|
 |
 |
 |
 |
|
T. V. Ramachandra Durga Madhab Mahapatra Karthick B. Citation: Ramachandra T V., Durga Madhab Mahapatra, Karthick B., Richard Gordon, 2009. Milking Diatoms for Sustainable Energy: Biochemical Engineering vs Gasoline Secreting Diatom Solar Panels, Ind. Eng. Chem. Res., Article ASAP, 10.1021/ie900044j |
| Abstract | |
 |
|
In the face of increasing CO2 emissions from conventional energy (gasoline), and the anticipated scarcity of crude oil, a worldwide effort is underway for cost-effective renewable alternative energy sources. Here, we review a simple line of reasoning: (a) geologists claim that much crude oil comes from diatoms; (b) diatoms do indeed make oil; (c) agriculturists claim that diatoms could make 10-200 times as much oil per hectare as oil seeds; and (d) therefore, sustainable energy could be made from diatoms. In this communication, we propose ways of harvesting oil from diatoms, using biochemical engineering and also a new solar panel approach that utilizes genomically modifiable aspects of diatom biology, offering the prospect of “milking” diatoms for sustainable energy by altering them to actively secrete oil products. Secretion by and milking of diatoms may provide a way around the puzzle of how to make algae that both grow quickly and have a very high oil content.
Keywords. sustainable energy, gasoline, diatoms, diatom nanotechnology, petroleum, solar energy, diatom solar panel, secretion, exocytosis
Briefs. Three methods are proposed for producing gasoline from diatoms, including genetically engineered secretion from leaf-like solar panels.
| Introduction: Diatoms as an Energy Source | |
 |
|
The recent soaring and crashing of oil prices and diminishing world oil reserves, coupled with enhanced greenhouse gases and the predicted threat of climate change, have generated renewed interest in using algae as alternative and renewable feedstock for energy production.(1-3) In fact, diatoms, which are single cell algae with silica shells,(4) may have created much of the purported global warming crisis by providing us with a convenient fossil source of energy in the form of much of the crude oil used to produce gasoline:(5, 6)
“The main primary producers within the phytoplankton today — in terms of net production — [and]... contributors to sedimentary organic matter... are the diatoms...”(7). |
Therefore, living diatoms may also point the way to a sustainable source of oil. Diatoms have become central to a new direction in nanotechnology in which we grow and harvest them for their hard silica parts, with the result that our knowledge of diatoms is increasing rapidly.(8-10) In our consideration of the question of exploiting diatoms for their oil, we will apply some of the concepts that are coming out of this new field of “diatom nanotechnology”.(4, 8-17)
The transparent diatom silica shell consists of a pair of frustules and a varying number of girdle bands(18, 19) that both protect and constrain the size of the oil droplets within, and capture the light needed for their biosynthesis.(20) We propose three methods: (a) biochemical engineering, to extract oil from diatoms and process it into gasoline; (b) a multiscale nanostructured leaf-like panel, using live diatoms genetically engineered to secrete oil (as accomplished by mammalian milk ducts), which is then processed into gasoline; and (c) the use of such a panel with diatoms that produce gasoline directly. The latter could be thought of as a solar panel that converts photons to gasoline rather than electricity or heat.
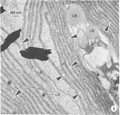
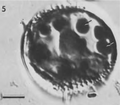
Over the past few decades, several thousand species of algae including diatoms have been screened for high lipid content.(1, 21-23) It was found that, on average, polyunsaturated fatty acid, which has a lower melting point than saturated fats,(24) constitutes 25% of algal mass. This content may vary noticeably between species, and, interestingly, the lipid content increases considerably (double or triples) when cells are subjected to unfavorable culture conditions, such as photo-oxidative stress or nutrient starvation.(25) This is due to the shift in lipid metabolism from membrane lipid synthesis to the storage of neutral lipids.(25) One lipid oil drop bearing a pennate diatom(26) (see Figure 1) has outwitted us by its extraordinary ability to proliferate; however, after we understand its secrets, we may be able to put it to work for us:
“The diatom Didymosphenia geminata (Bacillariophyceae) has garnered increased attention as a nuisance and invasive species in freshwater systems. Historically described as rare yet cosmopolitan, a suspected new variant of D. geminata has the capacity to inundate kilometres of river bottom during a bloom. Unlike most other bloom-forming algae, D. geminata proliferates under high water quality (i.e., low turbidity and low nutrient) conditions”.(27) |
|
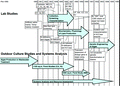
Physiological and genetic manipulations of diatoms have the potential to bring the concept of “diatoms for oil”(23, 25, 28, 29) closer to commercial reality. Indeed, genetic transformation of diatoms started with the goal of improving oil production(25, 30-32) (see Figure 2), and it may be time to resume this line of research with the large range of genomics tools being developed for diatoms.(33-44)
|
Agricultural oil crops, such as soybean and oil palm, are being used widely to produce biofuel; however, the amounts produced are <5% of the plants’ biomass. They also require high cropping area and extensive cultivation, with considerable concern about environmental impact and competition with food production and water use.(22, 45-48) Based on the photosynthetic efficiency and the growth potential of algae, theoretical calculations suggest that an annual oil production of >30 000 L (or
000 L (or  200 barrels) of algal oil per hectare of land may be achievable in mass culture of oleaginous algae, which is 100-200 times greater than that of soybeans.(21, 23) Estimates vary:
200 barrels) of algal oil per hectare of land may be achievable in mass culture of oleaginous algae, which is 100-200 times greater than that of soybeans.(21, 23) Estimates vary:
“The per unit area yield of oil from algae is estimated to be between 5000 and 20,000 gallons per acre [56 |
Diatoms, unlike other oil crops, grow extremely rapidly,(50) and some can double their biomass within 5 h(51) to 24 h.(52) Even “in the wild”, doubling times can be 2-10 days,(53, 54) which includes photosynthesis and photorespiration periods. Diatoms have been regarded as C3 photosynthesizers, and their photosynthetic efficiency is enhanced by concentrating CO2 around Rubisco, diminishing photorespiration. It is estimated that diatoms are responsible for up to 25% of global CO2 fixation.(9) Benthic diatoms, under favorable conditions, migrate toward sunlight and bloom in the presence of ample nutrients.(55) Similarly, during unfavorable conditions they sink downward, which constitutes the bulk of the organic flux. In addition, continuous upwelling (56) also replenishes nutrients, which end up in the next round of diatom blooms. Each diatom cell creates and then uses its own gas tank, so to speak, so perhaps diatoms could eventually keep our gas tanks full, and, of course, reabsorb CO2 in the process.
Diatoms may have a major role to play in the coming years, with regard to the mass production of oil. This entails appropriate cultivation and extraction of oil, using advanced technologies that mimic the natural process while cutting down the time period involved in oil formation. Here, we consider a simple line of reasoning:
(1) Geologists claim that much crude oil comes from diatoms.(57, 58)
(2) Diatoms do indeed make oil.
(3) Agriculturists claim that diatoms make 10 times as much oil per hectare as oil seeds,(59) with theoretical estimates reaching 200 times.(21, 23)
(4) Therefore, sustainable energy could be made from diatoms.
(5) We may be able to get diatoms to secrete their oil, perhaps even as gasoline, and therefore milk them, as we do cows.
We will review the evidence for these statements that is in the public domain. While some companies are forming to produce oil from unspecified “algae”,(60,61) diatoms have received scant mention,(62, 63) except by some hobbyists.(64) What we leave for a future study is a critical comparison of diatoms with other energy alternatives, such as nondiatom algae (cf. refs 23 and 65), other biofuels, solar, wind, tidal, geothermal, hydrogen, hydroelectric and nuclear power. It is possible that, unlike crop biofuels, diatoms would not compete with food crops(66) for arable land.
Clearly, if diatoms could be used to make gasoline, then we could continue using our gasoline-based motor vehicles without a major change in technology or our way of life. The private automobile becomes a sustainable proposition. We could continue to use the combustion engine, which would then remain a major competitor to other propulsion technologies. It sounds like an easy resolution to the current situation, a way to “have our cake and eat it too”. Thus, in this regard, diatoms are worthy of serious consideration. Let us see what we find, beyond a recent review:
“The characteristics of algal oil are similar to those of fish and vegetable oils, and can thus be considered as potential substitutes for the product of fossil oil. In the late 1940s, lipid fractions as high as 70-85% on a dry weight basis [were] ... reported in microalgae .... Nevertheless, only a few authors have reported lipid valorisation as biodiesel using diatoms with Hantzschia DI-60 (67) and with Chaetoceros muelleri.(68) A maximum yield of 400 mg total lipid L-1 in nitrogen-replete cultures was obtained. In the aim to produce biodiesel from microalgae, Cyclotella cryptica and Navicula saprophila were genetically manipulated (25) to optimise lipid production”.(69) |
The optimization of resource production via genetic manipulation seems to be appropriate for making diatom biotechnology viable and economically lucrative.
| Does Crude Oil Come from Diatoms? | |
 |
|
The recent genetic(70) and sedimentary(71) evidence suggests an origin of diatoms in the early Jurassic period, 185 million years ago.(70) Medlin et al. have suggested that their origin may be related to the end-Permian mass extinction, after which many marine niches were opened.(72)
The basic line of reasoning of geologists in attributing crude oil to diatoms is that they comprise the bulk of the ocean phytoplankton, so they must be a major source of the oil.(73-75) This view must be tempered by a comparison of the geological record of diatoms with the estimated ages of oil deposits. Clearly, those that preceded diatoms could not have been generated by them,(1) and, indeed, a wide variety of prehistoric organisms are suggested as sources of crude oil.(7) The pattern of fatty acids in recent sediments matches that of diatoms,(76) and the 24-norcholestane biomarkers are indicators of diatom-formed oil,(77, 78) as are many others.(79-84)
The notion that diatoms are major contributors to crude oil deposits has been around since 1839,(85-90) with evidence put forth(91) and then denied.(92) The upshot is that this period left us with uncertainty in the matter:
“The abundance of diatoms in parts of the Monterey formation has suggested that these organisms may have been the source of much of the oil of the formation. Tolman(89) has emphasized this view, and he had the charge of a research project of the American Petroleum Institute for the investigation of diatoms as a source of oil, but a final report on this work has not appeared”.(93) |
However, recent work seems to have firmly reinstated this hypothesis, based on the organic molecules (biomarkers) with over 20 C atoms that are often unique to diatoms.(76-84, 94, 95) Nevertheless, this may not account for the bulk of diatom oil, because many of these molecules may be membrane components,(95, 96) rather than the bulk fluid inside intracellular oil droplets. Indeed, “storage components, such as neutral lipids, and membrane-associated structural components, such as glycolipids and phospholipids, varied independently”.(97) Stratigraphic correlations of oil with diatoms are strong:(98)
“... diatomaceous sediments may themselves be important sources for petroleum,(99, 58) and there is now an “overwhelming consensus in the literature that the OM [organic matter] in the biosiliceous unit of the MF [Monterey Formation of California] is of marine origin”.(100) |
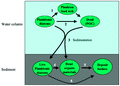
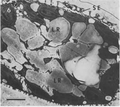
Despite all the evidence that diatoms are major contributors to crude oil formation, doubts continue with language such as: “A number of organic-rich sediments occur in the sedimentary record which contain abundant organic matter presumed to be of diatom origin”.(101) The root cause of this has been attributed to authoritarian pronouncements,(89) which may continue through our education system. On the other hand, a definitive story that both makes a definitive analysis of the oil in diatoms and evaluates the contribution of diatom oil, modified or not, to the worldwide petroleum reserves, apparently has not been written yet, although diatom biomarkers (24-norcholestanes) have been found in 109 crude oil basins.(77) Two recent models for the role of diatoms in producing sedimentary organic matter are shown in Figures 3 and 4, the latter of which indicates live diatoms in the sediment. What should be added to these flow diagrams is the upwelling(102-104) that leads to “the return of favorable conditions” for germination of the spores,(105) perhaps triggered by changes in day length,(103) at least of neritic diatoms. Also missing are the obligate benthic diatoms(106) and the possibility that some sinking diatoms or spores stop at a pycnocline.(107)
|
|
Perhaps the best “smoking gun” is the presence of oil inside fossil diatoms in sediments (see Figure 5):
“Based on experimental correlations between fluorescence microspectrometry and crude oils of known gross chemical composition (i.e., saturates, aromatics and resin-asphaltenes(108)), the ‘pure’ diatom oil contains an estimated 60-70% saturates. The diatom oils are likely to be rich in fatty acids, which during early diagenesis, reportedly transform into condensed lipids.(101, 109, 110) |
|
Nevertheless, the sedimentation of organic matter with diatom valves may be species- and season-dependent.(111) In any case, to date, no one has directly compared the oil in droplets inside fossil versus living diatoms, to determine how much diagenetic change, if any, has occurred. This could be tested in the laboratory, where diagenesis of the silica occurs within two years.(112)
Some of the contradictions may be resolved by noting that, although much of the organic material is reprocessed as it sediments down through the water column, (113, 114) “... most of the unsaturated [fatty] acids will be deposited directly into the sediment when the diatoms die, and hence the acids will largely escape the aerobic conditions at the surface. Preservation is then aided by the anaerobic conditions below the surface”.(76) Approximately 1% of the living diatoms in the ocean contribute to the sediment below, where the frustules travel downward live(115) and land mostly intact.(116) The freshest arrivals(117,118) include the cell contents, protected by two valves, consisting of live vegetative and spore cells (recall Figure 4). Perhaps even dead diatoms protect their oil contents from bacteria. Despite the darkness of the benthic zone, “...
most of the unsaturated [fatty] acids will be deposited directly into the sediment when the diatoms die, and hence the acids will largely escape the aerobic conditions at the surface. Preservation is then aided by the anaerobic conditions below the surface”.(76) Approximately 1% of the living diatoms in the ocean contribute to the sediment below, where the frustules travel downward live(115) and land mostly intact.(116) The freshest arrivals(117,118) include the cell contents, protected by two valves, consisting of live vegetative and spore cells (recall Figure 4). Perhaps even dead diatoms protect their oil contents from bacteria. Despite the darkness of the benthic zone, “... diatoms to a much greater extent than other phytoplankton groups escape degradation by heterotrophs [and]
diatoms to a much greater extent than other phytoplankton groups escape degradation by heterotrophs [and] ... to a greater extent than other groups sink out of the productive zone to the bottom
... to a greater extent than other groups sink out of the productive zone to the bottom ... particularly
... particularly ... in shallow boreal areas”.(119) After a “bloom, a large proportion of diatoms sink to the benthos” as spores(120) (although spores of some species sink more slowly than vegetative cells(107)). Here,(121) diatoms survive ingestion by pelagic deposit feeders (or maybe only diatom spores survive(107)), which, perhaps, is testament to their mechanical strength,(122) and “...
