|
Results and Discussion
Results and Discussion
Isolation and screening of ethanologenic wild yeast
strains
Fruits serve as microhabitats for a variety of yeast species.
Therefore bioprospecting of yeasts from fruits is advantageous
[32]. Yeasts isolated from 100 samples (98 fruit sources and two
fermented products) were screened in the current study. Colonies
were observed in 89 samples, and colony morphology was recorded
by incubating the strains at 35oC for 24h. Colony
morphology of isolated yeast presented elongated (24.72%), oval
(14.61%), rounded (60.67%) shape, Large (6.7%), medium (23.6%),
or small (69.66%) size, cream (39.33%) or white (57.3%) or
yellow (3.37%), irregular (48.31%) or regular (51.69%) borders,
bright (49.44%) or opaque (22.47%) or smooth (28.09%) texture.
Yeast exhibit variation in the ability to ferment and assimilate
various sugars, which also aids in the identification of yeast
than morphological and physiological characteristics. Certain
genera of yeast such as Saccharomyces, Torulaspora, and
Zygosaccharomyces ferment glucose readily, whereas;
Lipomyces and Sterigmatomyces are strictly non-fermentative
genera of the yeast. In this study, it was seen that about
74.16% and 71.91% of yeast strains readily fermented sucrose and
glucose, respectively. Glucose as a carbon source allows faster
growth within 24h, which is due to the presence of 20 different
glucose transporters in their plasma membrane [32]. Sucrose, a
disaccharide, is assimilated extracellularly by secreting enzyme
invertase. Lactose was fermented by 57.3% yeast strains,
galactose by 55.06% yeast strains, and lowest being maltose by
41.57% yeast. Production of biofuels from second and
third-generation biomass encourages isolation of yeast strains
capable of fermenting xylose. About 69.66% of yeast strains
fermented xylose in the current study. In a similar study[32],
45 yeast strains were isolated from fruits and chicken litter
and observed that yeast strains readily fermented glucose (22%),
sucrose (12.5%), lactose (2.1%), xylose (40%), galactose (8.3%)
and maltose (2.1%). Yeast strains were tested in glucose and
xylose medium as these sugars are major constituents across the
taxonomical groups of green macroalgae. Strains with the highest
biomass in glucose media (>0.5 OD600) and xylose
media (>0.1 OD600) were considered for further
investigations (Supplementary, S1). The screening was done using
the phenotypic microarray method by eliminating strains with
lower redox signal intensity (RSI) in glucose medium [37]. About
40.45% of yeast strains exhibited good growth in glucose media
with the biomass >0.5 OD, and 47.19% of yeast strains
obtained biomass > 0.1 OD in xylose medium. Yeast strain with
full gas production in Durham’s fermentation tube were selected.
About 19 yeast strains (CY, TY, CHY, MY, MFY, GVY, TNY, PLY
FBY2, BAY, FBJY, RJY, GWY, CKY, PWY, WTY, YKY, POY, CUIY) were
screened down for further characterization and fermentation
capabilities. A growth curve study was carried out for the
strains, incubated at 35oC for 24h with samples drawn
at every 1h interval and biomass growth observed at 600nm.
Strains exhibiting a more prolonged exponential phase
(Supplementary, S1) were selected, as it is a proxy for higher
ethanol production as most of the primary metabolites are formed
during this phase [38, 39].
Screening of multi-stress tolerant ethanologenic yeast
strain
Ethanol endurance is an important property that decides the
fermentation efficiency of the yeast strain. Ethanol tolerance
of yeast has been determined as the accumulation of ethanol
during the fermentation process, and ethanol is toxic to yeast
organisms as it inhibits the activity of crucial glycolytic
enzymes involved in ethanol production and hinders amino acid
and glucose transport leading to the loss of cell viability and
inhibition of cell growth [34, 35]. Isolated yeast growth was
recorded in a spectrophotometer (600nm) in terms of turbidity at
different ethanol concentrations. CHY had the highest ethanol
tolerance, up to 10%, followed by CY, TY, and MY, after which
the growth decreased (Supplementary, S2). In a similar study,
yeast strains CHY1011 and CHFY0901 belonging to Saccharomyces genera
exhibited ethanol tolerance up to 5% [42], comparable tolerance
level was recorded for baker’s yeast (positive control) in the
current study whereas, an isolated yeast (Y-1) from wine (Jiuqu)
had ethanol tolerance up to 14% [40]. S.cerevisiae
isolated from Nuruk [43], cashew [44], and soil [45] exhibited
tolerance in a medium containing 15% alcohol.
