white-nosed shrub frog Philautuscf .leucorhinus. Current Science, 90(3): 450-454.
- Gururaja, K.V., Sreekantha, Sameer Ali, Rao, G.R., Mukri, V.D. and Ramachandra, T.V. (2007), Biodiversity and Ecological Significance of Gundia River Catchment. CES Technical Report 117, Centre for Ecological Sciences, Indian Institute of Science, Bangalore.
- Gururaja K.V., Aravind N.A., Sameer Ali, Ramachandra T.V., Velavan T.P., Krishnakumar V. and Aggarwal R.K. (2007), A new frog species from the central Western Ghats of India and its phylogenetic position. Zoological Science, 24: 525- 534.
- Gururaja K. V., Sameer Ali, and Ramachandra T. V. (2008), Influence of land-use changes in river basins on diversity and distribution of amphibians, In Environment Education for Ecosystem Conservation, Ramachandra (ed.), Capital publishing company, New Delhi, Pp 33-42.
- Gurushankara H.P., Krishnamurthy S.V. and Vasudeva V. (2007), Morphological abnormalities in natural populations of common frogs inhabiting agroecosystems of central Western Ghats. Applied Hepetology, 4(1): 39 – 45.
- Inger R.F. and Dutta S.K. (1986), An overview of the amphibian fauna of India. J. Bomb. Nat. Hist. Soc. 83(suppl.), 135 – 146.
- Krishnamurthy S.V. (2003), Amphibian assemblages in undisturbed and disturbed areas of Kudremukh National Park, central Western Ghats, India. Environmental Conservation, 30(3): 274 – 282.
- Krishnamurthy S.V., Reddy A.H.M and Gururaja K.V. (2001) A new species of frog in the genus Nyctibatrachus (Anura: Ranidae) from Western Ghats, India. Curr Sci 80: 887 – 891.
- Kuramoto M. and Joshy S.H. (2003), Two new species of the genus Philautus (Anura: Rhacophoridae) from the Western Ghats, Southwestern India. Curr Herpetol 22: 51 – 60.
- Patil N. S. and Kanamadi R. D. (1997), Direct development in the rhacophorid frog, Philautus variabilis (Günther). Curr. Sci., 73: 697–701.
- Ramachandra T.V., Chandran M.D.S, Bhat H.R., Dudani S., Rao G.R., Boominathan M., Mukri V. and Bharath S. (2010), Biodiversity, Ecology and Socio-economic significance of Gundia River basin in the context of proposed Mega Hydro electric power project. CES Technical Report-122, Centre for Ecological Sciences, Indian Institute of Science, Bangalore.
- Savitha K.N. (2004), Population ecology and the threats for survival of Anuran populations in tropical rainforests of Western Ghats. PhD thesis, Department of Applied Zoology, Mangalore University.
- Shi Y.B. (2000), Amphibian Metamorphosis: From Morphology to Molecular Biology. John Wiley, New York, p. 288.
- Wilkinson M., Gower D.J., Govindappa V. and Venkatachalaiah G. (2007), A new species of Ichthyophis (Amphibia: Gymnophiona: Ichthyophiidae) from Karnataka, India. Herpetologica, 63(4): 511-518.
RIVERS OF CENTRAL WESTERN GHATS
The Western Ghats forms an important watershed providing water and food security for the entire peninsular India as it is the source of 37 west flowing rivers and three major east flowing rivers and their numerous tributaries. The Central Western Ghats also harbors many important perennial rivers and small streams. However, the freshwater ecosystems of the tropics and sub-tropics are undergoing rapid deterioration due to developmental pressures, opportunistic exploitation and neglect and hence, the challenging issues here are to improve the current knowledge of its biodiversity so that it would aid in sustainable management of the ecosystem through suitable conservation approaches (Ramachandra et al, 2010).The major rivers of the Central Western Ghats are –Cauvery, Sharavathi, Aghanashini, Kali, Bedthi, Netravathi, Kumaradhara and Gundia. Cauvery is an interstate river flowing in the eastern direction while the remaining rivers are west flowing rivers and drain into the Arabian Sea.
Narayana et al (2007) carried out systematic studies on radiation levels and distribution of radionuclides in the riverine environments of Kali, Sharavathi and Netravathi rivers of central Western Ghats, Karnataka and found the highest activity of 226Ra in riverbank soil samples of Sharavathi River whereas highest activities of 232Th and 40K were found in riverbank soil and sediment samples of Netravathi River. The median values of absorbed gamma dose rates in air of Kali, Sharavathi and Netravathi riverbanks were found to be 44 nGy h-1, 35 nGy h-1 and 57 nGy h-1, respectively. 40K was found to be the dominant gamma-emitting source in the soil. The radium equivalent activity, external hazard index (Hex) and internal hazard index (Hin) values in the Kali river basin exceeded the internationally recommended values.