... in shallow boreal areas”.(119) After a “bloom, a large proportion of diatoms sink to the benthos” as spores(120) (although spores of some species sink more slowly than vegetative cells(107)). Here,(121) diatoms survive ingestion by pelagic deposit feeders (or maybe only diatom spores survive(107)), which, perhaps, is testament to their mechanical strength,(122) and “... some cells survive several years(123-125, 126) especially at low temperatures.(127) As the sediment piles up on the diatoms, the pressure and time gradually transform the silica(128) until the diatom structure is no longer recognizable.(116, 129, 130) However, the record survival time under the accumulating sediment has not yet been established:
some cells survive several years(123-125, 126) especially at low temperatures.(127) As the sediment piles up on the diatoms, the pressure and time gradually transform the silica(128) until the diatom structure is no longer recognizable.(116, 129, 130) However, the record survival time under the accumulating sediment has not yet been established:
“In freshwater systems, Aulacoseira granulata resting cells that had been in anoxic sediments for 20 years were still able to germinate,(131) and other species have been found to survive in sediments from one to many decades(132, 133) [cf. ref 134]. Marine species have been germinated from depths of up to 50 m in sediments,(135) but the amount of time these cells had been buried is uncertain”.(136) |
These long-term survivors rapidly form storage products upon rejuvenation(131) and survive through heterotrophy.(137, 138) Some nonphotosynthetic diatoms are obligately heterotrophic.(139)
The selective loss of unsaturated lipids during simulated diagenesis(114, 140) suggests that these lower-melting-point organics would best be recovered from fresh or live diatoms. In any case, the inefficiency of natural sedimentation in capturing diatom oil is apparent,(130) and if we attribute substantial crude oil deposits to diatoms, it is clear that living diatoms have even more to offer. The live diatom cells at the bottom of the sea may survive on their stored oil and/or may be heterotrophic, if they are not in suspended animation. Diatom spores contain oil droplets (“more or larger vesicles of storage product(107, 141, 136)), which is consistent with the idea that their “primary role is probably related to nutrient stress”.(127) Up to 92% of cells form spores.(127) If, as suggested, (127) the survival time of spores is inversely related to temperature, because metabolism slows, then we would propose that death occurs after the reserve of oil inside spores is depleted via respiration,(107) perhaps delayed by an ability of diatoms to photosynthesize efficiently at low light levels.(106, 142) Metabolism under the high pressure of a water column may also be altered.(143)
| Why Do Diatoms Make Oil Droplets? | |
 |
|
It would have seemed obvious that the function of oil droplets in diatoms would be first and foremost to counter the weight of the dense silica shell (for Si/C mole ratios up to 0.81(144)) and provide buoyancy:
“The specific gravity of diatoms may also be lessened by the presence of an oil in the cells. Oil has been noted to accumulate in diatoms late in the vegetative period and, in addition to providing a food reserve, may also result in keeping them afloat while waiting return of conditions favorable for multiplication”.(145) |
However, this simple hypothesis is not the prevailing one for buoyancy, which suggests that ionic pumps instead somehow maintain buoyancy.(146, 147) Apart from that, extracellular secretions and the incorporation of the diatom cells in suspended organic matter may govern their buoyancy. Earlier, there were explanations invoking the importance of the carbohydrate pool as reserve products in light dark cycles.(148)
Indeed, nitrate depletion, which causes diatoms to increase oil production,(149-152) changes one species, Thalassiosira weissflogii, from neutrally buoyant to sinking,(153) and another to enter a faster sinking sexual phase,(154) so the correlation between oil and buoyancy is not so clear. Therefore, while auxospore formation is correlated with oil production,(110, 155) some auxospore sinks nevertheless.(146, 154) It may be that some diatoms respond by crenation(146) and others by oil droplet formation, or both occur, during auxospore formation. The problem here is that the oil content has never been quantified and compared to the mass and density of the other components (silica shell, cytoplasm, nucleus, vacuoles, and attached organic coat and extracellular material) for any diatom.
The puzzle was stated succinctly 4 decades ago, and it still seems to be unsolved:
“The nature of the problem is easily grasped. The specific weight of a diatom test [silica shell] has been reported to be 2.07, and that of protoplasm to range from 1.02 to 1.15.(156) The specific weight of seawater usually ranges from 1.020 to 1.028. It is evident that diatoms are heavier than seawater and will ordinarily sink rapidly unless adaptations to minimize the high frustule [shell] and protoplasmic weight load occur... |
The mean density of a diatom is the weighted sum of the densities of its components, where the mathematical weights are the volumes of the components. Whether it floats or sinks is dependent only on the difference of this mean density from that of the surrounding water (unless it is attached to material of a different buoyancy). Large marine diatoms can be positively or negatively buoyant.(158-161) Although shape, including spines and colony formation, can influence the rate of sinking, these features have no influence whatsoever on the direction (sink or float), unless they ensnare material of different mean density.
Again, one would suppose that the oil in diatoms might be a food store. However:
“Catabolism rate of lipids does not seem to be very important: 1 to 2% h-1, respectively, for the light and dark periods. On the other hand, significantly higher rates of catabolism of polysaccharides are found for both the light [20% h-1] and dark periods [8% h-1]. This... suggests that reserve products of this diatom population are mainly composed of polysaccharides”.(162) |
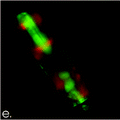
Perhaps the correct conclusion is that oil droplets assist the long-term survival of poor environmental conditions, whereas polysaccharides are for daily fare. However, polysaccharides are also secreted for microenvironment alteration,(163, 164) adhesion,(165) colony support,(166, 167) motility,(168-170) and possibly antiviral activity.(171) While spores generate their own substantial quantities of oil (see Figures 6 and 7),(172) so do some vegetative cells (see Figures 8 and 9), with “the oil droplets comprising most of the cell volume”.(173)
|
|
|
However, the “reason” an organism does something can be quite different from our initial guesses:
“Laboratory studies showed that polar diatoms |
Finally, we must keep in mind that oil production is far more ancient than diatoms:
“All plastid-containing organisms examined so far carry out de novo biosynthesis of fatty acids within the plastid via the type II fatty acyl synthase.(175) Diatoms can contain large proportions of polyunsaturated fatty acids. Armbrust et al.(176) identified a complete pathway for polyunsaturated fatty acid biosynthesis. After export from the plastid, lipid or oil droplets often accumulate in the vacuoles or cytoplasm under nutrient-limited conditions”.(174) |
| What Do We Know about Diatom Oil? | |
 |
|
Even if diatoms could produce enough oil (177) to satiate our drive for transportation, is it the right type of oil, or would it require much chemical processing before we would want to burn it? Microalgae generally are a potential source of energy, because the energy-rich storage lipid product of these plants is among the more useful natural products for conversion to alternate energy such as gasoline and diesel fuel,(178) besides being extremely efficient biomass producers with high photosynthetic efficiencies of 12%-16% and possessing often 50%-60% of their biomass weight in the form of lipids.(178-182) Lipid fractions as high as 70%-85% (on a dry weight basis) in microalgae have been reported.(177)
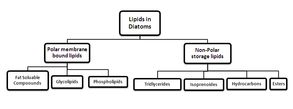
Certain microalgae cultures could result in higher quantities of storage lipids, such as triglycerides.(183) Diatoms have been regarded as useful neutral lipid sources of liquid-fuel precursors.(184) Besides high lipid and fatty acid content, there is an abundance of eicosapentaenoic acid (polyunsaturated fatty acids (PUFAs)) in diatoms.(185) Nitzschia laevis is a potential producer of eicosapentaenoic acid, as shown by extracting the lipid and analyzing it via thin layer chromatography (TLC) and gas chromatography (GC).(186) The lipids present are neutral lipids (accounting for  75%), glycolipids, and phospholipids (see Figure 10). Fatty acids that dominate the organisms include tetradecanoic acid, hexadecanoic acid, and palmiloteleic acid.(180)
75%), glycolipids, and phospholipids (see Figure 10). Fatty acids that dominate the organisms include tetradecanoic acid, hexadecanoic acid, and palmiloteleic acid.(180)
|
Diatoms of genera Haslea and Rhizosolenia biosynthesize a series of C25 highly branched isoprenoid (HBI) alkenes or haslenes, which evolved  90-146 million years ago in the Cretaceous period.(187-189) Isoprenoid-derived alkenes and alkanes are important fuel sources that are found in a wide variety of recent and ancient sediments and in some crude oils.(94, 190) Many of the C25 (haslene) and C30 (rhizene) alkenes are biosynthesized by a restricted number of diatom genera, particularly some species of Haslea, Rhizosolenia, Pleurosigma, and Navicula.(187, 190-194) In H. ostrearia, highly branched isoprenoid alkene (haslene) biosynthesis proceeds even under axenic conditions, indicating de novo biosynthesis.(195) Interestingly, HBI biosynthesis seems to have evolved at least twice in geological time in separate diatom genera.(190) The C26 24-norcholestanes(77) mark an increase in diatoms in the Oligocene-Miocene.(78) The spectrum of fatty acid and related molecules reported in diatoms is given in Table 1.
90-146 million years ago in the Cretaceous period.(187-189) Isoprenoid-derived alkenes and alkanes are important fuel sources that are found in a wide variety of recent and ancient sediments and in some crude oils.(94, 190) Many of the C25 (haslene) and C30 (rhizene) alkenes are biosynthesized by a restricted number of diatom genera, particularly some species of Haslea, Rhizosolenia, Pleurosigma, and Navicula.(187, 190-194) In H. ostrearia, highly branched isoprenoid alkene (haslene) biosynthesis proceeds even under axenic conditions, indicating de novo biosynthesis.(195) Interestingly, HBI biosynthesis seems to have evolved at least twice in geological time in separate diatom genera.(190) The C26 24-norcholestanes(77) mark an increase in diatoms in the Oligocene-Miocene.(78) The spectrum of fatty acid and related molecules reported in diatoms is given in Table 1.
|
Table 1. Fatty Acids and Related Organic Molecules Reported in Diatoms
a Blank lines mean no instances were found in our literature survey.
|
| How Much Oil Do Diatoms Produce? | |
 |
|
Diatoms would seem to fare, in average dry weight that they can synthesize as lipids, only a little better than green algae (24.5% vs 17.1%), although their average dry weight is enhanced by a factor of 2 to 3 by nitrogen deprivation.(65) However, the dry weight of silica in diatoms averages 60%.(227) Therefore, the corrected dry weight percentage of organic material in diatoms that is lipids is 24.5%/40% = 61%. This compares favorably to the record-holding nonsiliceous alga Botryococcus braunii (70%).(65) If we take the “stressed” lipid content of 44.6%, we end up with the apparent contradiction of >100% lipid organic matter,(23) which means that we cannot compute using cross-species averages. Nevertheless, this suggests that if we discount the silica shells, some diatoms may be far more productive for oil than other organisms.
Let us compare these calculations to actual observations:
“Living diatom plants will always be found to contain from two to ten shining oil globules. The bulk of this oil, in proportion to the size of the diatom, rarely falls below 5 percent; and the author has samples of diatom material in which a careful measurement of the contained oil shows a proportion of 50 percent”.(228) |
“... |
Oil droplets with diameters of 100-200 µm have been observed in and near chloroplasts in some diatoms, which suggests that they are synthesized within the chloroplasts.(26, 231-233) In the stationary phase, because of nitrogen depletion (but cf. ref 234) “the oil droplets could be clearly seen filling nearly the whole cell”, whereas the resumption of exponential growth led to a reduction in fats.(150) The source of nitrogen may matter, because, for Ditylum brightwellii, growth in the nitrate almost doubled lipid production, compared to ammonia, perhaps because “The most striking aspect was the use of nearly all ammonia before any nitrate was assimilated”.(235) Perhaps nitrate without ammonia present is a trigger for impending nitrogen depletion? On the other hand, a “... dramatic synthesis of lipid in the waters around Antarctica
dramatic synthesis of lipid in the waters around Antarctica ... [with] no evidence for nitrogen deficiency” has been observed.(236) The factors involved may be decreased temperature and irradiance, accompanied by increased salinity,(172, 236, 237) i.e., there may be multiple stimuli to lipid synthesis. Combining all these ups and downs of oil production versus nitrogen supply into a rational physiology is a task for the future:
... [with] no evidence for nitrogen deficiency” has been observed.(236) The factors involved may be decreased temperature and irradiance, accompanied by increased salinity,(172, 236, 237) i.e., there may be multiple stimuli to lipid synthesis. Combining all these ups and downs of oil production versus nitrogen supply into a rational physiology is a task for the future:
“A major research challenge is to conceptually integrate photorespiratory metabolism in diatoms into the framework of overall cellular energy balance, stress management, and C and N status and turnover”.(238) |
| Industrial Processing of Diatom Oil | |
 |
|
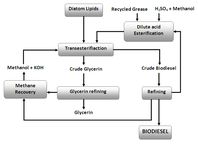
Lipid valorization as biodiesel using diatoms was reported with Hantzschia DI-60(67) and Chaetoceros muelleri.(68) The production of fuel (diesel, gasoline) through the transesterification and catalytic cracking of lipids accumulated in algal cells has been reported,(239) including diatoms.(28) The main raw material for diatom-based biodiesel is the enormous range of triglycerides (monoglycerides, diglycerides, and triglycerides), which are indeed compounds of fatty acids and glycerol. In the transesterification process, an alcohol (such as methanol) reacts with the triglyceride oils that are contained in diatom fats, forming fatty acid alkyl esters (biodiesel) and glycerin.(69)
Fuel oil formation can be achieved by base-catalyzed transesterification, acid-catalyzed transesterification (with simultaneous esterification of free fatty acids), and noncatalytic conversion via transesterification and esterification under supercritical alcohol conditions(240-243). The main process that can be used for biodiesel production from organisms is base-catalyzed transesterification.(244) The reaction requires heat and a strong base catalyst, such as sodium hydroxide or potassium hydroxide. The simplified transesterification reaction is depicted in Figure 11:
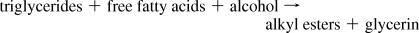
This chemical process converts the triglycerides (TGs) found in plants, microalgae, and animal fats to fatty acid methyl esters (FAMEs) in a multistep synthesis, with glycerol being liberated as a byproduct.(240, 245) More recently, it has been demonstrated that supercritical alcohol(246, 247) can esterify free fatty acids and transesterify triglycerides simultaneously with virtually no sensitivity to water content. A major advantage is that simultaneous esterification and transesterification makes use of diatoms, with elevated levels of free fatty acids,(248, 249) in less time.
