INTRODUCTION
“Hot-spots” are the regions of exceptionally rich species diversity and include 3.5% of the remaining primary forests in the world, containing about 34,000 endemic plant and 700,000 endemic animal species. Among the world’s 34 “hot-spots”, are the evergreen forests of Western Ghats of south India. The Western Ghats comprise a total area of 160,000km2 containing eight national parks and 39 wildlife sanctuaries in six states: Goa, Gujarat, Kerala, Maharashtra, Tamil Nadu and Karnataka. This region possesses rich species diversity and is one of the most threatened regions of south Asia. Two-thirds of the species found in the Western Ghats are endemic to this region, including the endangered Lion Tailed Macaque, Macaca silenus.
The Macaque monkeys, a genus of dozen or so species is important to humans as their contributions to laboratory medical sciences are well known. Contributions to evolution and biological regulation of animal societies have been dramatically enhanced by studies of wild macaques. Investigations of the relationship between structure and regulation of social groups of primates and the habitat in which they live have increasing relevance to the fate of man as we attempt to delineate the factors modulating our own social behaviours. Among such important macaques only the Lion tailed macaque is an obligate rainforest dweller. It has been uniquely classified as the only truly arboreal macaque, thus offering the opportunity to examine the behavioural correlates of arboreal living and a forest canopy habitat and thereby measure and contrast the effects of hereditary and environmental influences on behaviour.
Among many primate species living in the Western Ghats of Karnataka, Lion Tailed Macaque can be considered as an indicator species. This species is mainly arboreal and is an obligate evergreen forest species, feeding predominantly on fruits and insects. In Karnataka, habitat of this primate is threatened by human-caused disturbances and forest fragmentation (Krishnamurthy and Kiester, 1998).
All regions, where Lion Tailed monkeys have been reported, contain the lofty dense evergreen forests characterised by a large number of tree species reaching ~30 to 50m or more in height and forming a dense canopy. The vast majority of trees have large simple leaves. Giant climbers, epiphytic ferns, mosses and orchids are numerous at all levels from the canopy to the forest floor. The woody under storeys include saplings, smaller species of trees, shrubs and often a tangle of cane or bamboo-like reeds. Except in the ravines, the undergrowth is relatively free of herbaceous plants. The plant communities favoured by Macaca silenus have a very large, slightly buttressed tree occurring at elevations from 600 to 1400 m in areas over 3000 mm rainfall. The typical dominant canopy tree associations are Cullenia-Palaquium, Cullenia-Calophyllum, Poeciloneuron-Cullenia, and Palaquium-Mangifera (Champion and Seth, 1968). Lianas present are also a conspicuous feature; these are woody climbers with flexible stems, which may be a foot thickness in diameter, forming big disorderly curls. They have cauliflower-like canopies, in which both flowers and fruits sprout from the trunks or branches of trees and lianas. Trees and Lianas- and their seedlings – dominate the forest, where more than a half of the plant species are woody and herbs like orchids and ferns occur high up in the trees as epiphytes on the branches. The necessary conditions for its growth are:
- A tropical temperature almost throughout the year: found between 23°30’N and 23°30’S latitude. The land surfaces are within 1000 m altitude.
- High rainfall: at least 1800 mm per year (when evenly distributed). Such “ever-wet tropical” climate prevails, indicating that the earth was covered with this type of forest very long ago. It is hard to delineate precise boundaries for these forests (rainforest).
Peculiarities of rainforest: They contain large numbers of species of flora and fauna in an ecosystem. Their potential for utilisation is greatest in terms of quality, but smallest in terms of quantity. They are usually fragile and once damaged severely, do not recover, or recover very slowly.
Climate: The existence of this type of vegetation is mainly conditioned by climate. At the equator, the mean temperature at sea level is about 26°C and 12 hour-day length shows little seasonal variation. The tropical region is usually hot but it never becomes excessively hot. There is a close relationship between heat and moisture and the typical structure of a tropical rainforest with its giant trees and buttresses. In the humid tropics, the diurnal temperature fluctuation is around 7 to 12°C near the tropics of Cancer and Capricorn. The maximum temperature seldom exceeds 34°C. A mean temperature of 20°C in the coolest months of the year marks the boundary of the tropical rainforest. Temperature is related to latitude and altitude: temperature decreases by 0.61°C per 100 m increase in elevation.