SHARAVATHI RIVER
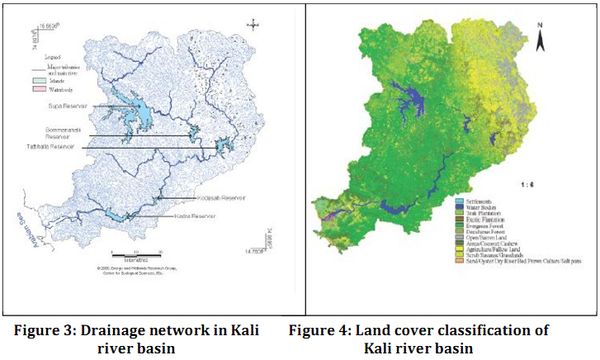
The Sharavathi River originates in Ambutirtha in Tirthahalli Taluk of Shimoga district and it is the one of the major West flowing river of Karnataka with a total length of about 132 km and a drainage area of 2771 sq km. It joins the Arabian Sea at Honnavar of Uttara Kannada distric and drops to a vertical fall of about 253 m in Jog. The major tributaries of Sharavathi in the upstream are Nandiholé, Haridravathi, Mavinaholé, Hilkunji, Yenneholé, Hurliholé, and Nagodiholé. The Sharavathi River basin lies in the latitude 75° 17'38” to 75° 17'38” and longitude 14° 25'08 to 13° 42'36”. The biodiversity exploration in the Sharavathi river ecosystem reveals a vast range of algae, micro and macro invertebrates, fishes, amphibians and an array of mammal and bird species (Ramachandra & Karthick, 2009).
The Sharavathi river basin has been harnessed for hydroelectric projects to its fullest potential. The Mahatma Gandhi Hydroelectric Project with present installed capacity of 120 MW was commissioned in 1948. This was followed by Sharavathi Generating Station (1035 MW) commissioned in 1964-65, the Linganamakki Dam Power House (55 MW) and the Sharavathi Tail Race Project (240 MW) at Gerusoppa in 2001. These account for about 45 % of the total installed capacity of hydroelectric power in the state. Sharavathi river alone, in its fullest potential, accounts for an estimated electricity generation of about 6,000 million units (kWh) per annum.
The river water is stored in three major reservoirs, at Linganamakki (14deg 10’ 24’’N, 74deg 50’ 54’’ E), Talakalale (14deg 11’ 10’’N, 74deg 46’ 55’’ E) and Gerusoppa (14deg 15’N, 74o 39’E). The areas submerged for these reservoirs are 326.34, 7.77 and 5.96 sq. km respectively. The Linganamakki reservoir resulted in the full or partial submergence of 99 villages in the Sagar and 76 villages in the Hosanagar taluks of Shimoga district, also causing the displacement of 12000 people. The Talakalale reservoir resulted in the full or partial submergence of 3 villages in the Sagar taluk. Whereas, the Gerusoppa reservoir did not affect any populated village or caused any displacement of humans, but the submergence of 5.96 sq. km of tropical evergreen to semi-evergreen forests. In addition, for the Sharavathi Tail Race project, 4.72 sq. km of forest and 0.08 sq. km of other lands was also acquired for the township, roads, etc.
The biological exploration of this river dates as early as 1958 for algae by Iyengar, (Randhawa, 1959) followed by Gandhi during 1959 to 1970. Iyengar laid the foundation of biological exploration with a description of 4 new species of green algae (Debarya jognsis, Zynemopsis saravatiensis, Zynemopsis jogensis, and Spirogyra jogensis). Subsequently Gandhi’s (1958, 1959a, 1960c, 1966 and 1970) meticulous work on taxonomy heralded further with description of 22 new species of diatoms (Ceratoneis jogensis, Cymbella rivularis, Cymbella sagarensis, Eunotia jogensis, Eunotia rivularis, Eunotia saravathense, Gomphonema sarvathense , Navicula jogensis, Neidium grandis, Neidium jogensis, Nitzschia pseudogracilis, Pinnularia mysorense, Pinnularia sagittata, Surirella capronioides, Gomphonema spiculoides, Gomphonema tenius , Navicula fridrrichii, Pinnularia balatoneis, Pinnularia pseudoluculenta, Synedra jogensis, Frustulia jogensis, Navicula subdapaliformis). This trend got major boost with recent work of Bhat and Jayaram (2004) describing a new fish species Batasiosharavathiensis and by Sreekantha et al. (2006) with a discovery of Schistura nagodiensis and Schistura sharavathiensis and Philautus neelanethrus sp. nov. (Gururaja et al., 2007).