|
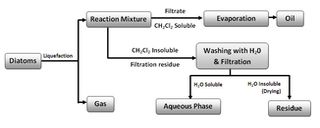
Liquefaction of algal diatom biomass can be accomplished by natural, direct and indirect thermal, and fermentation methods. Liquid hydrocarbon yields of close to 50% have been obtained using a very-high-temperature, high-pressure, catalyzed hydrogenation process. Reacting organic diatom biomass with near-critical (320-390 °C, 200-420 bar) or supercritical (400-500 °C, 400-550 bar) aqueous phases can altogether transform the organic compounds over short time periods (time frames from minutes to hours). The reductive process is conducted under anaerobic or near-anaerobic conditions. The lipids can be converted to a hydrocarbon mixture that is similar to crude petroleum, along with volatile alkane and alkene gases (C2-C5). This conversion allows the generation of a burnable fuel of very high calorific value.(250) Kerogens are converted to an oily substance under conditions of high pressure and temperature.(251-253) Besides n-alkanes, aromatics, napthenes, and alkyl benzenes(5) were found in the hydrocarbons produced from kerogen. The production of diesel fuel and gasoline through the transesterification and catalytic cracking of lipids accumulated in algal cells has been reported,(239) as has the production of energy from diatom biomass through fermentation and also via thermochemical liquefaction.(254)
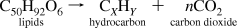
From this, it is evident that the direct extraction of diatom lipids is a more-efficient method for obtaining energy than fermentation. This can occur by having solvents such as CH2Cl2 (dichloromethane), through the direct expression of the liquid lipids, or a combination of both methods (see Figure 12). The thermochemical liquefaction process often results in a heavy oily or tarry material that is then separated into different fractions by catalytic cracking. As with hydrocarbons derived from other forms of renewable biomass, microalgal diatom lipids can be converted to suitable gasoline and diesel fuels through transesterification.
|
| Environmental and Genetic Manipulation of Diatoms | |
 |
|
Could we manipulate diatoms, by optimizing their environment(255) or genetics, to produce the type of oil that we want? Here are a few of the factors that have already been manipulated:
-
The aging of a sample of diatoms is reported to increase hydrocarbon content.(256)
-
The degree of unsaturation of C25 haslenes varies with temperature.(223)
-
Growth rate or salinity can affect C25 and C30 production,(225) and there is an optimum salinity for C20.(257)
-
Diatoms kept in the dark produce more oil droplets.(258) However, in the wild, there is “a small loss from lipid, during the night(162)”.(259)
-
Nitrogen depletion increases “fat” production,(149, 150, 255) sometimes within a day.(151, 152)
-
Drying or desiccation increases oil production,(260, 261) and slower drying allows cells to survive better.(261)
-
Mutants of fatty acid synthesis have been attained.(262)
-
Diatom species optimal for “?-3 polyunsaturated fatty acids (PUFAs) such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)”(263) have been selected.
-
Organic mercury and cadmium inhibit fatty acid and sterol production in diatoms.(264)
There is much variability between diatom species, such as, for instance, in their response to desiccation,(261) so the choice of optimal species for oil production is necessary.
An obligate photoautotrophic diatom has already been genetically transformed to a heterotroph.(37) Most, but not all, diatoms are photosynthesizers.(139) Regardless of whether one has obligatory(139) or facultative heterotrophs,(137, 138) the heterotrophic diatoms could then use other energy sources to make oil. An alternative point of view is that we may wish to use an obligate photoautotrophic diatom,(37) because it could possibly be engineered to avoid using its stored oil.
“‘You can make algae with a very high oil content and you can make algae that grows very quickly and, at the moment, no one can do both,’ said Robert Trezona, R&D director at the Carbon Trust”.(265) However, the answer to this may merely be switching the diatoms from exponental growth to “stress” that induces oil formation, both of which they can do rapidly. Nevertheless, there is a caveat:
“Prior to this program [Figure 2], little work had been done to improve oil production in algal organisms. Much of the program’s research focused attention on the elusive ‘lipid trigger.’ (Lipids are another generic name for TAGs [triacylglycerols], the primary storage form of natural oils.) This ‘trigger’ refers to the observation that, under environmental stress, many microalgae appeared to flip a switch to turn on production of TAGs. Nutrient deficiency was the major factor studied. Our work with nitrogen-deficiency in algae and silicon deficiency in diatoms did not turn up any overwhelming evidence in support of this trigger theory. The common thread among the studies showing increased oil production under stress seems to be the observed cessation of cell division. While the rate of production of all cell components is lower under nutrient starvation, oil production seems to remain higher, leading to an accumulation of oil in the cells. The increased oil content of the algae does not lead to increased overall productivity of oil. In fact, overall rates of oil production are lower during periods of nutrient deficiency. Higher levels of oil in the cells are more than offset by lower rates of cell growth”.(21) |
Thus, discussions so far highlight that diatoms are a potential source of oil and have the ability to grow rapidly. However, there is a need for technically sound methodologies that can serve as the infrastructure for implementing diatoms as a comprehensive source of energy in the form of oil. In the next section, a new solar panel approach that utilizes genomically modifiable aspects of diatom biology, offering the prospect of “milking” diatoms for sustainable energy, is discussed.
| A Diatom Solar Panel | |
 |
|
We do not harvest milk from cows by grinding them up and extracting the milk. Instead, we let them secrete the milk at their own pace, and selectively breed cattle and alter their environment to maximize the rate of milk secretion.(266-269) We do not simultaneously attempt to maximize their rate of reproduction. Perhaps we could do the same with diatoms. The milking of algae has been done by solvent extraction methods that do not kill the cells,(270, 271) but in which they are otherwise passive. Here, we propose altering cells so that they actively secrete their oil droplets.
Some diatoms are tough extremophiles that reside in assorted harsh environments(15) and diatoms have been raised in various microcosms;(272-277) therefore, it is plausible to consider confining a diatom colony to a solar panel. Unlike ordinary solar panels that produce electricity, a diatom solar panel would produce oil for us; therefore, in designing it, we would have to solve various optical and mass transport problems. We pose this here as an engineering and genomics challenge, rather than presuming to give a complete solution. Let us consider some of the questions and opportunities involved:
• Mammalian milk contains oil droplets that are exocytosed from the cells lining the milk ducts.(278-281) It may be possible to genetically engineer diatoms so that they exocytose their oil droplets. This could lead to continuous harvesting with clean separation of the oil from the diatoms, provided by the diatoms themselves. The reverse process of the uptake of hydrocarbons from the environment has already been demonstrated,(282) although we do not know if it occurs via endocytosis. Exocytosis of ß-carotene globules has been hypothesized as the mechanism of extraction into the biocompatible hydrophobic liquid dodecane(283) from the unicellular green alga Dunaliella, perhaps accelerated from the natural exocytosis mechanism of this species(284) by the presence of the dodecane.(283) Higher plants have oil secretion glands,(285-288) and diatoms already exocytose the silica contents of the silicalemma,(9, 289) adhesion and motility proteins, and polysaccharides,(166, 168, 290, 291) so the concept of secretion of oil by diatoms is not far-fetched.
• The concept of milking diatoms adds a third dimension to the inverse relationship between biomass and oil production. Therefore, the optimization of oil production(292) must be reconsidered. For example, a system that is closed except for oil secretion and gas exchange may be able to conserve micronutrients,(293) especially if little or no net growth of cells is occurring. This might solve the productivity gap by getting 10-200 times more oil, compared to oilseed crops.(23, 59)
• With at least a boundary layer(294) of water on the diatoms, secreted oil droplets would separate under gravity, rising to the top of a tilted panel, forming an unstable emulsion, which should progressively separate. The oil could then be removed, very similar to the cream that rises to the top of mammalian milk that has not been homogenized. The maximum size of the droplets might be limited by the diameters of the pores of the diatom valves (shells) or the width of the raphes.(166) It may prove wise to keep diatom oil in its natural droplet form, rather than produce a bulk liquid, because small droplets increase energy efficiency.(295, 296)
-
Diatoms require water, or at least high humidity.(297) Thus, we either need a water-impermeable chamber or a source of additional water for the panel. Diatoms can survive desiccation, and, indeed, drying or desiccation increases oil production.(260, 261) Because slower drying allows cells to survive better,(261) one could surmise that this gives time for more oil production: “fat-containing cells are more likely to survive drying than fat-free cells”.(297) Thus, wet/dry cycling might be a key to oil production.
-
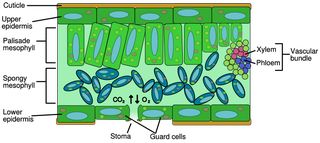
If we decide to keep the water in while allowing the entrance of CO2, then we either must use real stomata or design a replacement for them. After all, a solar panel with live diatoms inside (cf. refs 298 and 299) is not too different in structure and function from a plant leaf (see Figure 13), from which we could learn much.
-
Of course, many leaves have air spaces within them; therefore, contrary to the usual practice of growing diatoms immersed in water,(300) we might find it better to grow them on surfaces in air with high humidity. This also might enhance gas exchange.
-
We could go one step further and use actual leaves to support diatoms inside in their internal air spaces, similar to the relationship between a blue green alga (cyanobacterium) Anabaena and the plant Azolla.(301) The leaf would then provide and control water and gas exchange, temperature control, and directional control of light by solar tracking. Perhaps diatoms could substitute for the palisade mesophyll and/or spongy mesophyll (see Figure 13). Diatoms have entered naturally into many associations and symbioses,(18, 302-316) so the creation of such a new angiosperm leaf-diatom endophytic symbiosis may prove to be possible.
-
Of course, solar panels are subject to solar heating. This could be alleviated as a problem by starting with thermophilic diatoms, some of which have been reported to exist at temperatures up to 76 °C.(317-324) Temperature ranges for growth and photosynthesis of diatoms have only occasionally been investigated,(325) let alone for oil production.
-
Can we take advantage of the optical properties of diatoms and diatom colonies(18) to increase the photosynthetic yield of oil? The delivery of light to algal cultures has been addressed in a variety of ways, including fiber optics(326-328) and immersion optics;(329) however, in all cases, the algae have been passively involved. Diatoms may make use of natural light pipes, such as sponges do with spicules,(330) and colonial diatoms that are attached to each other in chains via silica or polysaccharide linkages(18) may share the light by having the entire colony acting as a light pipe.(9) In this case, we would be utilizing the multiscale optics of the diatoms themselves,(331) including their ability to focus(20) and redistribute(332) light, which may partially explain why diatoms photosynthesize efficiently at low light levels.(142) Diatoms can probably be oriented on grooved surfaces,(333) so that the deliberate optimization of light delivery may be achievable. The engineering of three-dimensional tissue scaffolds(334-338) may help contribute to optimizing the growth of adherent diatoms. Microfluidic scaffolds(339) could be designed to deliver time-cycled nutrients and perhaps remove oil.
-
Some motile colonial diatoms build their own tubular scaffolds, within which they orient themselves,(340, 341) perhaps forming dynamically adaptive light pipes. We could take advantage of the phototaxic motility(342-344) of raphid diatoms, to allow them to build their own optimal, perhaps constructal branched architectures.(15, 345, 346) Such self-assembled multiscale structures might simultaneously produce the optical properties needed, as well as the nutrient flow and oil removal microfluidics.
-
If our diatom panel is to have diatoms attached to a surface, then a monoraphid diatom(347, 348) might be best. This would be asymmetric (an example of heterovalvy(18)) and we might be able to arrange oil secretion from the araphid side, because of possible polarity to the cytoskeleton.(349)
-
It is generally assumed that more light means more productivity for photosynthesizing organisms; however, because diatoms flourish at low light levels,(293, 350-352) optimizing either their growth or oil production may require taking this into account. This ability, in nature, may be due to their facultative heterotrophy(352) or light level adaptation.(353) Low light may imitate conditions of nitrogen deprivation(354) and therefore improve the amount of oil per cell. On the other hand, we might want to select for diatoms that lack heterotrophic ability, if that ability uses part of the oil that they produce.