Precipitation: Second limiting factor is precipitation. Tropical rainforest can grow only in the presence of sufficient moisture but this is not the case all the time. In the tropics the extremes of wet and dry seasons are more pronounced than temperature variation between seasons.
Microclimate: Every vegetation creates its own microclimate. The taller and more closed the canopy is, the greater the relative protection it provides. The outside climate does eventually determine the climate inside. Since, the forest canopy is high and generally fairly dense, the protection inside a tropical rainforest is large, yet between the outside climate and the microclimate inside, one can identify a series of microclimates by examining the epiphyte growth on the trees. A forest tree with 5 microclimates (Longman & Jenik, 1974) is shown in Figure 1.
Figure 1
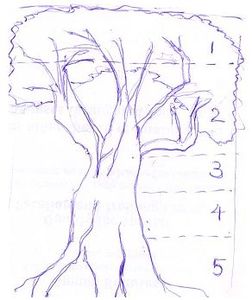 |
Top of the crown is exposed to weather with micro Epiphytes.
The protected part of crown and it is dominant zone of Epiphytes.
Driest upper part of the crown, with flat crust forming lichens.
Moist lower part of the trunk with best growth of lichens and mosses.
Trunk base with buttresses with moist shadowy holes with abundant moss growth. |
Temperature inside and outside the forest:
A temperature fluctuation inside the tropical rainforest is diurnal. The temperature in the forest is usually ~7 to 10°C less than that of outside. In plants, biochemical reactions at various heights, oxidation of humus through nitrate concentration varies due to temperature variations. Inside the forest, the temperature at depths between 5~40 cm is rarely lower than 23°C (Schulz, 1960). Therefore, all reactions in the soil, like root function, decomposition of organic matter or activities of the soil fauna, will be disturbed if normal conditions are altered such as opening of forest cover ( Jacobs, 1981).
1. Lion Tailed Macaques (LTM):
Lion tailed macaques belong to the Order-Primate (Gray, 1821), Family- Cercopithecidae (Gray, 1821) and Genus- Macaca (Linnaeus, 1758).
| Genus |
Species |
Family |
Diploid Chromosome no. |
| M. silenus |
Macaca silenus
(Linnaeus) |
Cecopithecidae |
42 (Bender & Chu, 1963; Napier & Napier, 1967) |
LTM belong to the Family Cercopithecidae, Subfamily Cercopithecinae. They are usually sexually dimorphic animals inhabiting a variety of habitats with an equal variety in diet. The monkey live in multimale, multifemale troops of usually ~10 to 20 animals, although troop size may vary from ~4 to 30 individuals (Roonwal and Mohnot, 1977). The dominant or largest male leads the group yet remains apart from it (Sugiiyama, 1968)(G.Mitchell & J.Erwin, 1986). They are handsome animals with full, almost white lion-like facial ruff or mane, may be called “Lion monkey”- the translation of the Sanskrit name Singalika. The tuft on the tail is found consistently only in adult males, developing with sexual maturation. Few female adults’ tails are tufted. The ruff characterises all members of this species, first appearing on youngsters of 5 to 6 month’s of age. Infants look like other macaque species, a dark dingy gray-brown. Adult males are larger than females, but are still among the smaller macaques, averaging about 55 cm in body length and 8 kg in weight.
It is considered as an indicator species. It is arboreal and obligate evergreen forest species, which feed on fruits and insects. (Krishnamurthy and Kioster, 1998). Lion Tailed Macaque (Macaca silenus) lives only in the Western Ghats Mountains of South India, in the tropical rainforest called Shola. Although sometimes mislabelled with a Sinhalese-name for Langur, “Waterloo”, the Lion Tailed Macaque has never occurred in Sri Lanka except as introduced commercially. It is one of India’s most endangered species. Their numbers have been so reduced by habitat fragmentation, human population pressures and hunting (legal and illegal) that extinction is imminent. The immediate measures to preserve them are to protect and preserve their natural habitats and to protect the monkeys.