Yeast growth at different temperatures were monitored.
Thermo-tolerance of yeast is evident up to 37oC, and
growth is inhibited at higher temperatures. PLY strain exhibited
consistent high growth up to 40oC, whereas TY strain
exhibited higher thermotolerance up to 45oC. In a
similar study earlier [46], Pichia kudriavzevii DMKU
3-ET15 isolated from fermented pork sausage displayed
thermo-tolerance up to 45 oC, which is comparable to
TY strain (Pichia kudriavzevii) in the current study
(Supplementary, S3). Candida tropicalis, Pichia
kudriavzevii, Candida orthopsilosis, Candida
glabrata and Kodamea ohmeri were reported as
thermotolerant, and high ethanol-producing yeasts strains [47].
Pichia caribbica isolated from ripe banana was
subjected to different temperatures, displayed good growth at
temperatures 28, 30, 40 oC, which declined at 45oC
[48], contrary to which CY strain (identified as P.carribbica)
exhibited good growth at 35, 40 and 45 oC, which
declined after 45 oC.
Bioethanol process from seaweeds encounters a high concentration
of NaCl due to its habitat [49]. Therefore, isolated yeast
strains were subjected to different salt concentrations, which
show a decline of cell growth with an increase in salt
concentration, similar to S.cerevisiae KCTC 1126. But,
S.cerevisiae KCTC 1126 adapted to NaCl and yielded an
ethanol concentration of 0.48 [50]. Issatchenkia
orientalis MF-121 produced 2.9% (w/v) ethanol in a
medium containing Na2SO4 (50g/L), while
tolerant to multi-stress factors such as temperature, ethanol,
and salt [51]. Halotolerance up to 14% NaCl concentration was
exhibited by WTY strain, however, the strain achieved a lower
ethanol conversion efficiency of glucose and xylose fermentation
(Supplementary, S4).
Multivariate analyses through Principal Component Analysis (PCA),
given in Fig. 1a, illustrate strains clustering into three
groups with the clustering of temperature tolerant strains and
overlapping of ethanol and salt-tolerant strains. Principal
components are accounting for 56.5% of the total variance, with
PC1 contributing 38.8% and PC2 contributing 17.7%. Salt tolerant
strains POY, YKY, CUIY, and PWY were located at the positive
side along PC1; on the other hand, thermotolerant species CY, TY
MY, MFY and CHY were closely loaded at the negative side along
PC1. Ethanol-tolerant strains were loaded at the positive side
of PC2. Ethanol production by yeast strains using
a synthetic medium
Fermentation of glucose is an established technology; however,
fermentation of xylose has been posing challenges. During the
fermentation process, 70% of the sugar is converted to ethanol,
whereas 20% assimilated by the yeast cells yield glycerol,
organic acids, etc. [52, 53]. Production of glycerol at a higher
concentration inside the yeast cell is stimulated by factors
such as higher pH, a lower flux of pyruvate (due to the
utilization of glycolytic intermediates), increase in osmotic
pressure, etc. with the formation of by-products (higher
alcohols and organic acids at lower level), affecting the
ethanol yield as the growth of yeast cells invariably directs
the glycolytic intermediates to corresponding pathways.
Ethanologenic yeast strains are evaluated based on the ability
of strains to utilize all sugars (glucose, xylose, galactose,
mannose, rhamnose, and arabinose) and convert to ethanol with
minimal by-product formation [54]. Conventionally, ethanol yield
at an industrial scale is calculated based on the total
sugar-fed into the fermentation system, and 90-93% ethanol
bioconversion is considered for an efficient ethanologenic
strain.