-
At the other extreme, excess and fluctuating light leads to stress and photoprotection reactions in diatoms;(355, 356) however, the relation of the “rapid (seconds to tens of minutes) regulation of a switch from a light harvesting to a photoprotecting state”,(174) to the postulated “switch” to oil production(21) seems unexplored. A full inquiry into the existence (and, if so, mechanism) of the “oil trigger”(21) is long overdue.
|
| Direct Production of Gasoline by Diatoms | |
 |
|
Low-molecular-weight C2-C6 hydrocarbons in diatoms unfortunately are treated only in a single report:(197)
“Laboratory experiments have been carried out(197) in order to assess and quantify the role of marine phytoplankton in the production of nonmethane hydrocarbons. They provided evidence that supports the hypothesis that some short-chain hydrocarbons are produced during diatom and dinoflagellate lifecycles. Their results suggest that ethane, ethene, propane and propene are produced during the autolysis of some phytoplankton, possibly by the oxidation of polyunsaturated lipids released into their culture medium. In contrast, isoprene and hexane appear during phytoplankton growth and are thus most likely produced either directly by the plankton or through the oxidation of exuded dissolved organic carbon. Studies(357) suggested that ethylene produced in estuarine water is produced biotically by phytoplankton and abiotically by the photolysis of polyunsaturated lipids in particulate and dissolved organic matter. They predicted that high concentrations of ethylene are produced in areas with high primary productivity.”(358) |
This is supported by observations of C2-C4 alkanes in ocean surface waters,(359) along with isoprene (C5), also attributed to algae(360) (mostly diatoms), and direct laboratory observation of isoprene production by diatoms.(199)
In the range of C7-C12,  1/3 of the tested diatom strains produced a,ß,?,d-unsaturated aldehydes.(201) With some optimism about the power of systems biology and how malleable microalgae might be,(361) perhaps we could engineer diatoms that would make these compounds, or the lower-molecular-weight alkanes and alkenes, in great quantities. The biochemical pathways of these polyunsaturated aldehydes are being worked out,(203, 204) and their production correlates with the onset of culture “decline”,(362) giving us another possible insight (cf. ref 363) into the long sought “switch” to oil production.(21) Thus, again, “stress” plays a role,(363, 364) especially mechanical damage,(365) which is imitated in the laboratory by sonication:(204)
1/3 of the tested diatom strains produced a,ß,?,d-unsaturated aldehydes.(201) With some optimism about the power of systems biology and how malleable microalgae might be,(361) perhaps we could engineer diatoms that would make these compounds, or the lower-molecular-weight alkanes and alkenes, in great quantities. The biochemical pathways of these polyunsaturated aldehydes are being worked out,(203, 204) and their production correlates with the onset of culture “decline”,(362) giving us another possible insight (cf. ref 363) into the long sought “switch” to oil production.(21) Thus, again, “stress” plays a role,(363, 364) especially mechanical damage,(365) which is imitated in the laboratory by sonication:(204)
“...LOX [lipoxygenase] activation in marine diatoms triggers a multiphase mechanism that is responsible for the synthesis of at least two classes of proapoptotic and teratogenic products, FAHs [fatty acid hydroperoxides] and hROS [highly reactive oxygen species], which can mimic the toxic effects of the microalgae on grazer copepods”.(202) |
“Although the effects of such toxins are less dramatic than those inducing direct poisoning and death of predators, they are nonetheless insidious, as they affect the future generation of grazers, inducing abortions, birth defects and reduced larval survivorship [though there is more to this story(366)]”(367)... |
“... |
Given that pathways exist for the production of many alkanes, starting with 12-alkane (see Table 1), the production of shorter alkanes within genetically manipulated diatoms might be plausible. If not, we could fall back on known organic chemistry reactions(370, 371) to convert the natural products to alkanes. Of course, we may have to select diatoms that can survive the very products that we are asking them to produce for us, but given the fact that extraction with biocompatible organic solvents works for some algae(270, 271, 283, 372) and diatoms can degrade petroleum hydrocarbons,(373) this should be possible.
| Conclusion | |
 |
|
Despite  170 years of research on the relationship between diatoms and crude oil, we still know very little about the oil inside diatoms itself. Therefore, we have collected some images from the literature, so we all know what we are looking for (recall Figures 1, 5, 6, 7, 8, and 9). To conduct a proper chemical analysis of the oil inside diatom oil droplets, a method for separating out oil droplets inside diatoms from the shell and cytoplasm must be developed. Sonication, centrifugation, freezing and crushing, extraction, induced exocytosis, etc. must be attempted.
170 years of research on the relationship between diatoms and crude oil, we still know very little about the oil inside diatoms itself. Therefore, we have collected some images from the literature, so we all know what we are looking for (recall Figures 1, 5, 6, 7, 8, and 9). To conduct a proper chemical analysis of the oil inside diatom oil droplets, a method for separating out oil droplets inside diatoms from the shell and cytoplasm must be developed. Sonication, centrifugation, freezing and crushing, extraction, induced exocytosis, etc. must be attempted.
With more than 200 000 species from which to choose, and all the combinatorics of nutrient and genome manipulation, finding or creating the “best” diatom for sustainable gasoline will be quite a task. Nevertheless, some guidelines for starting species can be guessed from our survey:
000 species from which to choose, and all the combinatorics of nutrient and genome manipulation, finding or creating the “best” diatom for sustainable gasoline will be quite a task. Nevertheless, some guidelines for starting species can be guessed from our survey:
-
Choose planktonic diatoms with positive buoyancy(158, 374, 375) or at least neutral buoyancy.(153, 235, 376, 377)
-
Choose diatoms that harbor symbiotic nitrogen-fixing cyanobacteria,(306, 378-380) which should reduce nutrient requirements.
-
Choose diatoms that have high efficiency of photon use, perhaps from those that function at low light levels.(106, 142)
-
Choose diatoms that are thermophilic,(317-324) especially for solar panels subject to solar heating.
-
Consider those genera that have been demonstrated by paleogenetics to have contributed to fossil organics.(381)
-
For motile or sessile pennate diatoms that adhere to surfaces, buoyancy may be much less important than survival from desiccation, which seems to induce oil production.(260, 261) Therefore, the reaction of these diatoms to drying is a place to start. The reaction of oceanic planktonic species to drying has not been investigated, although one would anticipate that they have no special mechanisms for addressing this (for them) unusual situation.
-
Genetic engineering of diatoms to enhance oil production has been attempted, but it has not yet been successful.(25, 30, 31, 382-384)
-
We could use a compustat for mutation and selection of diatoms that maximize oil droplet size and/or number.(9, 385)
-
Generally, cell proliferation seems to be counterproductive to oil production on a per-cell basis, which is a problem that has been expressed as an unsolved Catch-22.(62) However, this balance may shift in our favor when we start milking diatoms for oil instead of grinding them.
The need of the hour is to develop technologies for efficient, safer, less-polluting alternative oil sources, because the present stock of fossil oil is fast dwindling and its burning has accentuated human-caused global warming, which started 8000 years ago.(386) The mechanisms of crude oil formation by natural phenomena are now partially understood, and technology for crude oil synthesis is in the budding stage; however, because the majority of petroleum has its origins in algae, diatoms may play a vital role in sustainable oil production. The manipulation of microalgal or diatom lipid quantity and quality could be very significant and help us in effectively using this renewable resource as energy.
Energy is the prime mover for economic development. The global annual consumption of energy is now over 400 EJ(387) and the major share (79%) comes from fossil fuels.(388) Fossil fuels are nonrenewable resources that are used in the production of energy, and recent history shows that they have been consumed at increasing rates.(389) Fossil fuels include coal, natural gas, and oil, and the term might be extended to methyl hydrate.(390) During the 20th century, oil replaced coal as the dominant fossil fuel. Despite oil reserves that have been estimated to last until the year 2050,(391) the switch to an alternative energy source may occur as rapidly as the switch from the horse to the automobile(392) and, thus, may not take another generation. Therefore, it would seem wise to proceed with a rapid increase in biofuel use, from 0.3%(388) to the majority, well before the oil supply is depleted, picking up from the major aborted program of the 1980-1990s(21) (see Figure 2). On the other hand, estimates of fossil fuel reserves are “ambiguous” and “... the world consumption of oil, coal and gas has increased over the last half century. This trend is forecast to continue to 2030”,(393) so that if we put up with the predicted global warming, the pressure for biofuel use may prove more geopolitical than anything else. Certainly, the sale of crude oil has empowered some nations far beyond the role they would otherwise have in the world. We conclude that biofuel investment is a gamble in the short term, except for countries that are willing to eschew imported oil,(394) despite the latter’s possibly continued lower cost for a while. On the other hand, countries that master sustainable large-scale biofuel production now will be in a long-term position to sell the technology to others and ignore geopolitical pressures from fossil-oil-producing countries. “In [global oil] peaking there is certainly cause for significant concern, but not in our view for panic”.(395) We have an opportunity to plan ahead, or not. If each approach to sustainable energy sources, such as diatoms, must compete in price with fossil fuels before its proper investment occurs, we shall have to wait for the last drop of easily accessible oil to be extracted out of the ground, which is an approach to problem solving that has, for example, significantly reduced biodiversity.(396) If we subsidize the development of sustainable energy sources for reasons other than price,(397) there will be a gnashing of teeth and undermining of the effort by the oil-producing countries and those countries that maintain a dependence on them;(398) however, in the long run, we will have done the right thing. Just announced work(401) suggests that diatoms can triple the efficiency of electrical solar panels, an efficiency that should also apply to gasoline secreting solar panels.
the world consumption of oil, coal and gas has increased over the last half century. This trend is forecast to continue to 2030”,(393) so that if we put up with the predicted global warming, the pressure for biofuel use may prove more geopolitical than anything else. Certainly, the sale of crude oil has empowered some nations far beyond the role they would otherwise have in the world. We conclude that biofuel investment is a gamble in the short term, except for countries that are willing to eschew imported oil,(394) despite the latter’s possibly continued lower cost for a while. On the other hand, countries that master sustainable large-scale biofuel production now will be in a long-term position to sell the technology to others and ignore geopolitical pressures from fossil-oil-producing countries. “In [global oil] peaking there is certainly cause for significant concern, but not in our view for panic”.(395) We have an opportunity to plan ahead, or not. If each approach to sustainable energy sources, such as diatoms, must compete in price with fossil fuels before its proper investment occurs, we shall have to wait for the last drop of easily accessible oil to be extracted out of the ground, which is an approach to problem solving that has, for example, significantly reduced biodiversity.(396) If we subsidize the development of sustainable energy sources for reasons other than price,(397) there will be a gnashing of teeth and undermining of the effort by the oil-producing countries and those countries that maintain a dependence on them;(398) however, in the long run, we will have done the right thing. Just announced work(401) suggests that diatoms can triple the efficiency of electrical solar panels, an efficiency that should also apply to gasoline secreting solar panels.
| Acknowledgment | |
 |
|
We would like to thank Janhavi S. Raut and R. Venkataraghavan (Hindustan Unilever, Ltd.) for creating the opportunity that brought us together and led to this review. We also thank Ian R. Jenkinson and the referees for helpful comments. This work is not funded by any grant agency or commercial establishment, so we have no conflicts of interest to declare.
| References | |
 |
|
- Guliyev, I. S.; Feizulayev, A. A.; Huseynov, D. A., Isotope geochemistry of oils from fields and mud volcanoes in the South Caspian Basin, Azerbaijan. Petroleum Geoscience 2001, 7, (2), 201-209.
- Fortman, J. L.; Chhabra, S.; Mukhopadhyay, A.; Chou, H.; Lee, T. S.; Steen, E.; Keasling, J. D., Biofuel alternatives to ethanol: pumping the microbial well. Trends in Biotechnology 2008, 26, (7), 375-381.
- Gordon, R., The Glass Menagerie: Diatom Nanotechnology and Its Implications for Multi-Scale Manufacturing and Oil Production. In 2nd International Conference on Multi-Scale Structures and Dynamics of Complex Systems: Processes & Forces for Creation of Designer Materials with Multi-Scale Structures, 4-5 September, 2008, Bangalore, India, Raut, J. S.; Venkataraghavan, R., Eds. Unilever: Bangalore, 2008.
- Gordon, R.; Drum, R. W., The chemical basis for diatom morphogenesis. Int. Rev. Cytol. 1994, 150, 243-372, 421-422.
- Aoyagi, K.; Omokawa, M., Neogene diatoms as the important source of petroleum in Japan. Journal of Petroleum Science & Engineering 1992, 7, 247-252.
- Chisti, Y., Biodiesel from microalgae. Biotechnology Advances 2007, 25, (3), 294-306.
- Killops, S. D.; Killops, V. J., Introduction to Organic Geochemistry. 2nd ed.; Blackwell Publishing: Malden, MA, 2005.
- Gordon, R.; Sterrenburg, F. A. S.; Sandhage, K., A Special Issue on Diatom Nanotechnology. Journal of Nanoscience and Nanotechnology 2005, 5, (1), 1-4.
- Gordon, R.; Losic, D.; Tiffany, M. A.; Nagy, S. S.; Sterrenburg, F. A. S., The Glass Menagerie: diatoms for novel applications in nanotechnology [invited]. Trends in Biotechnology 2009, 27, (2), 116-127.
- Gordon, R., Diatoms and nanotechnology [Invited]. In The Diatoms: Applications for the Environmental and Earth Sciences, Smol, J. P.; Stoermer, E. F., Eds. Cambridge University Press: Cambridge, 2010; Vol. 2nd, submitted December 21, 2008
- Gordon, R.; Aguda, B. D., Diatom morphogenesis: natural fractal fabrication of a complex microstructure. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Part 1/4: Cardiology and Imaging, 4-7 Nov. 1988, New Orleans, LA, USA, Harris, G.; Walker, C., Eds. Institute of Electrical and Electronics Engineers: New York, 1988; pp 273-274.
- Parkinson, J.; Gordon, R., Beyond micromachining: the potential of diatoms. Trends Biotechnol (Tibtech) 1999, 17, (5), 190-6.
- Drum, R. W.; Gordon, R., Star Trek replicators and diatom nanotechnology. TibTech (Trends in Biotechnology) 2003, 21, (8), 325-8.
- Gebeshuber, L. C., Biotribology inspires new technologies. Nano Today 2007, 2, (5), 30-37.
- Sterrenburg, F. A. S.; Gordon, R.; Tiffany, M. A.; Nagy, S. S., Diatoms: living in a constructal environment. In Algae and Cyanobacteria in Extreme Environments. Series: Cellular Origin, Life in Extreme Habitats and Astrobiology, Vol. 11, Seckback, J., Ed. Springer: Dordrecht, The Netherlands, 2007; pp 141-172.
- Grachev, M. A.; Annenkov, V. V.; Likhoshway, Y. V., Silicon nanotechnologies of pigmented heterokonts. BioEssays 2008, 30, (4), 328-337.
- Kröger, N.; Poulsen, N., Diatoms: from cell wall biogenesis to nanotechnology. Annual Review of Genetics 2008, 42, 83-107.
- Round, F. E.; Crawford, R. M.; Mann, D. G., The Diatoms, Biology & Morphology of the Genera. Cambridge University Press: Cambridge, 1990.