In the IUCN Mammal Red Data Book - 76E (endangered) Macaca silenus, common name is Lion tailed macaque. It is endangered (E) taxa, which is in danger of extinction, i.e., whose survival is unlikely if the causal factors continue to operate. Included are taxa whose numbers have been so drastically reduced that they are deemed to be in immediate danger of extinction. It has also been listed in Appendix 1 of CITES (Convention on International Trade in Endangered Species of wild fauna and flora) which is exactly the same list of July 29,1983 CITES list. (G.Miechell & J.Erwin, 1986). It is protected under Indian Wildlife protection act 1972 (SCHEDULE – I: Rare and endangered species which are totally protected).
The LTM is a seriously endangered species and its total world population in nature has been variously estimated from 405(Green and Minkowki, 1977), to over 1200. Ali (1985) estimates its current minimum population at 915. Recent studies indicate population of 3,000 (Karanth 1985). The LTM are endemic lives only in the evergreen monsoon forests. It is highly susceptible to logging, disturbances and the conversion of natural forests to teak plantations. First of all, the primatological community has drawn both national and international attention to the plight of Lion tails, and this has resulted in definite conservation measures. The late prime minister of India, Smt. Indira Gandhi, acutely aware of its precarious status, enlarged Anamallai Wildlife sanctuary and relocated several villages to provide more security for groups in that area and also stopped a large-scale hydroelectric scheme at Silent Valley, a primary forest area in Kerala Border a prime habitat of LTM. One of the major problems in the conservation of this species has been the low rate of reproduction in natural and captive populations (Green and Minkowki, 1977; Lindberg, 1980a, b). The genetic and behavioural suitability of captive-born individuals for reintroduction scheme is also another major concern if these individuals are to contribute to the maintenance of wild populations (Kurt Benirschke, 1986). Long time study (local people) or continuous studies of two identifiable troops of Lion tailed macaques for a 19 month-period concede that the survival of this species depends on its access to a very large and continuous expanse of undisturbed rainforest. (Steven and Karen, 1977). The reason for its present endangered condition is due to the loss of habitats, due to deforestation, forest fragmentation (primary threat) and human-caused disturbance (encroachment and hunting, etc)
1.1 Habitat and distribution:
Lion tailed macaques have been sighted in the Anshighat, Jog Falls, Siddapur, Hulekal, Gerusoppa forest ranges and Mastimane Ghat of North-western Karnataka. They range mainly through mature forests. In Western Ghats, over 50% of the population is distributed in habitat fragments within <20 km2. In Karnataka, the habitat of Lion tailed macaque (including most of the study areas) is now within the reserved forests; providing habitat protection,– where still attempts have to be made to include more areas under reserved forest (Krishnamurthy and Kioster, 1998). Another habitat is Agastyamalai (Southern most part of Western Ghats). They are found only in fragmented patches within its former range.
The Ashambu hill is located between latitude of 8º32' to 8º 38' (N) where these animals are found. Other localities like Nilgiris, and Anamalais hills (11º30’) have similar requirements for their survival. Further north in Karnataka, the rainforest structure differs floristically. The canopy, here, is generally abundant with Dipterocarpus i.e., Lion tailed macaques are in less density.
Remnant patches of shola forest found in sheltered sites along watercourses among hills, generally used to connote any of the wet broad-leaved evergreen forest formation in Western Ghats. Annual rainfall of the areas is >175cm and < 250cm. Luxurious growth is at the altitude of 500 to1500 m. <300 to 600 m rainforests grade into low-lying moist deciduous types. Above 1500 to1800 m in altitude, it is Stunted Montane forest. Lion tailed macaques stay in lofty dense evergreen forests, which have a large number of tree species of heights 30 to less than 50m forming dense canopies. Majority of the trees have large simple leaves, giant climbers, epiphytic ferns, mosses and orchids, which are numerous at all levels of forest from canopy to floor. Woody under storeys have saplings, smaller species of trees, shrubs, cane or bamboo-like reeds. Except ravines, there is an undergrowth-free of herbaceous plants.