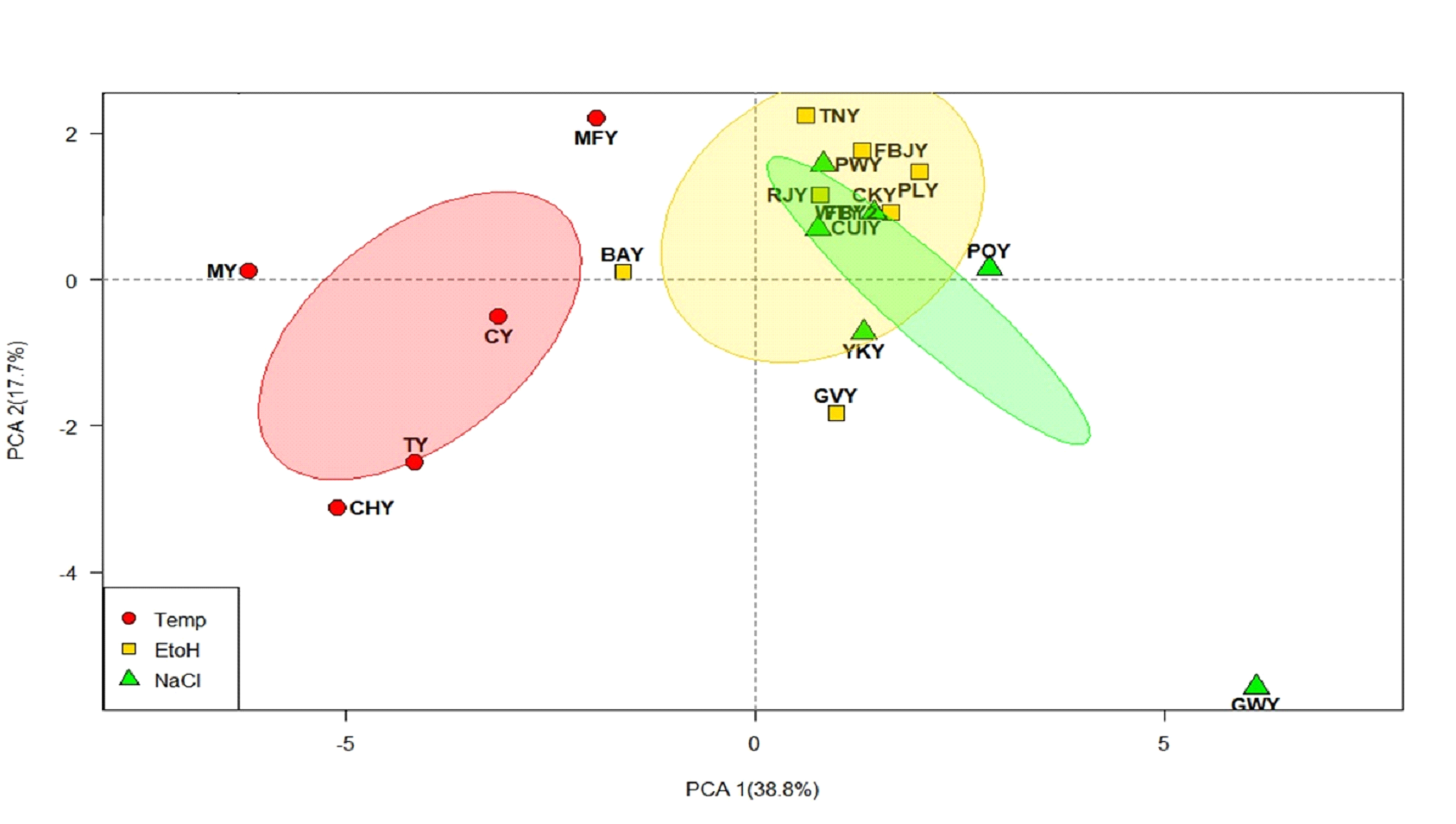
Fig. 1a. PCA score plot of yeast
strains tolerant to stress factors; temperature,
ethanol and salt concentration
Fig. 1b. Multivariate cluster
analysis of yeast strains
Glucose is abundantly found sugar in the feedstock and is readily
fermented by yeast microorganisms. Yeast prefers glucose over
xylose, and xylose uptake is regulated by glucose concentration
[55, 56]. Glucose is metabolized in a series of enzyme catalysed
reaction process called glycolysis, to yield two molecules of
three carbon compound pyruvate, under hypoxia or anaerobic
condition, pyruvate is decarboxylated and acetaldehyde is
reduced to ethanol through alcohol dehydrogenase [57]. Xylose is
converted to Xylulose and phosphorylated to Xylulose-5-phosphate
and further metabolized to glyceraldehyde-3-phosphate, and
fructose-6-phosphate, which then enters the glycolysis pathway
for subsequent pyruvate and ethanol production [58]. It was seen
that about 60-80% of glucose was assimilated and fermented by
yeast strains within 24 h except for CY and TY strains, which
consumed less glucose but achieved a higher conversion
efficiency of 83% and 94%, respectively, compared to other yeast
strains. The highest ethanol concentration of 5.04 g/L was
recorded for MY strain with 65.3% conversion efficiency. The
least ethanol concentration was recorded for PLY, CKY, and FBJY
strains.