- Cox, E. J., Identification of Freshwater Diatoms from Living Material. Chapman & Hall: London, 1996.
- De Stefano, L.; Rea, I.; Rendina, I.; De Stefano, M.; Moretti, L., Lensless light focusing with the centric marine diatom Coscinodiscus walesii. Optics Express 2007, 15, (26), 18082-18088.
- Sheehan, J.; Dunahay, T.; Benemann, J.; Roessler, P., A Look Back at the U.S. Department of Energy’s Aquatic Species Program: Biodiesel from Algae. Close-Out Report [NREL/TP-580-24190]. National Renewable Energy Laboratory: Golden, Colorado, 1998.
- Dismukes, G. C.; Carrieri, D.; Bennette, N.; Ananyev, G. M.; Posewitz, M. C., Aquatic phototrophs: efficient alternatives to land-based crops for biofuels. Current Opinion in Biotechnology 2008, 19, (3), 235-240.
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A., Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant Journal 2008, 54, (4), 621-639.
- Imahara, H.; Minami, E.; Saka, S., Thermodynamic study on cloud point of biodiesel with its fatty acid composition. Fuel 2006, 85, (12-13), 1666-1670.
- Dunahay, T. G.; Jarvis, E. E.; Dais, S. S.; Roessler, P. G., Manipulation of microalgal lipid production using genetic engineering. Applied Biochemistry and Biotechnology 1996, 57-8, 223-231.
- Moffat, M. C., An ultrastructural study of Didymosphenia geminata (Bacillariophyceae). Transactions of the American Microscopical Society 1994, 113, (1), 59-71.
- Kirkwood, A. E.; Shea, T.; Jackson, L.; McCcauley, E., Didymosphenia geminata in two Alberta headwater rivers: an emerging invasive species that challenges conventional views on algal bloom development. Canadian Journal of Fisheries and Aquatic Sciences 2007, 64, (12), 1703-1709.
- Nagle, N.; Lemke, P., Production of methyl ester fuel from microalgae. Applied Biochemistry and Biotechnology 1990, 24-25, (1), 355-361.
- Roberts, K.; Granum, E.; Leegood, R. C.; Raven, J. A., C3 and C4 pathways of photosynthetic carbon assimilation in marine diatoms are under genetic, not environmental, control. Plant Physiol 2007, 145, (1), 230-5.
- Dunahay, T. G.; Jarvis, E. E.; Zeiler, K. G.; Roessler, P. G.; Brown, L. M., Genetic engineering of microalgae for fuel production: scientific note. Applied Biochemistry and Biotechnology 1992, 34-5, 331-339.
- Roessler, P. G.; Brown, L. M.; Dunahay, T. G.; Heacox, D. A.; Jarvis, E. E.; Schneider, J. C.; Talbot, S. G.; Zeiler, K. G., Genetic engineering approaches for enhanced production of biodiesel fuel from microalgae. In Enzymatic Conversion of Biomass for Fuels Production, 1994; Vol. 566, pp 255-270.
- Dunahay, T. G.; Jarvis, E. E.; Roessler, P. G., Genetic transformation of the diatoms Cyclotella cryptica and Navicula saprophila. Journal of Phycology 1995, 31, (6), 1004-1012.
- Apt, K. E.; Kroth-Pancic, P. G.; Grossman, A. R., Stable nuclear transformation of the diatom Phaeodactylum tricornutum. Mol Gen Genet 1996, 252, (5), 572-9.
- Falciatore, A.; Casotti, R.; Leblanc, C.; Abrescia, C.; Bowler, C., Transformation of nonselectable reporter genes in marine diatoms. Mar. Biotechnol. 1999, 1, (3), 239-251.
- Zaslavskaia, L. A.; Lippmeier, J. C.; Kroth, P. G.; Grossman, A. R.; Apt, K. E., Transformation of the diatom Phaeodactylum tricornutum (Bacillariophyceae) with a variety of selectable marker and reporter genes. J. Phycol. 2000, 36, (2), 379-386.
- Scala, S.; Bowler, C., Molecular insights into the novel aspects of diatom biology. Cell Mol Life Sci 2001, 58, (11), 1666-73.
- Zaslavskaia, L. A.; Lippmeier, J. C.; Shih, C.; Ehrhardt, D.; Grossman, A. R.; Apt, K. E., Trophic conversion of an obligate photoautotrophic organism through metabolic engineering. Science 2001, 292, (5524), 2073-5.
- Scala, S.; Carels, N.; Falciatore, A.; Chiusano, M. L.; Bowler, C., Genome properties of the diatom Phaeodactylum tricornutum. Plant Physiol 2002, 129, (3), 993-1002.
- Grossman, A. R., Paths toward algal genomics. Plant Physiology 2005, 137, (2), 410-427.
- Poulsen, N.; Kröger, N., A new molecular tool for transgenic diatoms. Control of mRNA and protein biosynthesis by an inducible promoter-terminator cassette. FEBS J. 2005, 272, (13), 3413-3423.
- Poulsen, N.; Chesley, P. M.; Kröger, N., Molecular genetic manipulation of the diatom Thalassiosira pseudonana (Bacillariophyceae). J Phycol 2006, 42, (5), 1059-1065.
- Kroth, P.; León, R.; Gaván, A.; Fernández, E., Molecular biology and the biotechnological potential of diatoms. Advances in Experimental Medicine & Biology 2007, 616, 23-33.
- Kroth, P. G., Genetic transformation: a tool to study protein targeting in diatoms. Methods Mol Biol 2007, 390, 257-68.
- Sakaue, K.; Harada, H.; Matsuda, Y., Development of gene expression system in a marine diatom using viral promoters of a wide variety of origin. Physiol Plant 2008, 133, (1), 59-67.
- Koh, L. P.; Ghazoul, J., Biofuels, biodiversity, and people: Understanding the conflicts and finding opportunities. Biological Conservation 2008, 141, (10), 2450-2460.
- Müller, A.; Schmidhuber, J.; Hoogeveen, J.; Steduto, P., Some insights in the effect of growing bio-energy demand on global food security and natural resources. Water Policy 2008, 10, (S1), 83-94.
- Zhu, L., Impacts of food and energy price hikes and proposed coping strategies. China & World Economy 2008, 16, (6), 35-45.
- Hoogeveen, J.; Faurès, J. M.; Van de Giessen, N., Increased biofuel production in the coming decade: to what extent will it affect global freshwater resources? Irrigation and Drainage 2009, 58, (S1), S148-S160.
- Demirbas, A., Progress and recent trends in biodiesel fuels. Energy Conversion and Management 2009, 50, 14-34.
- Tadros, M. G.; Johansen, J. R., Physiological characterization of six lipid-producing diatoms from the southeastern United States. Journal of Phycology 1988, 24, (4), 445-452.
- Furnas, M. J., Net in situ growth rates of phytoplankton in an oligotrophic, tropical shelf ecosystem. Limnology and Oceanography 1991, 36, (1), 13-29.
- Lewin, J.; Hellebust, J. A., Heterotrophic nutrition of the marine pennate diatom Navicula pavillardi Hustedt. Can J Microbiol 1975, 21, (9), 1335-42.
- Brzezinski, M. A.; Villareal, T. A.; Lipschultz, F., Silica production and the contribution of diatoms to new and primary production in the central north Pacific. Marine Ecology-Progress Series 1998, 167, 89-104.
- Shipe, R. F.; Brzezinski, M. A.; Pilskaln, C.; Villareal, T. A., Rhizosolenia mats: an overlooked source of silica production in the open sea. Limnology and Oceanography 1999, 44, (5), 1282-1292.
- Furnas, M. J., In situ growth rates of marine phytoplankton: approaches to measurement, community and species growth rates. J. Plankton Res. 1990, 12, 1117-1151.
- Dugdale, R. C.; Wilkerson, F. P., Silicate regulation of new production in the equatorial Pacific upwelling. Nature 1998, 391, 270-273.
- Breger, D., Petroleum Education, Hydrocarbon Systems. Step 1 - Energy Capture. http://www.priweb.org/ed/pgws/systems/energy_capture/capture.html 2001.
- Krebs, W. N.; Gladenkov, A. Y.; Jones, G. D., Diatoms in oil and gas exploration. In The Diatoms: Applications for the Environmental and Earth Sciences [in preparation], 2nd ed.; Smol, J. P.; Stoermer, E. F., Eds. Cambridge University Press: Cambridge, 2010; in press.
- Whittington, T., Biodiesel Production and Use by Farmers: Is it Worth Considering? Department of Agriculture and Food, Government of Western Australia: Perth, 2006.
- Associated Press, Algae Emerges as a Potential Fuel Source http://www.nytimes.com/2007/12/02/us/02algae.html?_r=2&oref=slogin&oref=slogin 2007.
- Reuters, Sapphire Raises Over US$100 Mln for Algae Crude http://www.planetark.com/dailynewsstory.cfm/newsid/50277/story.htm 2008.
- Aguilera, M. C., Green Bullet: Scientists take aim with tiny algae and their giant promise as the biofuel solution of the future. http://explorations.ucsd.edu/Features/2008/Green_Bullet/images/12_2008_Feature.pdf 2008.
- Santhanam, N., Oilgae: Biodiesel from Algae Oil - Info, Resources, News & Links. http://www.oilgae.com/ 2008.
- Fossil Freedom, Algal BioButanol Conquers Colorado Continental Divide! http://www.fossilfreedom.com/ 2008.
- Shifrin, N. S.; Chisholm, S. W., Phytoplankton lipids: interspecific differences and effects of nitrate, silicate and light-dark cycles. Journal of Phycology 1981, 17, (4), 374-384.
- Berndes, G.; Hoogwijk, M.; van den Broek, R., The contribution of biomass in the future global energy supply: a review of 17 studies. Biomass & Bioenergy 2003, 25, (1), 1-28.
- Sriharan, S.; Bagga, D.; Sriharan, T. P., Effects of nutrients and temperature on lipid and fatty-acid production in the diatom Hantzshia DI-60. Applied Biochemistry and Biotechnology 1990, 24-5, (1), 309-316.
- McGinnis, K. M.; Dempster, T. A.; Sommerfeld, M. R., Characterization of the growth and lipid content of the diatom Chaetoceros muelleri. Journal of Applied Phycology 1997, 9, (1), 19-24.
- Lebeau, T.; Robert, J. M., Diatom cultivation and biotechnologically relevant products. Part II: current and putative products. Appl Microbiol Biotechnol 2003, 60, (6), 624-32.
- Kooistra, W. H.; Medlin, L. K., Evolution of the diatoms (Bacillariophyta). IV. A reconstruction of their age from small subunit rRNA coding regions and the fossil record. Mol Phylogenet Evol 1996, 6, (3), 391-407.
- Schieber, J.; Krinsley, D.; Riciputi, L., Diagenetic origin of quartz silt in mudstones and implications for silica cycling. Nature 2000, 406, 981-985.
- Medlin, L. K.; Kooistra, W.; Gersonde, R.; Sims, P. A.; Wellbrock, U., Is the origin of the diatoms related to the end-Permian mass extinction? Nova Hedwigia 1997, 65, (1-4), 1-11.
- Levorsen, A. I.; Berry, F. A. F., Geology of Petroleum. 2nd ed.; W. H. Freeman and Co.: San Francisco, 1967.
- Hunt, J. M., Petroleum Geochemistry and Geology. W. H. Freeman and Co.: San Francisco, 1979.
- North, F. K., Petroleum Geology. Allen & Unwin: Boston, 1985.
- Volkman, J. K.; Johns, R. B., The geochemical significance of positional isomers of unsaturated acids from an intertidal zone sediment. Nature 1977, 267, (5613), 693.
- Holba, A. G.; Tegelaar, E. W.; Huizinga, B. J.; Moldowan, J. M.; Singletary, M. S.; McCaffrey, M. A.; Dzou, L. I. P., 24-norcholestanes as age-sensitive molecular fossils. Geology 1998, 26, (9), 783-786.
- Rampen, S. W.; Schouten, S.; Abbas, B.; Panoto, F. E.; Muyzer, G.; Campbell, C. N.; Fehling, J.; Sinninghe Damsté, J. S., On the origin of 24-norcholestanes and their use as age-diagnostic biomarkers. Geology 2007, 35, (5), 419-422.
- Volkman, J. K.; Barrett, S. M.; Dunstan, G. A.; Jeffrey, S. W., Geochemical significance of the occurrence of dinosterol and other 4-methyl sterols in a marine diatom. Organic Geochemistry 1993, 20, (1), 7-15.
- Volkman, J. K.; Barrett, S. M.; Blackburn, S. I.; Mansour, M. P.; Sikes, E. L.; Gelin, F., Microalgal biomarkers: A review of recent research developments. Organic Geochemistry 1998, 29, (5-7), 1163-1179.
- Xu, Y. P.; Jaffe, R.; Wachnicka, A.; Gaiser, E. E., Occurrence of C25 highly branched isoprenoids (HBIs) in Florida Bay: Paleoenvironmental indicators of diatom-derived organic matter inputs. Organic Geochemistry 2006, 37, (7), 847-859.
- Belt, S. T.; Masse, G.; Rowland, S. J.; Poulin, M.; Michel, C.; LeBlanc, B., A novel chemical fossil of palaeo sea ice: IP25. Organic Geochemistry 2007, 38, (1), 16-27.
- Rampen, S. W.; Schouten, S.; Hopmans, E. C.; Abbas, B.; Noordeloos, A. A. M.; Geenevasen, J. A. J.; Moldowan, J. M.; Denisevich, P.; Sinninghe Damsté, J. S., Occurrence and biomarker potential of 23-methyl steroids in diatoms and sediments. Organic Geochemistry 2008, 40, (2), 219-228.
- Shiine, H.; Suzuki, N.; Motoyama, I.; Hasegawa, S.; Gladenkov, A. Y.; Gladenkov, Y. B.; Ogasawara, K., Diatom biomarkers during the Eocene/Oligocene transition in the Il'pinskii peninsula, Kamchatka, Russia. Palaeogeography Palaeoclimatology Palaeoecology 2008, 264, (1-2), 1-10.