The diet for Macaques in general: LTM’s diet varies from month to month but the tree species Cullenia exarillata and Artocarpus heterophylus (Jack fruit) are important year-round food. Recent field studies on other Macaques have showed that they are prominently fruitivorous (M.silenus, Green & Minkowski, 1977). Soil ingestion by Macaques was noted by Eudey (1978) and Lidberg (1977), which possibly provide nutritious elements to the diet and influence digestion (Donald G. Lindberg, 1980). They obtain their food from diverse undisturbed mature rainforest trees. They eat fruits of the top and the second storey of trees. They use every stratum of the forest and they consume flowers, fruits of climbers, small trees, and shrubs, leaves of reeds, grass and sedges. They also eat insects of specific life stages like adults, pupae and larvae, lizards and tree frogs; fungi are gleaned from foliage, exposed and snatched from inside dead wood, plucked underneath bark and rotting log falls.
Scattered aggregation of LTM’s along tree species during various months are:
March and April: Syzigium species, which grows on ridges and higher slopes.
May: Litsea wighitiata, which grows on steep valleys and reeds, breaks along stream banks.
June: Shallow wet soil at the base of precipitous cliffs, where later, ripening aggregation of Litsea sp. occurs.
July and August: LTM cover another area where there are gentle slopes and wide valleys with numerous trees like the fruiting Artocarpus are found. One of the most important things is that LTM’s diet varies in composition throughout the year.
1.2 Home range:
It ranges for over 5 km2 (Minkowski, 1977) of continuous rainforest in a year (in 1974 and 1975, the monkeys’ home range extended still further (O. Michael and J.F. Cates)). Each troop’s range includes perennial water resources. (Steven and Karen, 1977), moreover, they spend less than 1% of their time on the ground. Some arboreal forest animals have greater home range sizes. Grein & Minkowski (1977) give a figure of about 5 km2 for Macaca silenus.
Macaques are housed in pairs or in family groups (Crandall, 1964; Yadav, 1971), unimale groups (Kitchener, 1975~1976; Sargerson, 1977; Vreesvijik & Koning, 1978, Klos, 1981), and multimale/multifemale groups (Chance, 1956;Dumond, 1967;Gledhill, 1972; Desai and Malhotra, 1976; Deag, 1977; Vreeswijk and Koning, 1978; Kleiman, 1980; Klos, 1981), Though infants have been produced in groups of different sizes, both Gledhillm 1972; Kleiman, 1980, report that more than one male is needed in a group to stimulate breeding.
Competition: Food competitors for these animals are mainly arboreal mammals, namely, Nilgiri langur (presbytis johinii) and Malabar giant squerrel(Ratufa indicca).
Predation: Its terrestrial predators are Leopard, Dhole (Wild dog) including humans.
Disease: an introduction of disease causing viruses, bacteria and parasites may lead to the death of the animal.
1.3 Evolution
Male and female genital morphology seen in the silenus-sylvanus group of macaque species is ancestor for the genus. Considering this evidence along the date of early entry of macaques into Europe, it is apparent that they are related to M.sylvanus, however, it seems that this species is more distinct. It is linked to M.silenus and its relatives (Fooden, 1975) essentially by retention of similar ancestral conditions, and not by any shared or derived states, which would uphold its placement within the same species group. It may thus be tentatively recognised as a separate group.
After its origin in northern Africa, early macaques spread into Eurasia. One group entered southern Europe, where they are recognised as a succession of temporal sub species of Macaca sylvanus. A second group of macaques moved eastward, reaching India by the later Pliocene epoch. This group, like early M. sylvanus, would have been long-tailed, with silenus-type reproductive organs. The ancestral species probably spread from southern India, along the coast towards inland to Burma and into Malaya. As in Europe, climatic fluctuations affected Southern Asia and “Sundaland” (a collective term for Indonesia and the neighbouring margins of the Sunda shelf, see Medway, 1970). Brandon –Jones and Heiiemae (unpublished one) suggested that cool or arid phases (linked to drops in sea-level and thus to island connections, permitted migration of suitably adapted species) have led to restriction of forest in relict areas within this region (Eudy). Such phases would have altered several times during the past 2 million years and more rapidly after 0.5 million years with episodes of warmer and moist climate during which islands and their species were isolated from one another. The timing of this oscillation is quite unclear, but the relative sequence of evolutionary events postulated for macaca is estimated to be in best agreement with the underlying philosophy of known fossils and rare biogeographic evidence (suggested by Brandon-Jones and Hiiemae in the past). With alarming climatic amelioration and decay, several Asian macaque groups came into contact and competition. Long tailed early sinica group populations moved toward south and went into peninsular India, perhaps strongly competing with indigenous M.silenus. The sinica group was also moved toward China where they would have become larger or shorter-tailed. Later, M.silenus may have been restricted to its present south West Indian relict distribution, while the northern and southern members of the sinica group may have become partly and generally isolated.