Xylose is the main component (1/3) of lignocellulosic biomass,
and xylose is not fermented by Saccharomyces cerevisiae
due to lack of transport system. Yeast species capable of
fermenting xylose belong to the genera Brettanomyces,
Candida, Claviospora, Kluyveromyces, Pachyysolen,
Pichia, and Schizosacchzromyces. Among
which Candida shehatea, Pachysolen tannophilus, and
Pichia stipitis ferment xylose at high concentrations
[58], and studies are being carried out to isolate
xylose-fermenting yeast strains [59–61]. In the current study
(Table 1), a higher ethanol concentration of 28.2% was recorded
for GVY followed by CY strain with 15.3% conversion efficiency
indicating the ability to ferment xylose sugar. GVY strain
produced lower conversion efficiency in glucose medium.
Co-fermentation using two different yeast species has been
carried out to efficiently utilize both xylose and glucose [19,
62]. Co-fermentation of sugars present in lignocellulose biomass
was carried out using P.stipitis and
K.marxianus and P. stiptis, and S.cerevisiae yielded
31.87g/L and 29.45g/L of ethanol [15]. In this study,
co-fermentation of glucose and xylose using yeast strain was
carried out. Acid hydrolysis of biomass releases mixed sugars
into the medium, and therefore mixed sugar fermentation was
carried out, and CY strain produced 1.83 g/L of ethanol,
achieving 27% efficiency, whereas GVY strain produced 1.8g/L of
ethanol achieving 20.6% conversion efficiency. Ethanol
production was affected in other yeast strains due to xylose,
hence the lower ethanol yield despite the presence of glucose.
Studies report that in order to improve xylose fermentation,
isolated yeast strain was grown on xylose rich media, and
efficacy of yeast strain in ethanol production was evaluated by
supplementing xylose in concentration of 10g/L or 25g/L [63].
Ethanol production by prioritized strain using
macroalgal sugars
Fermentation of macroalgal hydrolysate was carried out to
validate the potential of isolated wild yeast strains for
selection of ethanol production. Hydrolysate of E.
intestinalis and U. lactuca obtained from acid
hydrolysis were neutralized and subjected to fermentation using
each of the screened yeast individually at 35oC for
24h on an orbital shaker with 100 rpm. Reducing sugar of Ulva and
Enteromorpha are illustrated in Table 1.
Table 1. Reducing sugar profile
of macroalgal feedstock |
Macroalgae |
Glucose |
Xylose |
Mannose |
Galactose |
Arabinose |
References |
Ulva sp. |
8.2 |
4.5 |
0.29 |
1 |
0.08 |
Wal et al., 2013, Yaich et al., 2011 |
Enteromorpha sp. |
26.3 |
3.5 |
|
6 |
|
Cho et al., 2010 |
Fermentation progress was determined by measuring the reducing
sugar (after the fermentation process) and comparing it with the
theoretical yield (51% of fermented sugar) by estimating the
sugar conversion efficiency of each strain, which is detailed in
Table 2. A higher conversion efficiency of 49.4% was obtained
for CY strain for E. intestinalis followed by TY strain
42.9%. A similar study was carried out using wild yeast strains
S.cerevisiae Y12 and YPS128, derived from clean
lineages with no alternations to their genome due to human
interventions or domestication [37, 64], and this strain
fermented the hydrolysate of U.lactuca producing 7g/L
of ethanol. A multi-tolerant strain of six
Saccharomyces strains was selected and utilized for
fermentation of lignocellulose hydrolysate. This study indicates
that the natural strains outcompeted other strains for specific
traits. Yeast strains isolated from the natural environment have
the potential for bioethanol production and superior to
industrial strains obtained by tweaking the strain through
breeding, experimental evolution, or genetic engineering [34].
Free amino acid nitrogen (FAN) content in green seaweeds is >
0.15 g/L, and this avoids the need to supply additional nitrogen
sources during fermentation [37]. The FAN required for yeast
growth during fermentation and metabolism is 0.15g/L. Hence,
fermentation of seaweed hydrolysate was carried out without the
addition of nitrogen sources. Multivariate cluster analysis was
performed by selecting biochemical compositions of fruit sources
(carbohydrate, protein, fat, dietary fibers, vitamins, moisture
content and minerals) as independent variables and ethanol
production from synthetic sugar as a dependent variable. Fig.
1(b) illustrates the clustering of strains CY and TY from the
rest of the isolated strains indicating its unique properties
with the higher performance capabilities. These two strains
achieved higher biomass, longer exponential growth, maximum
conversion efficiency concerning glucose fermentation, and
exhibited temperature tolerance. Based on these criteria,
strains CY and TY were prioritized for fermentation of
macroalgal sugar.