The ecological studies carried out by Naik (1992) highlighted the seasonal and spatial fluctuations in physic-chemical parameters of Sharavathi estuary and reveals the presence of 202 different species of phytoplanktons represented by the members of Bacillariophyceae, Chlorophyceae, Dinophyceae, Cyanophyceae, Euglenophyceae and Chrysophyceae. Kumar (2006) also analyzed the physic-chemical parameters of the water samples of Sharavathi reservoir and Different physico-chemical and biological parameters of the eight tributaries of Sharavathi in the upstream catchment have been found to be in a permissible limit (Karthick and Ramachandra, 2006).
Mesta (2008) investigated the regeneration status of endemic trees in the fragmented forest patches of Sharavathi river basin of central Western Ghats. He carried out systematic sampling in the study area with the help of quadrats and enumerated the tree species with girth more than 30 cm at GBH along with the shrub and herb layer. They recorded the presence of a total of 399 plant species belonging to 93 families out of which 203 were tree species belonging to 55 families. The families Euphorbiacea, Lauraceaea and Ebenaceae were found to be the three most dominant families in terms of tree composition whereas the families Ancardiaceae, Lauraceae, Clusiaceae and Ebenaceae were found to be most dominant in terms of the endemic species. Of the total sampled stems, 82% of the individuals were found to be evergreen with 40% of them being endemic to the Western Ghats. The estimated tree density per hectare ranged from 278 to 512 individuals across five evergreen classes and the IVI values differed with respect to different species and different classes. Among the endemics Knemma attenuata showed an excellent regeneration in the shrub and herb layer.
However, forest areas have been replaced by agricultural activities in the sub-basin region. The numerous streams and the banks of the river and its tributariesin the evergreen to semi-evergreen forest belt are lined with characteristic riparian vegetation of which the notable tree species are Calophyllum apetalum, Elaeocarpus tuberculatus, Mastixia arborea, Hydnocarpus wightiana, Madhuca neerifolia, etc. Towards the drier forests of the east the water bodies are lined with tree species such asPongamia pinnata, Madhuca neerifolia, Hopea wightiana, Bambusa sp,etc. The Linganamakki catchment region of Sharavathi River is rich in biodiversity and is found to be harbouring 232 different plant species in 21 different micro and macro habitats including large number of endemic species (Rao et al, 2005).
However, in the recent times the vegetation of this region has been pressurized by various anthropogenic factors, mainly by overexploitation of forests for collection of timber wood and NTFPs which has resulted in loss of diversity and basal area and replacement of herb layer by weeds (Rao et al, 2010). Hence, it has been proposed to take steps like reducing intensity of grazing inside forest, protecting forest from fire, discontinuing unsustainable extraction of timber or fuel wood from forests, maximize NTFP collection without affecting regeneration of forest and involve NGO’s, Government organizations and Research institutions in programs like JFM and CFM.
Sharavathi River Basin is also rich in animal diversity. The basin also covers part of Sharavathi Wildlife Sanctuary. The region is also adjacent to Mookambika Wildlife Sanctuary of Udupi District and Shettihalli Wildlife Sanctuary of Shimoga District. Many animals from these sanctuaries pass through the catchment area. Due to rising human pressures including creation of numerous monoculture plantations many of the migratory paths of the animals are disrupted.
The study area also harbours some of the endemic and endangered species of the Western Ghats. The major mammals found here are Gaur, Leopard, Sambar, Barking Deer, Spotted Deer, Mouse Deer, Common Langur, Wild Boar, etc. The Lion tailed macaque is an endangered endemic species found in dense evergreen forests. Tiger, Leopard, Wild Dog, Civet Cat, Sloth Bear, etc are rare animals in the study area. Some of the minor mammals are Indian Hare, Slender Loris, Flying Squirrel, Giant Squirrel, Common Indian Mongoose, Common Otter, Porcupine and Pangolin. Several species of bats, both frugivorous and insectivorous are also found.
A total of 140 species of birds has been sighted in the study area. Of the birds the order the most abundant are Passeriformes, which include the Flycatchers, songbirds, Warblers, etc. Notable endemic bird species include, Blue Winged Parakeet, Crimson Throated Barbet, Grey Headed Bulbul, Heart Spotted Woodpecker, Malabar Grey Hornbill, Malabar Pied Hornbill and Small Sunbird. The endangered Great Indian Hornbill was sighted near the Malemane village of Siddapur taluk.
The wetland habitats include the reservoirs and their environs, the Sharavathi estuary and several tanks in the study area. The wetlands are dominated by both Passeriformes and the Ciconiiformes, the latter includes the Egrets, Herons, Ducks, Kites, Kingfishers, Coots, etc. While the lands are dominated by resident birds the aquatic habitats have by both resident and migratory birds.
A spate of construction activities in the lower catchment of Sharavathi river and ongoing and threatened fragmentation from the burgeoning human population are perilous to the habitats of several forest birds. There has also been considerable decline in the mangrove vegetation threatening the estuarine birds as well.