Dispersal of species in Asia
Silenus-sylvanus group: The distinct distribution of extant species extends from the Atlas Mountains of North Africa eastward to Sulawesi, suggesting that the silenus-sylvanus group of macaques experienced the earliest dispersal throughout Asia (Fooden, 1975).
Fooden (1975) infers primarily for tail length and secondarily from skull morphology that Macaca silenus is morphologically most similar to the ancestral population of the Asian sector of the silenus-sylvanus group. Today M. silenus is reduced to a relic population with perhaps no more than 800 individuals in south Indian higher ranges (Mohnotr, 1976). Fooden considers that the M. silenus subgroup originated in peninsular India from where it dispersed northward to reach continental and subsequently insular Southeast Asia. According to Fooden (1975), the initial dispersal northward may have provided the impetus for tail reduction that expresses itself in a west to east gradient in the subgroup. At present, a gap of about 2000 km separates M.silenus in peninsular India from M.nemestrina leonina in Assam and Burma. M.silanus may have been driven to refugium in south India from which it did not recover due to the subsequent expansion of M. radiata in Peninsular India. The dispersal of the silenus group to the major islands of Indonasia, the Mentawai Islands and Sulawesi appears to be intimately associated with the glacial phenomena on Sundaland. The Sunda shelf or Sundaland is that area of the present sea floor in the western part of the East Indian archipelago, which emerged from the sea during the Pleistocene glacial maxima (Donald G. Lindberg, 1980).
1.4 Threats:
Analysis showed various patterns of habitat fragments like linear strips, semi circular and irregular polygons. For example, forest fragmentation concentrated around areas where previously disturbance had occurred, like, the presence of several human habitations within lion tailed macaque’s habitat, and the expansion of human population (Krishnamurthy and Kiester, 1998). Activities of these animals are limited in hillsides, and they may less extensively invade the habitats within Shola on a seasonal basis, seeking fruits of gregarious palm (Bentinckia coddapanna), which grows on the narrow edges of rocky precipices in the southern most Ghats. They also make brief seasonal excursions into the low-lying semi-evergreen areas. The lowest zone is the heaviest hunting zone, thus, they need to be protected. One troop on the Sharavati river north bank (Uttara Kannada district, Karnataka) is most frequently observable.
Hunting: This animal is being hunted for
- food
- as a source of medicinal products; this use of primates is closely related to hunting for food (and may be nothing more than a by-product of such hunting), which involves the use of certain body parts for their supposed medicinal value. For example, in South India, the meat of LTM is said to have an aphrodisiac value with other medicinal properties.
- LTM occurs in less diverse primate faunal areas and require special conservation measures of their own (G. Mitchell &. Erwin, 1986), which is seldom considered, making them more viable to be hunted.
The conversion of forest into another land use or the long-term reduction of the tree canopy cover (below 10% threshold) results in the loss of vegetation cover. Deforestation implies to the long-term or permanent loss of forest cover transforming it into another land use. Such a loss can only be caused and maintained by a continued human-induced or natural perturbation. Deforestation includes areas of forest converted to agriculture, pasture, water reservoirs and urban areas. The term specifically excludes areas where the trees have been removed as a result of harvesting or logging, and where the forest is expected to regenerate naturally or with the aid of silvi-cultural measures. Unless logging is followed by the clearing of the remaining logged-over forest for the introduction of alternative land uses, or the maintenance of the clearings through continued disturbance, forests commonly regenerate, although often to a different, secondary condition. In areas of shifting agriculture, forest, forest fallow and agricultural lands appear in a dynamic pattern where deforestation and the return of forest occur frequently in small patches. To simplify reporting of such areas, the net change over a larger area is typically used. Deforestation also includes areas where, for example, the impact of disturbance, over utilisation or changing environmental conditions affect the forest to an extent that it cannot sustain a tree cover above the 10 percent threshold.