- Ehrenberg, C. G., Ueber die Dysodil genannte Mineralspecies also ein Pruduct aus Infusorienschalen. Poggendorff's Annalen der Physik und Chemie, Ser. 2 1839, 48, (Pt. 4, No. 12), 573-575.
- Whitney, J. D., Geological Survey of California. Vol. 1, Geology. Sherman and Co.: Philadelphia, 1865.
- Whitney, J. D., On the fresh water infusorial deposits of the Pacific coast, and their connection with the volcanic rocks. Proc. Calif. Acad. Nat. Sci., Ser. 1 1867, 3, 319- 324.
- Anderson, F. M., Origin of California petroleum. Bull. Geol. Soc. Amer. 1926, 37, 585-614.
- Tolman, C. F., Biogenesis of hydrocarbons by diatoms. Econ. Geol. 1927, 22, (5), 454-474.
- Hanna, G. D., An early reference to the theory that diatoms are the source of bituminous substances. Bull. Amer. Assoc. Petrol. Geol. 1928, 12, 555-556.
- Phleger Jr., F. B.; Albritton Jr., C. C., Diatoms as a source for California petroleum: a summary review. Field and Laboratory 1937, 6, (November), 25-32.
- Ver Wiebe, W. A., How Oil is Found. Edward Bothers: Wichita, 1951.
- Bramlette, M. N., The Monterey Formation of California and the Origin of Its Siliceous Rocks [Professional Paper 212]. U.S. Geological Survey, United States Department of the Interior: Washington, D.C., 1946.
- Rowland, S. J.; Robson, J. N., The widespread occurrence of highly branched acyclic C20, C25 and C30 hydrocarbons in recent sediments and biota—A review. Marine Environmental Research 1990, 30, (3), 191-216.
- Johns, L.; Belt, S.; Lewis, C. A.; Rowland, S.; Massé, G.; Robert, J. M.; König, W. A., Configurations of polyunsaturated sesterterpenoids from the diatom, Haslea ostrearia. Phytochemistry 2000, 53, (5), 607-611.
- Gotoh, M.; Miki, A.; Nagano, H.; Ribeiro, N.; Elhabiri, M.; Gurnienna-Kontecka, E.; Albrecht-Gary, A. M.; Schmutz, M.; Ourisson, G.; Nakatani, Y., Membrane properties of branched polyprenyl phosphates, postulated as primitive membrane constituents. Chemistry & Biodiversity 2006, 3, (4), 434-455.
- Palmisano, A. C.; Lizotte, M. P.; Smith, G. A.; Nichols, P. D.; White, D. C.; Sullivan, C. W., Changes in photosynthetic carbon assimilation in Antarctic sea-ice diatoms during spring bloom - variation in synthesis of lipid classes. Journal of Experimental Marine Biology and Ecology 1988, 116, (1), 1-13.
- Gürgey, K., Correlation, alteration, and origin of hydrocarbons in the GCA, Bahar, and Gum Adasi fields, western South Caspian Basin: geochemical and multivariate statistical assessments. Marine and Petroleum Geology 2003, 20, (10), 1119-1139.
- Mertz Jr., K. A., Origin of hemipelagic source rocks during the early and Middle Miocene, Salinas Basin, California. AAPG Bulletin: American Association of Petroleum Geologists 1989, 73, (4), 510-524.
- Johnson, K. M.; Grimm, K. A., Opal and organic carbon in laminated diatomaceous sediments: Saanich Inlet, Santa Barbara Basin and the Miocene Monterey Formation. Marine Geology 2001, 174, (1-4), 159-175.
- Gelin, F.; Volkman, J. K.; Largeau, C.; Derenne, S.; Sinninghe Damsté, J. S.; de Leeuw, J. W., Distribution of aliphatic, nonhydrolyzable biopolymers in marine microalgae. Organic Geochemistry 1999, 30, (2-3), 147-159.
- Garrison, D. L., Monterey Bay phytoplankton: II. Resting spore cycles in coastal diatom populations. J. Plankton Res. 1981, 3, (1), 137-156.
- Eilertsen, H. C.; Sandberg, S.; Tøllefsen, H., Photoperiodic control of diatom spore growth; a theory to explain the onset of phytoplankton blooms. Marine Ecology-Progress Series 1995, 116, (1-3), 303-307.
- Wetz, M. S.; Wheeler, P. A.; Letelier, R. M., Light-induced growth of phytoplankton collected during the winter from the benthic boundary layer off Oregon, USA. Marine Ecology-Progress Series 2004, 280, 95-104.
- Suto, I., The explosive diversification of the diatom genus Chaetoceros across the Eocene/Oligocene and Oligocene/Miocene boundaries in the Norwegian Sea. Marine Micropaleontology 2006, 58, (4), 259-269.
- McGee, D.; Laws, R. A.; Cahoon, L. B., Live benthic diatoms from the upper continental slope: extending the limits of marine primary production. Marine Ecology-Progress Series 2008, 356, 103-112.
- Hargraves, P. E.; French, F. W., Diatom resting spores: significance and strategies. In Survival Strategies of the Algae, Fryxell, G. A., Ed. Cambridge University Press: Cambridge, 1983; pp 49-68.
- Stasiuk, L. D.; Snowdon, L. R., Fluorescence micro-spectrometry of synthetic and natural hydrocarbon fluid inclusions: Crude oil chemistry, density and application to petroleum migration. Applied Geochemistry 1997, 12, (3), 229-241.
- Bourdon, S.; Laggoun-Défarge, F.; Disnar, J. R.; Maman, O.; Guillet, B.; Derenne, S.; Largeau, C., Organic matter sources and early diagenetic degradation in a tropical peaty marsh (Tritrivakely, Madagascar). Implications for environmental reconstruction during the Sub-Atlantic. Organic Geochemistry 2000, 31, (5), 421-438.
- Stasiuk, L. D.; Sanei, H., Characterization of diatom-derived lipids and chlorophyll within Holocene laminites, Saanich Inlet, British Columbia, using conventional and laser scanning fluorescence microscopy. Organic Geochemistry 2001, 32, (12), 1417-1428.
- Vargas, G.; Ortlieb, L.; Pichon, J. J.; Bertaux, J.; Pujos, M., Sedimentary facies and high resolution primary production inferences from laminated diatomacous sediments off northern Chile (23oS). Marine Geology 2004, 211, (1-2), 79-99.
- Michalopoulos, P.; Aller, R. C.; Reeder, R. J., Conversion of diatoms to clays during early diagenesis in tropical, continental shelf muds. Geology 2000, 28, (12), 1095-1098.
- Wakeham, S. G.; Lee, C., Production, transport, and alteration of particulate organic matter in the marine water column. In Organic Geochemistry: Principles and Applications, Engel, M.; Macko, S. A., Eds. Plenum Press: New York ; London, 1993; pp 145-169.
- Harvey, H. R.; Macko, S. A., Kinetics of phytoplankton decay during simulated sedimentation: changes in lipids under oxic and anoxic conditions. Organic Geochemistry 1997, 27, (3-4), 129-140.
- Hayakawa, K.; Handa, N.; Ikuta, N.; Fukuchi, M., Downward fluxes of fatty acids and hydrocarbons during a phytoplankton bloom in the austral summer in Breid Bay, Antarctica. Organic Geochemistry 1996, 24, (5), 511-521.
- Hein, J. R.; Scholl, D. W.; Barron, J. A.; Jones, M. G.; Miller, J., Diagenesis of late Cenozoic diatomaceous deposits and formation of the bottom simulating reflector in the southern Bering Sea. Sedimentology 1978, 25, (2), 155-181.
- Colombo, J. C.; Silverberg, N.; Gearing, J. N., Amino acid biogeochemistry in the Laurentian Trough: vertical fluxes and individual reactivity during early diagenesis. Organic Geochemistry 1998, 29, (4), 933-945.
- DeMaster, D. J.; Dunbar, R. B.; Gordon, L. I.; Leventer, A. R.; Morrison, J. M.; Nelson, D. M.; Nittrouer, C. A.; Smith Jr., W. O., Cycling and accumulation of biogenic silica and organic matter in high-latitude environments: the Ross Sea. Oceanography 1992, 5, (3), 146-153.
- Josefson, A. B.; Hansen, J. L. S., Quantifying plant pigments and live diatoms in aphotic sediments of Scandinavian coastal waters confirms a major route in the pelagic-benthic coupling. Marine Biology 2003, 142, (4), 649-658.
- McQuoid, M. R.; Nordberg, K., Composition and origin of benthic flocculent layers in Swedish fjords following the spring bloom - contribution of diatom frustules and resting stages. Nova Hedwigia 2006, 83, (1-2), 1-16.
- Zgurovskaya, L. N., Species composition and distribution of planktonic algae in bottom sediments of Black Sea. Okeanologiya 1978, 18, (4), 716-721.
- Hamm, C. E.; Merkel, R.; Springer, O.; Jurkojc, P.; Maier, C.; Prechtel, K.; Smetacek, V., Architecture and material properties of diatom shells provide effective mechanical protection. Nature 2003, 421, (6925), 841-843.
- Itakura, S.; Imai, I.; Itoh, K., ‘‘Seed bank’’ of coastal planktonic diatoms in bottom sediments of Hiroshima Bay, Seto Inland, Japan. Mar. Biol. 1997, 128, (3), 497-508.
- Lewis, J.; Harris, A. S. D.; Jones, K. J.; Edmonds, R. L., Long-term survival of marine planktonic diatoms and dinoflagellates in stored sediment samples. Journal of Plankton Research 1999, 21, (2), 343-354.
- McQuoid, M. R., Pelagic and benthic environmental controls on the spatial distribution of a viable diatom propagule bank on the Swedish west coast. Journal of Phycology 2002, 38, (5), 881-893.
- Hansen, J. L. S.; Josefson, A. B., Ingestion by deposit-feeding macro-zoobenthos in the aphotic zone does not affect the pool of live pelagic diatoms in the sediment. Journal of Experimental Marine Biology and Ecology 2004, 308, (1), 59-84.
- Durbin, E. G., Aspects of the biology of resting spores of Thalassiosira nordenskioeldii and Detonula confervacea. Mar. Biol. 1978, 45, 31-37.
- Gendron-Badou, A.; Coradin, T.; Maquet, J.; Fröhlich, F.; Livage, J., Spectroscopic characterization of biogenic silica. Journal of Non-Crystalline Solids 2003, 316, (2-3), 331-337.
- Iijima, A.; Tada, R., Silica diagenesis of Neogene diatomaceous and volcaniclastic sediments in northern Japan. Sedimentology 1981, 28, (2), 185-200.
- Henchiri, M., Sedimentation, depositional environment and diagenesis of Eocene biosiliceous deposits in Gafsa basin (southern Tunisia). Journal of African Earth Sciences 2007, 49, (4-5), 187-200.
- 131. Sicko-Goad, L.; Stoermer, E. F.; Fahnenstiel, G., Rejuvenation of Melosira granulata (Bacillariophyceae) resting cells from the anoxic sediments of douglas lake, michigan. I. Light microscopy and 14C uptake. Journal of Phycology 1986, 22, (1), 22-28.
- Nipkow, F., Ruheformen planktischer Kieselalgen in Geschichteten Schlamm Des Zürichsees. Schweiz. Z. Hydrol. 1950, 12, 263-270.
- Stockner, J. G.; Lund, J. W. G., Live algae in postglacial lake deposits. Limnology and Oceanography 1970, 15, (1), 41-58.
- Davis, J. S., Survival records in the algae, and the survival role of certain algae pigments, fat, and mucilaginous substances. The Biologist 1972, 54, (2), 52-93.
- Zgurovskaya, L. N., Effect of addition of nutrients on growth of spores and division of planktonic algae from bottom sediments. Okeanologiya 1977, 17, (1), 119-122.
- McQuoid, M. R.; Hobson, L. A., Diatom resting stages. Journal of Phycology 1996, 32, (6), 889-902.
- White, A. W., Uptake of organic compounds by two facultatively heterotrophic marine centric diatoms. Journal of Phycology 1974, 10, (4), 433-438.
- White, A. W., Growth of two facultatively heterotrophic marine centric diatoms. Journal of Phycology 1974, 10, (3), 292-300.
- Li, C.-W.; Volcani, B. E., Four new apochlorotic diatoms. Br. Phycol. J. 1987, 22, 375-382.
- Belt, S. T.; Allard, W. G.; Rintatalo, J.; Johns, L. A.; van Duin, A. C. T.; Rowland, S. J., Clay and acid catalysed isomerisation and cyclisation reactions of highly branched isoprenoid (HBI) alkenes: Implications for sedimentary reactions and distributions. Geochim. Cosmochim. Acta 2000, 64, (19), 3337-3345.
- Doucette, G. J.; Fryxell, G. A., Thalassiosira antarctica: vegetative and resting stage chemical composition of an ice-related marine diatom Marine Biology 1983, 78, (1), 1-6.
- Hawes, I.; Schwarz, A. M., Photosynthesis in an extreme shade environment: benthic microbial mats from Lake Hoare, a permanently ice-covered Antarctic lake. Journal of Phycology 1999, 35, (3), 448-459.
- Michels, P. C.; Clark, D. S., Pressure dependence of enzyme catalysis. ACS Symposium Series 1992, 498, 108-121.
- Nelson, D. M.; Treguer, P.; Brzezinski, M. A.; Leynaert, A.; Queguiner, B., Production and dissolution of biogenic silica in the ocean: Revised global estimates, comparison with regional data and relationship to biogenic sedimentation. Global Biogeochem. Cycle 1995, 9, 359-372.
- Sverdrup, H. U.; Johnson, M. W.; Fleming, R. H., The Oceans, Their Physics, Chemistry and General Biology. Prentice-Hall: New York, 1942.
- Gross, D.; Zeuthen, E., The buoyancy of planktonic diatoms: a problem of cell physiology. Proc. Roy. Soc. Edinb. 1948, 135, 382-389.
- Waite, A. M.; Thompson, P. A.; Harrison, P. J., Does energy control the sinking rates of marine diatoms? Limnology and Oceanography 1992, 37, (3), 468-477.