The notable reptiles are crocodiles (in the reservoir), turtles, King Cobra, Python, Saw Scaled Viper, Russel’s Viper, Malabar Pit Viper, Striped Keel-back, Johnson/s Boa and the Flying Snake.
The notable amphibians and Caecilians observed in the river basin are, Rana tigrina, Rana limnocharis, Rana curtipes, Philautus sps, Ansonia sps, Ichthyophis sps, etc.
A total of 134 species of Butterflies were reported from the study area. Some are endangered and some are endemic species. Notable among the butterflies are, Fluffy Tit, Monkey Puzzle, Southern Birdwing (the largest south Indian butterfly), Malabar Raven, , Paris Peacock, Malabar Banded Peacock, etc. The diversity of butterflies is correlated to the diversity of host plants. The varied kind of human impacts are posing threats to several rare plants thereby also affecting their dependent butterflies. Examples are Crimson Rose, Malabar Rose, Southern Birdwing, (dependent on members of Aristolochia family), Blue Nawab (host plants unknown), Malabar Banded Swallow Tail (dependent on Acronychia and Euodia). Therefore while redesigning vegetation in the human impacted landscape of the catchment attention should be paid to the rehabilitation of these rare and endangered butterflies by planting their host plants.
Except for stray references no systematic study has ever been undertaken of the beetles, of which there is an amazing diversity in the Sharavathi catchment area with 122 beetle species belonging to 26 families. Many of these beetles are very specific to their microhabitats. The beetles have important role in the ecosystem, as they are pollinators of several plant species including the endangered Myristica of the swamps.
Sreekantha and Ramachandra (2005)recorded the presence of 51 different fish species from Linganamakki reservoir which formed an important source of income for local people as fishing was their main occupation. The reservoir fish yield was found to be 610.76 kg/sq.km at full reservoir level while the average annual income of an individual permanent fisherman was calculated to be Rs. 22042. Ali et al (2005)discovered the presence of an endangered and endemic freshwater fish species – Schistura nilgiriensis (Menon) from the south-western part of Sharavathi River basin. Soon after, Sreekantha et al (2006)also discovered the presence of two new fish species – Schistura nagodiensis sp. nov. andSchistura sharavathiensis sp. nov. belonging to family Balitoridae in the Sharavathi River. The analysis of fish species composition, distribution and ecological status highlighted that the endemic fish species preferred perennial streams with their catchments having evergreen to semi-evergreen forests with higher levels of plant endemism. However, in contrast to that, the streams having catchments with moist deciduous forests and low levels of endemism were harboring fishes with wider distribution ranges and few endemic species (Sreekantha et al, 2007).
BHADRA RIVER
Bhadra River is a tributary of Tungabhadra river and arises near Samse in the Aroli hill range of Kudremukh in Karnataka state (Kumara et al, 2010). Bhadra River initially flows east, changing course towards north and then joins Tunga at Kudli in Shimoga district. The Tungabhadra River formed by the confluence of Tunga and Bhadra rivers flows upto 298 kms through Karnataka and some parts of Andhra Pradesh and joins river Krishna (Kumar et al, 2010). The catchment and command area of Bhadra River covers three districts in Karnataka namely Shimoga, Chikmagalur and Davangere. The Kudremukh National Park and the Bhadra Wildlife Sanctuary are two important conservation areas in the reach of Bhadra River. The Bhadra River, the Bhadra Reservoir and the catchment provide critically important resources for the wildlife in Kudremukh National Park and Bhadra Tiger Reserve (Karanth, 1985; Karanth, 1992; Karanth et al, 2001). Downstream of Kudremukh, the Bhadra river flows past the recently established Bhadra Tiger Reserve which is rich in moist deciduous forests and serves as a habitat for several large mammals and is particularly rich in avifauna and drains into the Bhadra reservoir which is one of the important irrigation storage projects in Karnataka.
Kumara et al (2005)analyzed the physic-chemical properties of the surface and sub-surface water samples of the Bhadra River basin for ascertaining its suitability in domestic and irrigational purposes. The calcium and bicarbonates were found to be the dominant cations and anions, respectively in the surface and sub-surface waters. The total dissolved solids present in the sub-surface water were four to six times higher than the surface waters. The sub-surface water of the Bhadra river basin was found to be suitable for using in domestic purposes with few exceptions. The quality assessment of surface water indicated that the water was excellent to good quality and could be used for agricultural purposes.
Mannjappa et al (2006) investigated the metal speciation in the water and sediments of Bhadra River by collecting samples from four different stations at monthly intervals. The analysis of the samples revealed that all the metals except Fe and Mn at the Railway bridge and New bridge stations were well within the limits of BIS standards. This can be attributed to the discharge of industrial effluents and municipal wastes and in the long run might prove to be harmful for organisms present in water as well as other animals and human beings.