2. Fragmentation
Fragmentation is the breaking up of a habitat or land type into smaller parcels (Forman, 1995). It is implicit that the pieces are somewhat widely and usually unevenly separated. Carving up or subdividing an area with equal width lines is dissection. Dissection could be considered as a special case of fragmentation. Alternatively, the fragmentation concept has been used in a narrower sense, of being essentially the combination of 'habitat loss' and 'isolation'. However, both habitat loss and isolation increase with all these fragmentation processes. Furthermore, habitat loss can take place with or without fragmentation and likewise it can happen in isolation too. In essence, habitat loss and isolation are both useful concepts but different and broader in meaning than fragmentation. Many additional spatial and ecological characteristics result from or are correlated with fragmentation. (Forman, 1997)
Fragmentation is caused by natural processes as well as human activities. For example, herbivore population explosions could break a continuous habitat into small patches just as effectively as logging and suburbanisation. Some human activities cause fragmentation, whereas others, such as the spread of irrigation or severe degradation due to overgrazing typically change the land without fragmentation.
Furthermore, each mechanism operates at a range of scales. Thus, a nesting of fragment sizes over a range of scales is probably a normal result of land fragmentation. The finer scale pattern of habitat fragments is suggested to be especially detrimental to small organisms, specialist species, and ecosystem functions. Finally, it warrants emphasis that scattered patches result from several mechanisms.
- fragmentation of previously continuous habitat.
- a patchy substrate of different soils and slopes.
Alternatively, scattered patches may be due to colonisation into new separate locations. Thus, climate in short, fragmentation is one of several important spatial processes in land transformation. Space, species, and other parameters introduced below result from this process.
Two types of spatial processes are categorised in one category are roads, railroads, power lines wind breaks, etc. and in the other category are logged clearings, cultivated fields, hosing tracts, pastures, and the alike. The ecological effects of dissection and fragmentation may be similar, or highly dissimilar, depending on whether the dissecting corridor is a barrier to movement of the species or otherwise a different process is involved.
Fragmentation affects either the whole area or a patch within it. Patch number or density in the landscape increases with fragmentation, average patch size decreases with it and the total amount of interior habitat normally drops with it. Connectivity across the area in continuous corridors or matrix typically decreases with fragmentation.
Other spatial processes include Perforation, which means the process of making holes in an object such as a habitat or land type. An extensive forest is perforated by logged or blow down clearings, and a desert-grassland is perforated by scattered or clustered houses. Shrinkage means the decrease in size of objects, such as patches. For instance, remnant woodlots shrink as portions are removed for farming or building houses. Attrition means the disappearance of objects like patches and corridors. Usually, small patches disappear, although the occasional disappearance of large patches is apt to be especially ecologically significant (Forman, 1997).
Fragmentation is considered to have two components:
- Reduction of the total extent of habitat type, or perhaps of all natural habitats in a landscape; and
- Apportionment of the remaining habitat into smaller and more isolated patches.
Habitat fragmentation occurs when human development or some other force eliminates large areas of contiguous natural habitat, leaving habitat “islands” thereby conforming that the remaining species of plants and animals are left in a limited space, isolated from other similar communities and habitats. Examples of this condition are natural spaces (parks or undeveloped lots) that are surrounded by urban infrastructure, remnant patches of wilderness that are left when a forest is cleared for farming, or elevated terrestrial habitats that suddenly become scattered islands when a landscape is flooded. While some plant and animal species initially remain on the habitat fragments, the long-term stability of the isolated ecosystems is in question. Habitat fragmentation is a pervasive problem that has generally been recognised as the primary cause of the loss of biodiversity. Understanding fragmentation processes and its effects has a vast practical importance with respect to sustainable use of forest ecosystems and sustaining wildlife populations.