Table. 2. Fermentation
capacities of yeast strains for synthetic sugar:
Glucose, Xylose and Glucose+ Xylose |
Glucose |
Xylose |
Glucose + Xylose |
Yeast strains |
Initial sugar g |
Fermented sugar g |
EtOH g |
Theoretical yield |
%
Conversion |
Fermented sugar g |
EtOH g |
Theoretical yield |
%
Conversion |
Fermented sugar g |
EtOH g |
Theoretical yield |
%
Conversion |
POY |
20 |
17.01±0.06 |
1.80±0.04 |
8.68 |
20.8 |
14.63±0.01 |
0.07±0.01 |
7.47 |
0.1 |
18.08±0.01 |
0.33±0.05 |
7.47 |
3.6 |
BAY |
16.36±0.14 |
3.71±0.10 |
8.34 |
44.6 |
15.63±0.01 |
0.01±0.00 |
7.98 |
0.1 |
18.27±0.01 |
1.28±0.17 |
7.98 |
13.8 |
GVY |
16.78±0.06 |
0.98±0.20 |
8.56 |
11.5 |
15.69±0.01 |
2.25±0.04 |
8.00 |
28.2 |
17.50±0.01 |
1.83±0.05 |
8.00 |
20.6 |
F3 |
15.26±0.22 |
0.48±0.01 |
7.78 |
6.2 |
13.43±0.03 |
0.05±0.02 |
6.85 |
0.8 |
17.05±0.06 |
0.08±0.02 |
6.85 |
1.0 |
TY |
2.97±0.18 |
1.42±0.02 |
1.52 |
93.7 |
16.01±0.1 |
0.04±0.01 |
8.17 |
0.6 |
16.63±0.03 |
0.02±0.01 |
8.17 |
0.3 |
CY |
3.21±0.24 |
1.35±0.04 |
1.83 |
82.5 |
18.40±0.04 |
1.43±0.01 |
9.39 |
15.3 |
13.35±0.03 |
1.83±0.05 |
9.39 |
27.0 |
CHY |
14.20±0.01 |
4.16±0.22 |
7.24 |
57.5 |
10.36±0.01 |
0.02±0.01 |
5.29 |
0.5 |
13.96±0.02 |
0.04±0.01 |
5.29 |
0.6 |
MY |
15.12±0.04 |
5.04±0.03 |
7.72 |
65.3 |
9.54±0.06 |
0.03±0.01 |
4.87 |
0.7 |
11.57±0.03 |
0.05±0.02 |
4.87 |
0.9 |
MFY |
15.25±0.02 |
4.64±0.03 |
7.78 |
59.7 |
10.21±0.12 |
0.02±0.01 |
5.21 |
0.5 |
11.57±0.11 |
0.04±0.02 |
5.21 |
0.8 |
|
Table. 3. Fermentation
capacities of yeast strains for acid treated hydrolysate
of E. intestinalis and U. lactuca
|
Yeast strains |
Enteromorpha intestinalis
|
Ulva lactuca |
Initial sugar g |
Fermented sugar g |
Ethanol g |
Theoretical yield |
%Conversion |
Initial sugar g |
Fermented sugar g |
Ethanol g |
Theoretical yield |
%Conversion |
CY |
3.74±0.15 |
3.23±0.01 |
0.81±0.01 |
1.6 |
49.4 |
3.74±0.24 |
1.97±0.01 |
0.42±0.02 |
1.0 |
41.8 |
TY |
3.20±0.02 |
0.70±0.02 |
1.6 |
42.9 |
2.02±0.05 |
0.59±0.01 |
1.0 |
57.1 |
CHY |
3.39±0.01 |
0.57±0.02 |
1.7 |
33.2 |
2.75±0.02 |
0.46±0.02 |
1.4 |
32.8 |
MY |
3.32±0.01 |
0.42±0.03 |
1.7 |
24.8 |
2.85±0.01 |
0.36±0.04 |
1.5 |
25.2 |
MFY |
3.32±0.01 |
0.50±0.02 |
1.7 |
29.5 |
2.61±0.02 |
0.42±0.03 |
1.3 |
31.8 |
GVY |
3.12±0.03 |
0.38±0.01 |
1.6 |
24.3 |
2.82±0.01 |
0.23±0.02 |
1.4 |
16.4 |
BAY |
3.08±0.01 |
0.46±0.04 |
1.6 |
29.6 |
2.73±0.03 |
0.43±0.01 |
1.4 |
31.5 |
YKY |
3.14±0.03 |
0.51±0.01 |
1.6 |
32.3 |
2.51±0.01 |
0.18±0.01 |
1.3 |
14.7 |
POY |
3.01±0.04 |
0.24±0.02 |
1.5 |
16.1 |
2.62±0.01 |
0.44±0.03 |
1.3 |
33.6 |
Yeast Identification
The identity of the prioritized yeast strains CY was confirmed as
Meyerozyma (Pichia) caribbica and TY as Pichia
kudriavzevii based on 16S rRNA
nucleotide sequences homology match within the NCBI GenBank
(Supplementary, S5). The yeast cells
were stained with methylene blue and observed under an Olympus
BX-51 bright field, phase contrast microscope, live cells reduce
the dye (methylene blue) [65] and remain colorless, whereas dead
cells retain the color and are stained blue (Fig 2 a-d). Pichia
kudriavzevii cells are oval or ellipsoidal to elongate
in the study. P.kudriavzevii is a thermo-tolerant yeast
strain isolated from fruits and food sources. In contrast, Meyerozyma
caribbica are isolated from fermented beverages having
the capabilities to ferment xylose with high efficiency [66],
which was observed in this study as well.