The fish diversity of the Tunga and Bhadra rivers was studied by Ahmad (2008) in which he recorded the presence of 77 different fish species represented by 5 orders, 18 families and 44 genera out of which 8 species were in critical category, 10 species were in high risk category, 36 species were moderately risked and 28 species were in low risk category. 36 fish species were found to be endemic to the Western Ghats, 12 species were endemic to India and 26 species were endemic to the Indian sub-continent. Shahnawaz et al (2010) assessed the water quality and fresh water fish diversity of the Bhadra River in central Western Ghats of Karnataka. They recorded the presence of 56 different fish species representing 31 genera and 15 families with Cyprinids being the most dominant group. A positive correlation was observed between the fish species richness and physic-chemical parameters of the water. The variations in the species diversity at the sampling stations indicated that the altered habitats supported less biodiversity whereas less disturbed habitats supported more diversity of the fish fauna.
NETRAVATHI AND KUMARADHARA RIVERS
Netravathi and Kumaradhara are two west flowing rivers in the Central Western Ghats of Karnataka. The Netravathi river, flowing only in Karnataka state in the western direction, originates at an altitude of 1720m in the Western Ghats region of Kudremukh hill ranges in Chikmagaloor district whereas Kumaradhara river originates in the Coorg district and joins the Netravathi river in Uppinangadi wherefrom, these two rivers flow westwards merging with Arabian Sea near Mangalore (Ramachandra et al, 2010). Before joining Kumaradhara river, Netravathi river forms some important tributaries namely - Charmudi hole, Neria hole, Shishla hole, Belthangdi hole, etc. whereas Gundia hole forms an important tributary of Kumaradhara part of Netravathi. A catchment area of size 3,300 sq.km.is drained by river Netravathi and its tributaries and the average annual rainfall received by this area is recorded to be 5000 mm. The Netravathi basin can be divided into three basins namely - Netravathi basin which is typically umbrella shaped, Gundia basin and Kumaradhara basin.
Rajashekhara et al (2005) carried out systematic analysis of soil, sediment and rock samples collected from the riverine environment of Netravathi and Kali river in Karnataka. They found that the concentration of 232Th, 226Ra and 40K in the Netravathi river sediment was higher than the Kali river sediment and the concentration of 232Th was more than twice of the 226Ra concentration.
Avvannavar and Shrihari (2008) collected water samples from eight different sites along the stretch of Netravathi river and analyzed that for six different parameters viz. pH, DO, BOD, MPN, turbidity and TDS for developing a water quality index. The overall Water Quality Index (WQI) along the stretch of river basin was calculated based on Bhargava WQI and Harmonic Mean WQI indexes.
The Gundia River is one of the most important tributaries of river Kumaradhara originating at an elevation of about 1400 m in Saklasheshpura taluka in Hassan district and is formed by the streams namely Yettinaholé and Kempholé to which the streams Kadumaneholé and Hongadahallé join in the course (Ramachandra et al, 2010). The Gundia catchment region is surrounded Hemavathi river water-shed on its right, Barapole river catchment on its left and Netravathi River on downstream side.
Figure 2 – Gundia River Basin
According to Pascal et al (1982), Gundia river basin is situated along the narrow belt of evergreen and semi-evergreen climax and potentially related forests which is of two categories: Dipterocarpus indicus – Kingiodendron pinnatum- Humboldia brunonis type of low elevation (0-850 m elevation) and Mesua ferrea – Palacuim ellipticum type of medium elevation (650-1400 m).
This region is extremely rich in terms of biodiversity which involves the presence of 239 angiosperms, 54 pteridophytes, 44 butterflies, 4 odonates, 56 fishes, 23 amphibians, 32 reptiles, 91 birds and 22 mammals (Ramachandra et al, 2010). This region is also a very rich centre of endemism with almost 36% of plant species, 87% of amphibians and 41% of fishes endemic to Western Ghats being present here (Gururaja et al, 2007; Ramachandra et al, 2010).
Gundia basin forms an important link of two Traditional Elephant Migratory paths and Bisle reserve forest forms a vital part of the Mysore Elephant Reserve. Considering the tangible and intangible benefits derived from fifty year old forests, based on the values given by Das (1980), the value of eco-services provided by the forests in Gundia basin works out to be 195 billion Rs./year (with food and water security) while aiding the livelihood of ecosystem people. This region is the ‘hottest hotspot’ of biodiversity and considering the ecological significance and rich biodiversity, this region should be declared as an Eco-sensitive region as per sub-section (1) with clause (v) of sub-section (2) of section 3 of the Environment (Protection) Act, 1986 (29 of 1986) and clause (d) of sub-rule (3) of rule 5 of the Environment (Protection) Rules, 1986 in concurrence with the provisions of the Indian Forests Act, 1927 (16 of 1927) and Forest (Conservation) Act, 1980 (69 of 1980) the Wildlife (Protection) Act, 1972 (53 of 1972) (Gururaja et al, 2007; Ramachandra et al, 2010).