- Post, A. F.; Dubinsky, Z.; Wyman, K.; Falkowski, P. G., Kinetics of light-intensity adaptation in a marine planktonic diatom. Marine Biology 1984, 83, (3), 231-238.
- Collyer, D. M.; Fogg, G. E., Studies on fat accumulation by algae. J. Exp. Bot. 1955, 6, (2), 256-275.
- Badour, S. S.; Gergis, M. S., Cell division and fat accumulation in Nitzschia sp grown in continuously illuminated mass cultures. Archiv fur Mikrobiologie 1965, 51, (1), 94-102.
- Werner, D., Die Kieselsäure im Stoffwechsel von Cyclotella cryptica Reimann, Lewin und Guillard. Arch. Mikrobiol. 1966, 55, (3), 278-308.
- Coombs, J.; Darley, W. M.; Holm-Hansen, O.; Volcani, B. E., Studies on the biochemistry and fine structure of silica shell formation in diatoms. Chemical composition of Navicula pelliculosa during silicon-starvation synchrony. Plant Physiol. 1967, 42, (11), 1601-1606.
- Richardson, T. L.; Cullen, J. J., Changes in buoyancy and chemical composition during growth of a coastal marine diatom: Ecological and biogeochemical consequences. Marine Ecology-Progress Series 1995, 128, (1-3), 77-90.
- Waite, A.; Harrison, P. J., Role of sinking and ascent during sexual reproduction in the marine diatom Ditylum brightwellii. Marine Ecology-Progress Series 1992, 87, (1-2), 113-122.
- Belt, S. T.; Massé, G.; Allard, W. G.; Robert, J. M.; Rowland, S. J., Effects of auxosporulation on distributions of C25 and C30 isoprenoid alkenes in Rhizosolenia setigera. Phytochemistry 2002, 59, (2), 141-148.
- Jacobs, W., Das Schweben der Wasserorganismen. Ergeb. Biol. 1935, 11, 131-218.
- Smayda, T. J.; Boleyn, B. J., Experimental observations on the flotation of marine diatoms. I. Thalassiosira CF. Nana, Thalassiosira rotula and Nitzschia seriata. Limnol Oceanogr 1965, 10, (4), 499-509.
- Villareal, T. A., Buoyancy properties of the giant diatom Ethmodiscus. J. Plankton Res. 1992, 14, 459-463.
- Villareal, T. A.; Lipschultz, F., Internal nitrate concentations in single cells of large phytoplankton from the Sargasso Sea. Journal of Phycology 1995, 31, (5), 689-696.
- Villareal, T. A.; Woods, S.; Moore, J. K.; Culver-Rymsza, K., Vertical migration of Rhizosolenia mats and their significance to NO3- fluxes in the central North Pacific gyre. Journal of Plankton Research 1996, 18, (7), 1103-1121.
- Villareal, T. A.; Joseph, L.; Brzezinski, M. A.; Shipe, R. F.; Lipschultz, F.; Altabet, M. A., Biological and chemical characteristics of the giant diatom Ethmodiscus (Bacillariophyceae) in the central north Pacific gyre. J. Phycol. 1999, 35, (5), 896-90.
- Lancelot, C.; Mathot, S., Biochemical fractionation of primary production by phytoplankton in Belgian coastal waters during short-term and long-term incubations with 14C-bicarbonate .1. Mixed diatom population. Marine Biology 1985, 86, (3), 219-226.
- Gautier, C.; Livage, J.; Coradin, T.; Lopez, P. J., Sol-gel encapsulation extends diatom viability and reveals their silica dissolution capability. Chem Commun (Camb) 2006, (44), 4611-3.
- Tesson, B.; Gaillard, C.; Martin-Jézéquel, V., Brucite formation mediated by the diatom Phaeodactylum tricornutum. Marine Chemistry 2008, 109, (1-2), 60-76.
- Faraloni, C.; De Philippis, R.; Sili, C.; Vincenzini, M., Carbohydrate synthesis by two Navicula strains isolated from benthic and pelagic mucilages in the Tyrrhenian Sea (Tuscan Archipelago). Journal of Applied Phycology 2003, 15, (2-3), 259-261.
- Wustman, B. A.; Lind, J.; Wetherbee, R.; Gretz, M. R., Extracellular matrix assembly in diatoms - (Bacillariophyceae) - III. Organization of fucoglucuronogalactans within the adhesive stalks of Achnanthes longipes. Plant Physiology 1998, 116, (4), 1431-1441.
- McConville, M. J.; Wetherbee, R.; Bacic, A., Subcellular location and composition of the wall and secreted extracellular sulphated polysaccharides/proteoglycans of the diatom Stauroneis amphioxys Gregory. Protoplasma 1999, 206, (1-3), 188-200.
- Gordon, R.; Drum, R. W., A capillarity mechanism for diatom gliding locomotion. Proc Natl Acad Sci USA 1970, 67, (1), 338-344.
- Gordon, R., A retaliatory role for algal projectiles, with implications for the mechanochemistry of diatom gliding motility. J. Theor. Biol. 1987, 126, 419-436.
- Haynes, K.; Hofmann, T. A.; Smith, C. J.; Ball, A. S.; Underwood, G. J.; Osborn, A. M., Diatom-derived carbohydrates as factors affecting bacterial community composition in estuarine sediments. Appl Environ Microbiol 2007, 73, (19), 6112-24.
- Lee, J. B.; Hayashi, K.; Hirata, M.; Kuroda, E.; Suzuki, E.; Kubo, Y.; Hayashi, T., Antiviral sulfated polysaccharide from Navicula directa, a diatom collected from deep-sea water in Toyama Bay. Biol Pharm Bull 2006, 29, (10), 2135-9.
- Doucette, G. J.; Fryxell, G. A., Thalassiosira antarctica (Bacillariophyceae): vegetative and resting stage ultrastructure of an ice-related marine diatom Polar Biology 1985, 4, (2), 107-112.
- Fahl, K.; Kattner, G., Lipid-content and fatty-acid composition of algal communities in sea-ice and water from the Weddell Sea (Antarctica). Polar Biology 1993, 13, (6), 405-409.
- Wilhelm, C.; B¸chel, C.; Fisahn, J.; Goss, R.; Jakob, T.; Laroche, J.; Lavaud, J.; Lohr, M.; Riebesell, U.; Stehfest, K.; Valentin, K.; Kroth, P. G., The regulation of carbon and nutrient assimilation in diatoms is significantly different from green algae. Protist 2006, 157, (2), 91-124.
- Waller, R. F.; Keeling, P. J.; Donald, R. G.; Striepen, B.; Handman, E.; Lang-Unnasch, N.; Cowman, A. F.; Besra, G. S.; Roos, D. S.; McFadden, G. I., Nuclear-encoded proteins target to the plastid in Toxoplasma gondii and Plasmodium falciparum. Proc Natl Acad Sci U S A 1998, 95, (21), 12352-7.
- Armbrust, E. V.; Berges, J. A.; Bowler, C.; Green, B. R.; Martinez, D.; Putnam, N. H.; Zhou, S.; Allen, A. E.; Apt, K. E.; Bechner, M.; Brzezinski, M. A.; Chaal, B. K.; Chiovitti, A.; Davis, A. K.; Demarest, M. S.; Detter, J. C.; Glavina, T.; Goodstein, D.; Hadi, M. Z.; Hellsten, U.; Hildebrand, M.; Jenkins, B. D.; Jurka, J.; Kapitonov, V. V.; Kröger, N.; Lau, W. W.; Lane, T. W.; Larimer, F. W.; Lippmeier, J. C.; Lucas, S.; Medina, M.; Montsant, A.; Obornik, M.; Parker, M. S.; Palenik, B.; Pazour, G. J.; Richardson, P. M.; Rynearson, T. A.; Saito, M. A.; Schwartz, D. C.; Thamatrakoln, K.; Valentin, K.; Vardi, A.; Wilkerson, F. P.; Rokhsar, D. S., The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science 2004, 306, (5693), 79-86.
- Princen, L. H., Alternate industrial feedstocks from agriculture. Economic Botany 1982, 36, (3), 302-312.
- Neenan, B.; Feinberg, D.; Hill, A.; McIntosh, R.; Terry, K., Fuels from Microalgae: Technology Status, Potential, and Research Requirements”. Publ. No. SERI/SP-231-2550. Solar Energy Research Institute: Golden, CO, 1986.
- Meier, R. L., Biological cycles in the transformation of solar energy into useful fuels. In Solar Energy Research, Daniels, R.; Duffie, J. A., Eds. University of Wisconsin Press: Madison, WI, 1955; pp 179-184.
- Formo, M. W., Physical properties of fats and fatty acids. In Bailey’s Industrial Oil and Fat Products, 4th ed.; Swern, D., Ed. Wiley & Sons: New York, 1979; Vol. 1, pp 177-190.
- Cheremisinoff, N. P.; Cheremisinoff, P. N.; Ellerbusch, F., Biomass: Applications, Technology, and Production. M. Dekker: New York, 1980; p vii, 221 p.
- Hall, D. O.; House, J. I., Biomass: a modern and environmentally acceptable fuel. Solar Energy Materials and Solar Cells 1995, 38, 521-542.
- Opute, F. I., Lipid and fatty-acid composition of diatoms. Journal of Experimental Botany 1974, 25, (87), 823-835.
- Ahlgren, G.; Lundstedt, L.; Brett, M.; Forsberg, C., Lipid composition and food quality of some freshwater phytoplankton for cladoceran zooplankters. Journal of Plankton Research 1990, 12, (4), 809-818.
- Renaud, S. M.; Parry, D. L.; Thinh, L. V., Microalgae for use in tropical aquaculture I: Gross chemical composition and fatty acid composition of twelve species of microalgae from the Northern Territory, Australia. Journal of Applied Phycology 1994, 6, (3), 337-345.
- Chen, G. Q.; Jiang, Y.; Chen, F., Fatty acid and lipid class composition of the eicosapentaenoic acid-producing microalga, Nitzschia laevis. Food Chemistry 2007, 104, (4), 1580-1585.
- Belt, S. T.; Allard, W. G.; Massé, G.; Robert, J. M.; Rowland, S. J., Structural characterisation of C30 highly branched isoprenoid alkenes (rhizenes) in the marine diatom Rhizosolenia setigera. Tetrahedron Letters 2001, 42, (32), 5583-5585.
- Belt, S. T.; Massé, G.; Rowland, S. J.; Rohmer, M., Highly branched isoprenoid alcohols and epoxides in the diatom Haslea ostrearia Simonsen. Organic Geochemistry 2006, 37, (2), 133-145.
- Kooistra, W. H. C. F., The evolution of the diatoms. In Handbook of Biomineralization, Baeuerlein, E.; Behrens, P.; Epple, M.; Pickett-Heaps, J., Eds. Wiley-VCH: 2007; pp 95-112.
- Sinninghe Damsté, J. S.; Muyzer, G.; Abbas, B.; Rampen, S. W.; Massé, G.; Allard, W. G.; Belt, S. T.; Robert, J. M.; Rowland, S. J.; Moldowan, J. M.; Barbanti, S. M.; Fago, F. J.; Denisevich, P.; Dahl, J.; Trindade, L. A. F.; Schouten, S., The rise of the rhizosolenid diatoms. Science 2004, 304, (5670), 584-587.
- Volkman, J. K.; Barrett, S. M.; Dunstan, G. A., C25 and C30 highly branched isoprenoid alkenes in laboratory cultures of two marine diatoms. Organic Geochemistry 1994, 21, (3-4), 407-413.
- Sinninghe Damsté, J. S.; Schouten, S.; Rijpstra, W. I. C.; Hopmans, E. C.; Peletier, H.; Gieskes, W. W. C.; Geenevasen, J. A. J., Structural identification of the C25 highly branched isoprenoid pentaene in the marine diatom Rhizosolenia setigera. Organic Geochemistry 1999, 30, (12), 1581-1583.
- Belt, S. T.; Allard, W. G.; Massé, G.; Robert, J. M.; Rowland, S. J., Highly branched isoprenoids (HBIs): Identification of the most common and abundant sedimentary isomers. Geochim. Cosmochim. Acta 2000, 64, (22), 3839-3851.
- Grossi, V.; Beker, B.; Geenevasen, J. A. J.; Schouten, S.; Raphel, D.; Fontaine, M. F.; Sinninghe Damsté, J. S., C25 highly branched isoprenoid alkenes from the marine benthic diatom Pleurosigma strigosum. Phytochemistry 2004, 65, (22), 3049-3055.
- Wraige, E. J.; Johns, L.; Belt, S. T.; Massé, G.; Robert, J. M.; Rowland, S., Highly branched C25 isoprenoids in axenic cultures of Haslea ostrearia. Phytochemistry 1999, 51, (1), 69-73.
- Colomb, A.; Yassaa, N.; Williams, J.; Peeken, I.; Lochte, K., Screening volatile organic compounds (VOCs) emissions from five marine phytoplankton species by head space gas chromatography/mass spectrometry (HS-GC/MS). Journal of Environmental Monitoring 2008, 10, (3), 325-330.
- McKay, W. A.; Turner, M. F.; Jones, B. M. R.; Halliwell, C. M., Emissions of hydrocarbons from marine phytoplankton - some results from controlled laboratory experiments. Atmospheric Environment 1996, 30, (14), 2583-2593.
- Ratte, M.; Bujok, O.; Spitzy, A.; Rudolph, J., Photochemical alkene formation in seawater from dissolved organic carbon: Results from laboratory experiments. Journal of Geophysical Research-Atmospheres 1998, 103, (D5), 5707-5717.
- Moore, R. M.; Oram, D. E.; Penkett, S. A., Production of isoprene by marine phytoplankton cultures. Geophys. Res. Lett. 1994, 21, (23), 2507-2510.
- Milne, P. J.; Riemer, D. D.; Zika, R. G.; Brand, L. E., Measurement of vertical distribution of isoprene in surface seawater, its chemical fate, and its emission from several phytoplankton monocultures. Marine Chemistry 1995, 48, (3-4), 237-244.
- Pohnert, G., Diatom/copepod interactions in plankton: the indirect chemical defense of unicellular algae. Chembiochem 2005, 6, (6), 946-959.