|
Fig 2a. Microscopic
image and Scanning electron micrographs of (CY) Meyerozyma
(Pichia) caribbica |
Fig 2b. Microscopic image and
Scanning electron micrographs of (TY) Pichia
kudriavzevii |
|
Fig 3. (a) Effect of different
concentration of salt on CY strain growth and ethanol
production (RS : Reducing sugar) |
Fig 3. (b) Effect of different
concentration of salt on TY strain growth and
ethanol production (RS : Reducing sugar) |
Fermentation of macroalgal sugar using prioritized yeast
strainsSeparate Hydrolysis (acid) and
Fermentation (SHF)
Reducing sugar from E. intestinalis and U.
lactuca was obtained by using 0.7N and 0.5N H2SO4 concentration.
About 22.4% and 19.2% sugar conversion with respect to biomass
were achieved for E. intestinalis and U.
lactuca, respectively (Table 4). The acid hydrolysate
obtained was subjected to fermentation using prioritized yeast
strains CY and TY in different combinations at 35oC,
100 rpm for 24h. Fermentation of E. intestinalis hydrolysate
using CY and TY produced 0.14g/L and 0.16 g/L of ethanol with
fermentation efficiencies of 46.9% and 51.8%, respectively.
Co-fermentation of E. intestinalis hydrolysate using CY
and TY yielded lower fermentation efficiency of 33%.
Candida sp. isolated from marine fermented red algae,
Kappaphycus alvarezii acid hydrolysate achieving 50%
fermentation efficiency [27]. E.intestinalis subjected
to SHF produced 8.6 g/L of ethanol with 30% conversion
efficiency within 48h (Cho et al., 2013). SHF of
K.alvarezii [67] and G.amansii [68] yielded
0.25g and 3.33 g of ethanol achieving 55.9% and 74.7% efficiency
respectively. Acid hydrolysis (1% v/v,
H2SO4 for 90 min) of sugarcane bagasse
pith were subjected to fermentation obtaining 2.58 g/L of
ethanol in 30h. Fermentation time for lignocellulose biomass is
longer than macroalgal biomass due to the presence of complex
polysaccharide lignin. Fermentation of U. lactuca hydrolysate
yielded lower ethanol of 0.04g/L and 0.05g/L for both CY and TY
strain with fermentation efficiencies 24 % and 48.7%
respectively. Bioethanol has been obtained from all the three
types of algae, however appropriate microorganism is yet to be
isolated which consumes pentose sugar and mixed sugar [69].
Lower ethanol yield in this study can be attributed to inhibitor
formation during acid hydrolysis. Simultaneous
Saccharification and Fermentation (SSF)
Acid pre-treated macroalgal biomass was subjected to enzyme
hydrolysis using enzyme extracted from V.
parahaemolyticus [14] and subjected to the
subsequent fermentation. SSF of E. intestinalis and
U. lactuca using CY strain produced ethanol of 0.12g/L
and 0.08g/L achieving conversion efficiencies of 45.5% and 48.7%
(Table 5). Higher conversion efficiency of 80.9% was achieved
for U. lactuca followed by 65.2% for E.
intestinalis biomass using TY yeast strain indicating
its thermo-tolerance capabilities. Similarly, Kluyveromyces
marxianus was recognized as a safe (GRAS)
thermo-tolerant yeast strain with tolerance range of 38-45oC and
producing high ethanol concentration SSF process [66].