KALI RIVER
The catchment basin of River Kali lies between 74deg 05’ 7.63” to 74deg 57’ 39.05” East longitude and 14deg 43’ 11.8” to 15deg 33’ 44.9” North latitude and the river basin extends over an area of 4943.43 sq. km. and covers the entire taluks of Supa, Haliyal, Karwar and partially covers the district of Ankola and Yellapur from the Uttar Kannada District. The Karwar beaches receive the water from the river. Kali river extending to a length of 184 kilometer earlier originated near the village Diggi in Supa taluk, as Karihole. After the construction of the dam near Supa, the entire region has disappeared and the taluk, which was once Supa, is now submerged in the reservoir.
Two branches of the main stream - the Pandri and the Ujli originated in the extreme north. The two streams join at Supa, about 32 km, in the south east of the source of Pandri. Later the stream Tattihalla also joins it (the Tattihalla is a stream with a winding southerly course of about 56 km to the north of Haliyal). Near the confluence of these streams is the stepped Lalguli falls. Below its meeting with the Tattihalla, the Kali flows 16 km west where it is joined on the right by the Nujji, which originates from the southeast course of about 40 km from Goa. The Kaneri and the Vaki are its two tributaries. Kaneri originates near the village Kundal in the Supa taluk. Vaki starts near Nujji in the same taluk, takes a southeast direction and finally joins Kali near Tulasgeri. Near Kadra, Thananala (originating from Goa) joins the river. In all, the catchment area of the river is about 5,104 sq. km and the annual river discharge is 6,537 million cu. M. There are four major dam projects on this river now - the Supa reservoir near the headwaters, the Bommanhalli reservoir near the Dandeli forests, the Kodasalli dam near Ganeshgudi and finally, one at Kadra, which is the part of the Kaiga nuclear project and the other two minor dams being at Kaneri and Tattihala. The six dams together generate 1200 MW of electricity and an additional 400 MW are generated by the Kaiga power plant. The river Kali has paper mill at Dandeli which discharges a majority of its effluents into the river apart from a sugar mill which draws water from the river.
According to Nalawadi (1993)mid-kali river basin covers an area of 1849.26 sq.km. and comprises of rugged mountainous terrain, with low order streams on high altitude. The fluctuation in sediment mean size along the Kali River indicates aggradation and degradational fluvial processes due to the presence of many major tributaries transporting and depositing eroded materials all along Kali river course.

Figure 5: Kali river basin
Balakrishna (1998) analyzed the presence of U-th series radionuclides and heavy metals in the particulate and dissolved phases of Kali River, its estuary and adjoining coastal environments. The study was carried out in the Uttara Kannada district where the soil samples from Kaiga region, water and suspended particulate matter (SPM) samples of the river were subjected to geochemical and radiochemical analysis. The Kali river water was found to be depleted in most of the elements studied during post monsoon when compared to monsoon season suggesting that the solubilization of weathered products was less during post monsoon due to less rain and meager river discharge. The Kali estuarine sediments were found to be having lower amounts of 226Ra, 232Th and 40K when compared with adjacent Sharavathi and Netravathi rivers. The SPM of the Kali river was found to be enriched with most of the Rare earth elements (REEs) when compared to the world river SPM and major rock types of Kali river basin.
Yadav et al (2008) documented the biodiversity and assessed the ecological significance of the flood plains of Kali River in central Western Ghats. Random opportunistic sampling was done to record the vegetation, systematic survey was employed for documenting the amphibian diversity, birds were observed and water samples collected were analyzed for various parameters. The vegetation of the region included 67 tree species 26 shrubs, 11 climbers, 78 herbs and one fern species belonging to 33, 15, 8, 23 and 1 family respectively. 45 plants endemic to Western Ghats and 73 plants endemic to both Western Ghats and Sri Lanka were recorded from the study area. The amphibians of the region were represented by 20 species belonging to seven families with 45% endemic species while the birds were represented by 50 different species belonging to 31 different families. The water quality of the streams in the Kali floodplain was found to be in pristine condition.
BEDTHI AND AGHANASHINI RIVERS
The Bedthi river is formed by the confluence of two streams one by the name Shalmala which has its origin near Someshwara temple, south of Dharwad district and the other Bedti stream, originating in the Hubli taluk (Figure 6). These two streams join near Kalghatgi in Dharwad district and then, it is named the Bedthi. Then it flows for about 25 km westwards and enters the Uttara Kannada district where it travels a fairly straight south-westerly course of about 32 km and then falls into the sea, about 32 km south of Kalinadi. At the village of Magod, about 40 km from where it enters the district, the Bedti river dashes over the western face of Sahyadri hills forming the Magod Fall.
