l |
l |
l |
Energy and Wetlands Research Group, Centre for Ecological Sciences, Indian Institute of Science, Bangalore – 560 012, India
Email: cestvr@ces.iisc.ac.in, supriya@ces.iisc.ac.in
l |
l |
l |
l |
l |
l |
Materials and methods
Study area
Hesarghatta lake, the chosen sampling site for microalgae, is situated at a distance of 18 kms to the North West of Bengaluru city, Karnataka in India This sampling site was chosen so as to get maximum microalgae diversity and to enable characterization of lipid.
Collection of samples
Water quality: 500 ml of water sample was collected and bought to Aquatic ecology lab for the water quality analysis. The physical parameters such as pH, temperature (oC), salinity (mg L-1), total dissolved solids (mg L-1) and electrical conductivity (µScm-1) was measured on the site using Extech pH/conductivity EC500. The nitrate and phosphate concentration of the sample was measured in the laboratory using standard protocols as given in APHA (APHA, 1998).
Microalgal biomass: The epiphytic (Hydrilla sp.) microalgal sample was collected and stored in non-reactive plastic bottles following the procedure given by Kelly et al., 2008.
Analysis of samples
Water quality analysis: The water quality parameters such as pH, salinity, total dissolved solids, electrical conductivity, nitrate concentration, phosphate concentration of triplicate samples were determined according to the standard protocols. The parameters were measured once in 2 days for the indoor and outdoor culture samples.
Community structure analysis: In order to study the variation in microalgal community for the cultured samples, the sample was analyzed two days once by observing 1 ml of the samples under microscope (40X magnification). The community structure of natural sample on 1st, 3rd, 5th, 7th, 9th, 11th, 13th and 15th day samples were analyzed.
In situ culturing
The culturing of the microalgae was done using Chu’s freshwater medium (Bold & Wynne, 1978), since the sample showed presence of large number of diatom species. WC vitamin solution (Guillard & Lorenzen, 1972) was added to this medium to minimize the growth of bacteria in the inoculums (Debenest et al., 2009).
Experimental setup
The experimental setup of outdoor and indoor culture conditions was maintained for comparative analysis of lipid quality with diverse community in each of the individual setup.
Outdoor culturing: Outdoor culturing refers to culturing of microalgal species in natural conditions. The outdoor culturing was maintained for 15 days with 2 replicates per day. 100mL media was taken in sterilized cotton plugged culture flask and to each 15 mL of inoculum (microalgal sample) was added.
Indoor culturing: The term indoor culturing refers to the culturing of microalgae in fixed laboratory conditions. Alike outdoor conditions, the culture was maintained for 15 days 2 replicates per day. 100mL media was taken in sterilized cotton plugged culture flask and to each flask 15 mL of inoculum (microalgal sample) was added. The light intensity of 1320 lx was provided using Compact fluorescent lamps (Philips Genie, Made in India) with 16:8 hours light and dark phase respectively.Analysis
The growth rate of microalgal community was analyzed. The percentage relative abundance for each alga every odd day was calculated using the following formula:

Lipid analysis (Fig 1)
Pre-thin layer chromatography: 25 ml of the microalgal sample was sonicated (Pernet & Tremblay, 2003) in water bath for 2 hours at room temperature in order to disrupt the cell membranes, chloroform: methanol (2:1) was added as the extraction solvent. The chloroform layer was evaporated using rotary evaporator (Eppendorf Vacuum Concentrator 5301) to obtain lipids. This step was important since lipids are highly sensitive to hydrolysis and oxidation processes during storage (Sasaki & Capuzzo, 1984).
Thin layer chromatography: All samples were reconstituted in chloroform to make stock solutions. The stock solutions were spotted in bands onto silica gel TLC plates (Merck KGaA). The mobile phase consisted of a solvent system of hexane/diethyl ether/acetic acid (70:30:1 by volume) [Maloney, 1996]. The plates were developed by exposing the vapors of iodine crystals to stain the plates for visualizing neutral lipids. The samples were extracted and stored in -20 oC until further analysis (Mansour et al., 2005).
Gas chromatography-mass spectrometry analysis: After the initial thin layer chromatography (TLC) lipid screening, the extracts were converted into fatty acid methyl esters (FAME) using Boron trifluoride-methanol and was heated in water bath at a temperature of 60oC for 1 hour. The methylated sample was then purified further for GC-MS. The main focus of using GC-MS was purely for lipid identification rather than quantification. The injector and detector temperatures were set at 250oC while the initial column temperature was set at 40oC for 1 min. A 1 µL sample volume was injected into the column and ran using a 50:1 split ratio. After 1 min, the oven temperature was raised to 150oC at a ramp rate of 10 oC min-1. The oven temperature was then raised to 230oC at a ramp rate of 30C min-1, and finally the oven temperature was increased to 300oC at a ramp rate of 10oC min-1 and maintained at this temperature for 2 min. The total run time was programmed for 47.667 min. The mass spectra were acquired and processed using Agilent Chem Station (5975 C; Agilent, USA).
Fig. 1. Process for the extraction and purification of lipids from microalgae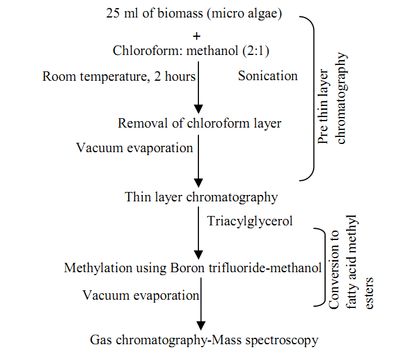
| * Corresponding Author : | |||
| Dr. T.V. Ramachandra Energy & Wetlands Research Group, Centre for Ecological Sciences, Indian Institute of Science, Bangalore – 560 012, INDIA. Tel : 91-80-23600985 / 22932506 / 22933099, Fax : 91-80-23601428 / 23600085 / 23600683 [CES-TVR] E-mail : cestvr@ces.iisc.ac.in, energy@ces.iisc.ac.in, Web : http://wgbis.ces.iisc.ac.in/energy |
|||