Higher sugar conversion efficiency by these non-domesticated
(“wild”) strains Pichia kudriavzevii and Meyerozyma
caribbica indicate potential to be used at industrial
level, with strain improvement through experimental evolution,
hybridization, or genetic engineering.
Table. 4. Separate
Hydrolysis and Fermentation of dilute acid hydrolysis of
macroalgal biomass |
Seaweed
hydrolysate |
Biomass
(g) |
Acid pretreatment |
Yeast strain and fermentation
Process condition |
Initial sugar
(g) |
Fermented sugar
(g) |
Ethanol (g) |
Theoretical yield |
%Conversion efficiency |
E. intestinalis |
5 |
0.7N
H2SO4, 121oC for 45min
|
CY (35oC, 100rpm for 24h) |
1.12±0.03 |
0.58±0.03 |
0.14±0.02 |
0.30 |
46.9 |
TY (35oC, 100rpm for 24h) |
0.61±0.01 |
0.16±0.02 |
0.31 |
51.8 |
CY & TY (35oC, 100rpm for
24h) |
0.48±0.08 |
0.08±0.01 |
0.25 |
33.0 |
U. lactuca |
0.5N
H2SO4, 121oC for 45min
|
CY (35oC, 100rpm for 24h)
|
0.96±0.07 |
0.30±0.07 |
0.03±0.01 |
0.15 |
24.0 |
TY (35oC, 100rpm for 24h)
|
0.20±0.04 |
0.05±0.01 |
0.11 |
48.7 |
CY & TY (35oC, 100rpm for
24h) |
|
0.30±0.01 |
0.06±.01 |
0.16 |
40.4 |
Effect of salt on ethanol production
Marine yeast is utilized in several applications as they thrive
in harsh conditions hence tolerate higher process conditions
(salinity and temperature) [70]. In the bioethanol process,
pretreatment using dilute acid hydrolysis of marine macroalgal
biomass results in salty hydrolysate, which requires a
desalination process when employing terrestrial yeast strains,
but in the case of halotolerant yeast strains, the hydrolysate
is directly fermented to bioethanol.
Table. 5. Simultaneous
Saccharification and Fermentation of acid pre-treated
macroalgal biomass |
Seaweed
hydrolysate |
Biomass
(g) |
Acid pretreatment |
Enzyme and
Yeast strain
Fermentation process condition |
Initial sugar (g) |
Fermented sugar
(g) |
Ethanol
(g) |
Theoretical yield |
%Conversion efficiency |
E. intestinalis
|
2 |
0.7N
H2SO4, 121oC for 45min
|
S9 &CY (55oC 100rpm
for 24h) |
0.87±0.08 |
0.53±0.02 |
0.12±0.02 |
0.27 |
45.48 |
S9 & TY (55oC 100rpm for 24h) |
0.31±0.02 |
0.10±0..01 |
0.16 |
65.19 |
S9, CY &TY (55oC 100rpm for 24h)
|
0.33±0.01 |
0.06±0.01 |
0.17 |
39.04 |
U. lactuca
|
0.5N
H2SO4, 121oC for 45min
|
S9 &CY (55oC, 100rpm for 24h)
|
0.86±0.01 |
0.33±0.04 |
0.08±0.01 |
0.17 |
48.74 |
S9 & TY (55oC 100rpm for 24h) |
0.34±0.01 |
0.14±0.02 |
0.18 |
80.94 |
S9, CY &TY (55oC 100rpm for 24h)
|
0.21±0.02 |
0.04±0.01 |
0.11 |
44.67 |
CY: Cashew yeast (P. caribbica); TY:
Toddy yeast (P. kudriavzevii); S9: V.
parahaemolyticus |
Several industrial
applications have utilized salt-tolerant yeast strains such as
Debaryomyces hansenii and Zygosaccharomyces
rouxii, but not all the yeast have the ability
to tolerate high salt conditions. However, in this study, it was
seen that CY strain has consistent ethanol production till 4%
salt concentration, produced the highest ethanol of 2.6g/L from
5.95 g/L of reducing sugar achieving 88.8% fermentation
efficiency and reduced at 5% and 6% salt concentration along
with biomass. Similar results of luxuriant growth was observed
for Candida sp. isolated from a marine source in the
presence of 2-13% salt, which subsequently decreased at 14 and
15% salt [27]. Dabaryomyces, Rhodotorula Candida and
Saccharomyces exhibit tolerance to NaCl ranging from
0-16% [71]. The TY strain (Fig 3b) biomass gradually
decreased with the increase in salt concentration and
intermittent ethanol production. Highest
ethanol of 2.5g/L from 11.94g/L fermented sugar was obtained at
3% salt concentration achieving 41.45% fermentation efficiency.