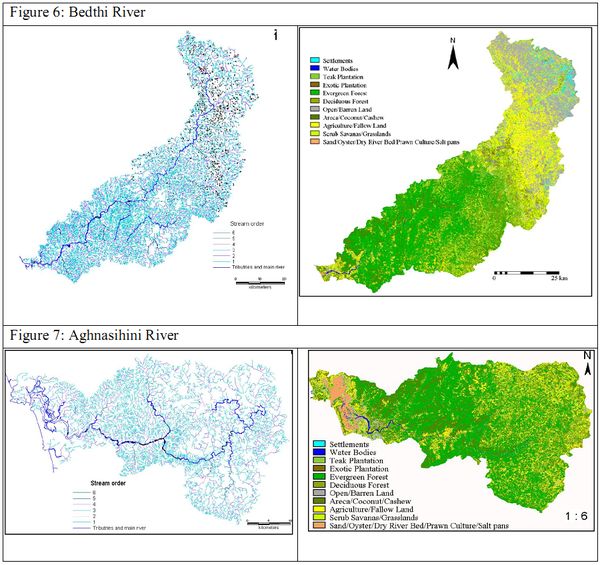
The Aghanashini or Tadri River (total length 121 km) originates in the Sirsi taluk of Uttara Kannada district in the central Western Ghats of Karnataka State (Figure 7). It has two sources – the Bakurhole rising in a pond at Manjguni about 25 km west of Sirsi and the Donihalla whose source is close to Sirsi. The streams meet near Muthalli about 16 km south of Sirsi and under the name of Donihalla it flows about 24 km south of Sirsi with a winding westerly course to the western face of the Sahyadris. Five km from Heggarne, in Siddapur taluk, at Unchalli, it leaps into the Lushington falls. Winding its way through deep gorges and valleys the river meets the tides of the Arabian Sea and forms a large estuarine expanse (13 km long and 2 to 6 km wide) in the coastal taluk of Kumta.
CAUVERY RIVER
Cauvery is one of the most important and a scared river of southern India rising in the Brahmagiri hills of the Western Ghats in the Kodagu district of Karnataka. It is an east flowing river which passes through the states of Karnataka and Tamil Nadu, descends in the Eastern Ghats and empties itself in the Bay of Bengal. About 42.2 per cent of the area of the Cauvery basin (81,155 sq km) lies in Karnataka. This basin covers 18 per cent of the State area comprising seven districts. Its major tributaries in Karnataka are the Hemavati, Lakshmanatirtha, Harangi, Kabini, Suvarnavati, Lokapavani, Shimsha and the Arkavati.
Sunil et al (2010) studied the riparian vegetation of Cauvery river basin by laying down 27 sampling plots across 133 km stretch of Cauvery river basin and recorded the presence of 84 tree species in the sampling sites. The riparian vegetation of the forest zone was more diverse than the agro ecosystem zone in the river basin and the concentration of dominance (Cd) revealed that tree species in both the areas are heterogeneous in distribution dominated by a few species showing a mixed type of tropical forest.
Begum et al (2008) found that due to movement of fertilizers, agricultural ashes, industrial effluents and anthropogenic wastes, large scale effluents were added in the downstream of the river and the heavy metal concentration was found to be maximum in sediments, phytoplanktons and fishes.
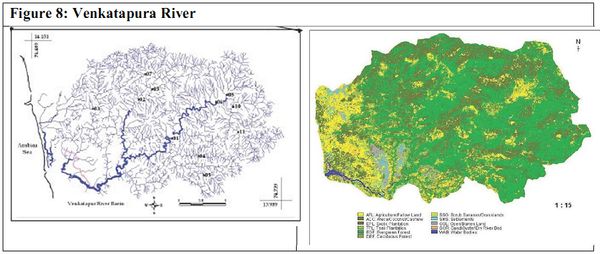
VENKATAPURA RIVER
The Venkatapura river is located between 13.98° - 14.15° N and 74.48°- 74.73° E in the southern part of Uttara Kannada district of Karnataka, India (Figure 8). It originates in Western Ghats and confluence into Arabian Sea after a course of 45 km near Venkatapura with a catchment of 335 km2 (Kamath, S.U., 1985). Forest and agriculture are the major land use in the catchment. The river basin is divided in to six sub basins namely Venkatapura tributary, Chitihalla, Katagar Nala, Basti Halla, Kitrehole and Venkatapura river (Figure 2) based on major tributaries.
References:
- Avvannavar S.M. and Shrihari S. (2008), Evaluation of the water quality index for drinking purposes for river Netravathi, Mangalore, South India. Env Monit Assess 143: 279 – 290.