A decrease in the overall area of habitat is quite serious, but when combined with fragmentation, it can undermine the integrity of all ecosystems. Roads, urbanisation and agriculture are among the main human activities, which break up natural areas, often with disastrous implications for flora and fauna. The frequency of occurrence and its long lasting effects are also difficult to reverse. The results of this include inadequate forest interior for edge sensitive species, inadequate size of contiguous forest for area sensitive species, inability of some forest species populations to receive and provide genetic material to other isolated populations. This affects animals as it leads to changes in behaviour of animals, decrease in reproductive fitness (inbreeding), and possibility of extinction of keystone species, which could be fatal on the environment. Also fragmentation affects plants, as they are susceptible to extinction through biomass loss, and decimation of tree communities and disruption of species distribution, i.e., their interdependence, disruption of symbiotic relationships. Fragmentation specifically causes an increase in emission levels of greenhouse gases as it has been shown that certain types of man made fragmentations in central America are more detrimental to the environment because they show a greater increase in greenhouse gas emissions (Radzicki, 2002).
2.1 Species most vulnerable to habitat fragmentation
- Rare species
- Species with a large home range
- Species with limited dispersal
- Species with low reproductive potential
- Species with short life cycles
- Species depending on resources that are unpredictable in time or space
- Ground nesting birds
- Species of interior habitats
- Species exploited or persecuted by people
2.2 Consequences of habitat fragmentation:
Biotic: isolation, edge effects
Abiotic: sunlight, temperature, wind and water flux
Biotic
i) Isolation has the following elements
· time since isolation;
· distance from other remnants (MacArthur and Wilson 1963);
· size (MacArthur and Wilson 1963);
· connectivity;
· surrounding landscape;
· changes in dispersion;
· shifts in abundance patterns to favour weedy species;
· changes in species composition:
· extinction of native species,
· colonisation by invaders;
· changes in gene frequencies (heterogozygosity decrease).
ii) Edge effects are characterised by:
· ecological trap;
· nest predation;
· increased species diversity;
· elimination of interior species;
· shape.
Abiotic
i) Sunlight increase
· shade tolerant species move to interior;
· pioneer species invade edges;
· changes in temperature and water regimes;
ii) temperature fluctuations increase
· daytime temperature is higher => increased transpiration
· night temperature is lower => behavioural changes
iii) impact of wind increases
· higher evapotranspiration => changes in nutrient status in soil (add biomass)
· damage to existing vegetation => creation of gaps (pioneer species invade)
iv) changes in water flux
· rainfall interception; evapotranspiration; increased surface and ground water flows
Habitat fragmentation, or the subdivision of a continuous habitat into smaller patches, has three components, viz., direct removal of suitable habitat, reduction in patch size, and increasing isolation of the remaining patches (Andren, 1994).
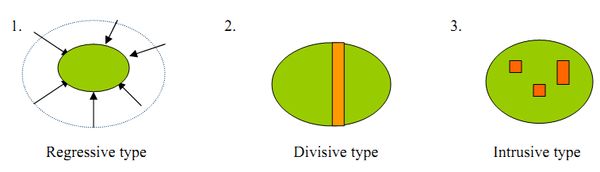
There are three types of habitat fragmentations: regressive type, divisive type and intrusive type.
- Regressive type: an area of a land is decreased due to the reduction of the size of the area.
- Divisive type: an area of a land is divided into two halves ( could be due to a road passing through the area).
- Intrusive type: a few patches of fragme nted areas inside the land.
A range of techniques could be adopted to increase the connectivity in fragmented landscapes. These include creating corridors, buffers and stepping-stones to aid the movement of different organisms. Stepping-stones are patches of habitat, which ease movement through the landscape without necessarily creating direct links. Buffer zones around woodlands may help to reduce the edge effect, and protect the interior of the woods from disturbances caused by activities such as agrochemical applications on an adjacent land. Additional solutions include creating a matrix of other semi-natural habitats such as scrubland, which may still be favourable to some woodland fauna. Species-specific links, such as badger tunnels and aerial runways for squirrels, are also used to help these animals to negotiate roads. There is a "Time lag" in the effects of fragmentation since some of such effects may not come apparent for decades or centuries, like certain ecological processes (nutrient cycling, soil formation, etc.), and the ability of species in isolated fragments to track changes in habitat conditions related to changing climate.