At 5 and 6%, sugars were left unutilized by TY strain.
Screening of cellulolytic yeast and ethanol production
by CBP
A single strain of microorganisms that expresses cellulolytic
activity and fermentation capabilities is of potential interest
in bioethanol production as it brings down the economic burden
of enzyme production and the overall bioethanol production and
is regarded as the low-cost biomass processing [72, 73]. In this
study, prioritized strains were isolated on plates comprising 1%
CMC as the sole source of carbon (Fig 4). Hydrolytic activity
was recorded for both strains CY: 2.06 and TY: 2.69. Enzyme
activity of 1.15 U/ml and 1.19 U/ml was recorded for M.
caribbica and P. kudriavzevii at 24h
respectively (Table 6).
.png) Fig. 3 a Fig. 3 a Efect of diferent concentrations of salt on CY strain
growth and ethanol production
(RS, reducing sugar). b Efect
of diferent concentrations of
salt on TY strain growth and ethanol production (RS, reducing sugar) In CBP, cellulase production, cellulose hydrolysis, and
fermentation of subsequent sugar occur in a single reactor by a
single microbial community [74] compared to SSF. The advantage
of CBP is lower or zero capital costs for enzyme production and
compatibility of enzymatic and fermentation processes. Anaerobic
bacteria have been tested for ethanol production via CBP.
However, lower ethanol tolerance (<2%) of bacteria is a
limitation for its application at an industrial scale. In this
study, wild ethanologenic yeast strains M. caribbica
and P. kudriavzevii were used to ferment the
pre-treated macroalgal biomass E. intestinalis and U.
lactuca (Table 7). Higher ethanol conversion efficiency
was recorded for P. kudriavzevii fermenting E.
intestinalis and U. lactuca compared to the
conversion efficiency achieved through SSF process.
K.marxianus PT -1 isolated from grape fermented
Jerusalem artichoke tuber flour consisting of inulin at
40oC for 48h and achieved 90% conversion efficiency
through CBP [39]. CBP was carried out for brown algae Saccharina
japonica using engineered E.coli (BAL1611)
bacteria for 150h and obtained 4.7% ethanol [75].
SEM analysis was carried out for macroalgal biomass after CBP
process, the initial dilute-acid pretreatment provided surface
area for the yeast cells to attach and secret enzymes to degrade
the biomass. Biomass was disintegrated after CBP process
indicating the cellulolytic yeast activity (Fig 5).
CBP- compatible microorganism extensively studied is S.cerevisiae;
however, it is not suitable while employing second-generation
feedstock as it only yields higher ethanol from hexose and not
from pentose sugar [72]. Therefore, there is a need to explore
wilder ethanologenic yeast strains that exhibit higher
cellulolytic activity and be employed for CBP of
third-generation macroalgal feedstock and second-generation
feedstock.
Table 6. Enzyme activity
of yeast strains at 24h |
Yeast Strain |
Protein (mg) |
Total activity (U/ml) |
Specific activity (U/mg) |
Meyerozyma caribbica, CY |
5.23 |
1.15 |
0.22 |
Pichia kudriavzevii, TY |
5.73 |
1.19 |
0.21 |
Table 7. Fermentation
of pre-treated macroalgal biomass using cellulolytic
yeast strain through CBP |
Seaweed |
Acid Pretreatment |
Fermentation |
Initial sugar
g |
Fermented sugar
g |
Ethanol g |
Theoretical yield |
%Conversion efficiency |
E. intestinalis |
0.7N H2SO4,
121oC for 45min |
CY (35oC, 100rpm for 72h) |
1.07±0.09 |
0.56±0.02 |
0.12 |
0.29 |
43.06 |
TY (35oC, 100rpm for 72h) |
0.19±0.01 |
0.07 |
0.10 |
74.14 |
U. lactuca |
0.5N H2SO4,
121oC for 45min |
CY (35oC, 100rpm for 72h) |
1.51±0.09 |
0.95±0.02 |
0.32 |
0.49 |
64.22 |
TY (35oC, 100rpm for 72h) |
0.94±0.01 |
0.46 |
0.49 |
94.84 |
Fig 5. SEM micrographs of the
interaction between cellulolytic yeast strains Meyerozyma
caribbica on (A) E.intestinalis and
(B) U.lactuca, Pichia kudriavzevii on (C) E.intestinalis
and (D) U.lactuca
|