- Balakrishna K. (1998), U-th series radionuclides and heavy metals in Kaiga region and Kali river, Karnataka state and their adjoining Arabian Sea. PhD thesis, Department of Marine Geology, Mangalore University.
- Begum A., Ramaiah M., Harikrishna, Khan I. and Veena K. (2009), Heavy metal pollution and chemical profile of Cauveri river water. E-Journal of Chemistry, 6(1): 47 – 52.
- Boominathan M., Subhashchandran M.D. and Ramachandra T.V. (2008), Economic valuation of the Bivalves in Aghanashini estuary, West coast, Karnataka, ENVIS Technical Report no.30, Centre for Ecological Sciences, Indian Institute of Science, Bangalore.
- Desai S.R., Chandran M.D.S. and Ramachandra T.V. (2008), Phytoplankton diversity in Sharavathi river basin, Central Western Ghats. Icfai University Journal of Soil and Water Sciences, 1(1): 7 – 28.
- Gururaja, K.V., Sreekantha, Sameer Ali, Rao, G.R., Mukri, V.D. and Ramachandra, T.V. (2007), Biodiversity and Ecological Significance of Gundia River Catchment. CES Technical Report 117, Centre for Ecological Sciences, Indian Institute of Science, Bangalore.
- Kamath S.U. (1985), Karnataka State Gazetteer: Uttara Kannada district. V.B. Soobaiah & Sons, Bangalore.
- Karthick B. and Ramachandra T. V. (2006), Water quality status of Sharavathi river Basin, Western Ghats. Envis Technical report – 23, Centre for Ecological Sciences, Indian Institute of Science, Bangalore.
- Kumar H. (2006), Studies on the ecological characteristics of Sharavathi Reservoir. PhD thesis, Department of Environmental Science, Kuvempu University, Shimoga.
- Kumar K.H., Kiran B.R., Purushottam R., Puttaiah E.T. and Manjappa S. (2006), Length-Weight relationship of Cyprinid fish, Rasbora daniconius (Hamilton – Buchanan) from Sharavathi reservoir, Karnataka. Zoos’ Print Journal, 21(1): 2140 – 2141.
- Kumara V., Narayana J., Puttaiah E.T. and Babu K. (2005), Assessment of surface and sub-surface water of Bhadra River basin near Bhadravathi town, Karnataka. J. Ecotoxicol. Environ. Monit., 15(3): 253 – 261.
- Manjappa S., Puttaiah E.T. and Manjunath N.T. (2006), Metal speciation in water and sediments of the river Bhadra near Bhadravathi town, Karnataka. J. Ecotoxicol. Environ. Monit. 16(1): 1 – 7.
- Rajashekhara K.M., Narayana Y., Karunakara N. and Siddapa K. (2005), Transportation of radionuclides from Western Ghats to Arabian Sea through some major rivers of South India. International Congress Series, 1276: 348–349.
- Rajashekhara K.M., Narayana Y. and Siddapa K. (2008), Distribution of 210Po and 210Pb in the riverine environs of coastal Karnataka. Journal of Radioanalytical and Nuclear Chemistry, 277, No.2 379–388.
- Ramachandra T.V., Chandran M.D.S, Bhat H.R., Dudani S., Rao G.R., Boominathan M., Mukri V. and Bharath S. (2010), Biodiversity, Ecology and Socio-economic significance of Gundia River basin in the context of proposed Mega Hydro electric power project. CES Technical Report-122, Centre for Ecological Sciences, Indian Institute of Science, Bangalore.
- Rao G. R., Subhashchandran M. D. and Ramachandra T. V., (2005), Habitat approach for conservation of herbs, shrubs, and climbers in the Sharavathi River Basin, The Indian Forester, 131 (7): 885-900
- Rao G.R., Subash Chandran M.D. and Ramachandra T.V., (2010), Plant Diversity in the Sharavathi River Basin in Relation to Human Disturbance. The Indian Forester 136(6): 775-790.
- Shahnawaz A., Venkateshwarlu M., Somashekhar D.S. and Santosh K. (2010), Fish diversity with relation to water quality of Bhadra river of Western Ghats (India). Environ Monit Assess, 161: 83 – 91.
- Sunil C., Somashekhar R.K. and Nagaraja B.C. (2010), Riparian vegetation assessment of Cauveri river basin of south India. Environ Monit Assess, 545 – 553.
- Yadav A.S., Gururaja K.V., Karthik B., Rao G. R., Vishnu Mukri, Subash Chandran M.D. & Ramachandra T.V. (2008), Ecological Status of Kali river Flood plain, ENVIS Technical Report - 29, Centre for Ecological Sciences, Indian Institute of Science, Bangalore.
|
|