- Fontana, A.; d'Ippolito, G.; Cutignano, A.; Romano, G.; Lamari, N.; Gallucci, A. M.; Cimino, G.; Miralto, A.; Ianora, A., LOX-induced lipid peroxidation mechanism responsible for the detrimental effect of marine diatoms on Zooplankton grazers. Chembiochem 2007, 8, (15), 1810-1818.
- Wichard, T.; Gerecht, A.; Boersma, M.; Poulet, S. A.; Wiltshire, K.; Pohnert, G., Lipid and fatty acid composition of diatoms revisited: Rapid wound-activated change of food quality parameters influences herbivorous copepod reproductive success. Chembiochem 2007, 8, (10), 1146-1153.
- Barofsky, A.; Pohnert, G., Biosynthesis of polyunsaturated short chain aldehydes in the diatom Thalassiosira rotula. Org Lett 2007, 9, (6), 1017-20.
- Garg, A.; Bhosle, N. B., Abundance of macroalgal organic matter in biofilms: Evidence from n-alkane biomarkers. Biofouling 2004, 20, (3), 155-165.
- Tornabene, T. G.; Kates, M.; Volcani, B. E., Sterols, aliphatic hydrocarbons, and fatty acids of a nonphotosynthetic diatom, Nitzschia alba. Lipids 1974, 9, (4), 279-84.
- Volkman, J. K.; Eglinton, G.; Corner, E. D. S., Sterols and fatty acids of the marine diatom Biddulphia sinensis. Phytochemistry 1980, 19, (8), 1809-1813.
- Nichols, D. S.; Nichols, P. D.; Sullivan, C. W., Fatty-acid, sterol and hydrocarbon composition of Antarctic sea-ice diatom communities during the spring bloom in McMurdo Sound. Antarctic Science 1993, 5, (3), 271-278.
- Viso, A. C.; Marty, J. C., Fatty acids from 28 marine microalgae. Phytochemistry 1993, 34, (6), 1521-1533.
- Suzuki, T.; Matsuyama, Y., Determination of free fatty acids in marine phytoplankton causing red tides by fluorometric high-performance liquid chromatography. Journal of the American Oil Chemists‘ Society 1995, 72, (10), 1211-1214.
- Whyte, J. N. C.; Ginther, N. G.; Townsend, L. D., Formation of domoic acid and fatty acids in Pseudonitzschia pungens f. multiseries with scale of culture Journal of Applied Phycology 1995, 7, (2), 199-205.
- Reuss, N.; Poulsen, L. K., Evaluation of fatty acids as biomarkers for a natural plankton community. A field study of a spring bloom and a post-bloom period off West Greenland. Marine Biology 2002, 141, (3), 423-434.
- Pel, R.; Floris, V.; Gons, H. J.; Hoogveld, H. L., Linking flow cytometric cell sorting and compound-specific 13C-analysis to determine population-specific isotopic signatures and growth rates in cyanobacteria-dominated lake plankton. Journal of Phycology 2004, 40, (5), 857-866.
- Massé, G.; Belt, S. T.; Rowland, S. J.; Rohmer, M., Isoprenoid biosynthesis in the diatoms Rhizosolenia setigera (Brightwell) and Haslea ostrearia (Simonsen). Proceedings of the National Academy of Sciences of the United States of America 2004, 101, (13), 4413-4418.
- Sinninghe Damsté, J. S.; Rijpstra, W. I. C., Identification of a novel C25 highly branched isoprenoid thiophene in sediments. Organic Geochemistry 1993, 20, (3), 327-331.
- Summons, R. E.; Barrow, R. A.; Capon, R. J.; Hope, J. M.; Stranger, C., The structure of a new C25 isoprenoid alkene biomarker from diatomaceous microbial communities. Australian Journal of Chemistry 1993, 46, (6), 907-915.
- Belt, S. T.; Cooke, D. A.; Robert, J. M.; Rowland, S., Structural characterisation of widespread polyunsaturated isoprenoid biomarkers: a C25 triene, tetraene and pentaene from the diatom Haslea ostrearia Simonsen. Tetrahedron Letters 1996, 37, (27), 4755-4758.
- Wraige, E. J.; Belt, S. T.; Lewis, C. A.; Cooke, D. A.; Robert, J. M.; Massé, G.; Rowland, S. J., Variations in structures and distributions of C25 highly branched isoprenoid (HBI) alkenes in cultures of the diatom, Haslea ostrearia (Simonsen). Organic Geochemistry 1997, 27, (7-8), 497-505.
- Wraige, E. J.; Belt, S. T.; Massé, G.; Robert, J. M.; Rowland, S. J., Variations in distributions of C25 highly branched isoprenoid (HBI) alkenes in the diatom, Haslea ostrearia: influence of salinity. Organic Geochemistry 1998, 28, (12), 855-859.
- Johns, L.; Wraige, E. J.; Belt, S. T.; Lewis, C. A.; Massé, G.; Robert, J. M.; Rowland, S. J., Identification of a C25 highly branched isoprenoid (HBI) diene in Antarctic sediments, Antarctic sea-ice diatoms and cultured diatoms. Organic Geochemistry 1999, 30, (11), 1471-1475.
- Sinninghe Damsté, J. S.; Rijpstra, W. I. C.; Schouten, S.; Peletier, H.; van der Maarel, M.; Gieskes, W. W. C., A C25 highly branched isoprenoid alkene and C25 and C27 n-polyenes in the marine diatom Rhizosolenia setigera. Organic Geochemistry 1999, 30, (1), 95-100.
- Belt, S. T.; Masse, G.; Allard, W. G.; Robert, J. M.; Rowland, S. J., C-25 highly branched isoprenoid alkenes in planktonic diatoms of the Pleurosigma genus. Organic Geochemistry 2001, 32, (10), 1271-1275.
- Rowland, S. J.; Belt, S. T.; Wraige, E. J.; Massé, G.; Roussakis, C.; Robert, J. M., Effects of temperature on polyunsaturation in cytostatic lipids of Haslea ostrearia. Phytochemistry 2001, 56, (6), 597-602.
- Massé, G.; Belt, S. T.; Allard, W. G.; Lewis, C. A.; Wakeham, S. G.; Rowland, S. J., Occurrence of novel monocyclic alkenes from diatoms in marine particulate matter and sediments. Organic Geochemistry 2004, 35, (7), 813-822.
- Rowland, S. J.; Allard, W. G.; Belta, S. T.; Massé, G.; Robert, J. M.; Blackburn, S.; Frampton, D.; Revill, A. T.; Volkman, J. K., Factors influencing the distributions of polyunsaturated terpenoids in the diatom, Rhizosolenia setigera. Phytochemistry 2001, 58, (5), 717-728.
- Rampen, S. W.; Volkman, J. K.; Hur, S. B.; Abbas, B. A.; Schouten, S.; Jameson, I. D.; Holdsworth, D. G.; Bae, J. H.; Sinninghe Damsté, J. S., Occurrence of gorgosterol in diatoms of the genus Delphineis. Organic Geochemistry 2009, 40, (1), 144-147.
- Sicko-Goad, L. M.; Schelske, C. L.; Stoermer, E. F., Estimation of intracellular carbon and silica content of diatoms from natural assemblages using morphometric techniques. Limnology and Oceanography 1984, 29, (6), 1170-1178.
- Mann, A., The economic importance of the diatom. Ann. Rept. Smithsonian Inst. 1916, 386-397.
- Fritsch, F. E., The Structure and Reproduction of the Algae. V. 1. Introduction, Chlorophyceae, Xanthophyceae, Chrysophyceae, Bacillariophyceae, Cryptophyceae, Dinophyceae, Chloromonadineae, Euglenineae, Colorous Flagellata. Cambridge University Press: Cambridge, 1935.
- South, G. R.; Whittick, A., Introduction to Phycology. Blackwell Scientific: Oxford, 1987.
- Coulon, F., Über die Fettbildung und den Plastidenformwechsel bei Nitzschia palea. Arch. Protistenk. 1956, 101, 443-476.
- Gibbs, S. P., The ultrastructure of the pyrenoids of algae, exclusive of the green algae. J. Ultrastr. Res. 1962, 7, (3-4), 247-261.
- Drum, R. W., The cytoplasmic fine structure of the diatom, Nitzschia palea. J. Cell Biol. 1963, 18, 429-440.
- Fogg, G. E., Photosynthesis and formation of fats in a diatom. Ann. Bot. 1956, 20, (78), 265-285.
- Strickland, J. D. H.; Holm-Hansen, O.; Eppley, R. W.; Linn, R. J., The use of a deep tank in plankton ecology. I. Studies of the growth and composition of phytoplankton crops at low nutrient levels. Limnol Oceanogr 1969, 14, 23-34.
- Smith, A. E.; Morris, I., Synthesis of lipid during photosynthesis by phytoplankton of the Southern Ocean. Science 1980, 207, (4427), 197-199.
- Palmisano, A. C.; Sullivan, C. W., Physiology of sea ice diatoms .I. Response of 3 polar diatoms to a simulated summer-winter transition. Journal of Phycology 1982, 18, (4), 489-498.
- Allen, A. E.; Vardi, A.; Bowler, C., An ecological and evolutionary context for integrated nitrogen metabolism and related signaling pathways in marine diatoms. Curr Opin Plant Biol 2006, 9, (3), 264-73.
- Feinberg, D. A., Technical and economic analysis of liquid fuel production from microalgae. In Symposium Papers: Energy from Biomass and Wastes IX, Presented January 28 - February 1, 1085, Lake Buena Vista, Florida, Klass, D. L., Ed. Institute of Gas Technology: Chicago, 1984; pp 1225-1244.
- Ma, F. R.; Hanna, M. A., Biodiesel production: a review. Bioresource Technology 1999, 70, (1), 1-15.
- Kusdiana, D.; Saka, S., Methyl esterification of free fatty acids of rapeseed oil as treated in supercritical methanol. Journal of Chemical Engineering of Japan 2001, 34, (3), 383-387.
- Kusdiana, D.; Saka, S., Kinetics of transesterification in rapeseed oil to biodiesel fuel as treated in supercritical methanol. Fuel 2001, 80, (5), 693-698.
- Gercel, H. F.; Pütün, A. E.; Pütün, E., Hydropyrolysis of extracted Euphorbia rigida in a well-swept fixed-bed tubular reactor. Energy Sources 2002, 24, (5), 423-430.
- Otera, J., Transesterification. Chemical Reviews 1993, 93, (4), 1449-1470.
- Lotero, E.; Liu, Y. J.; López, D. E.; Suwannakarn, K.; Bruce, D. A.; Goodwin, J. G., Synthesis of biodiesel via acid catalysis. Industrial & Engineering Chemistry Research 2005, 44, (14), 5353-5363.
- Kusdiana, D.; Saka, S., Effects of water on biodiesel fuel production by supercritical methanol treatment. Bioresource Technology 2004, 91, (3), 289-295.
- López, D. E.; Goodwin, J. G.; Bruce, D. A.; Lotero, E., Transesterification of triacetin with methanol on solid acid and base catalysts. Applied Catalysis a-General 2005, 295, (2), 97-105.
- Bunyakiat, K.; Makmee, S.; Sawangkeaw, R.; Ngamprasertsith, S., Continuous production of biodiesel via transesterification from vegetable oils in supercritical methanol. Energy & Fuels 2006, 20, (2), 812-817.
- van Kasteren, J. M. N.; Nisworo, A. P., A process model to estimate the cost of industrial scale biodiesel production from waste cooking oil by supercritical transesterification. Resources Conservation and Recycling 2007, 50, (4), 442-458.
- Enssani, E., A method for the extraction of liquid hydrocarbons from microalgal biomass. In Proceedings of the 25th Intersociety Energy Conversion Engineering Conference, August 12-17, 1990. IECEC-90, IEEE: 1990; Vol. 6, pp 250-255.
- Ishiwatari, R.; Ishiwatari, M.; Kaplan, I. R.; Rohrback, B. G., Thermal alteration of young kerogen in relation to petroleum genesis. Nature 1976, 264, 347-349.
- Espitalié, J.; Madec, M.; Tissot, B., Role of mineral matrix in kerogen pyrolysis: influence on petroleum generation and migration. AAPG Bulletin-American Association of Petroleum Geologists 1980, 64, (1), 59-66.
- Takeda, N.; Asakawa, T., Study of petroleum generation by pyrolysis--I. Pyrolysis experiments by Rock-Eval and assumption of molecular structural change of kerogen using 13C-NMR. Applied Geochemistry 1988, 3, (5), 441-453.
- Demirbas, A., Global biofuel strategies. Energy Education Science & Technology 2006, 17, (1-2), 27-63.
- Chelf, P., Environmental control of lipid and biomass production in two diatom species. J. Appl. Phycol. 1990, 2, (2), 121-129.
- Clarke, H. T.; Mazur, A., The lipids of diatoms. J. Biol. Chem. 1941, 141, (1), 283-289.
- Chen, G. Q.; Jiang, Y.; Chen, F., Salt-induced alterations in lipid composition of diatom Nitzschia laevis (Bacillariophyceae) under heterotrophic culture condition. Journal of Phycology 2008, 44, (5), 1309-1314.
- Smith, G. M., Cryptogamic Botany. V. 1. Algae and Fungi. 2nd ed.; McGraw-Hill: New York, 1955.
- Wainman, B. C.; Smith, R. E. H.; Rai, H.; Furgal, J. A., Irradiance and lipid production in natural algal populations. In Lipids in Freshwater Ecosystems, Arts, M. T.; Wainmann, B. C., Eds. Springer: 1999; pp 45-70.
- Evans, J. H., The survival of fresh-water algae during dry periods . Part I. An investigation of the algae of 5 small ponds. Journal of Ecology 1958, 46, (1), 149-167.
- Evans, J. H., The survival of fresh-water algae during dry periods . Part II. drying experiments . Part III. Stratification of algae in pond margin litter and mud. Journal of Ecology 1959, 47, (1), 55-81.
- Chaturvedi, R.; Uppalapati, S. R.; Alamsjah, M. A.; Fujita, Y., Isolation of quizalofop-resistant mutants of Nannochloropsis oculata (Eustigmatophyceae) with high eicosapentaenoic acid following N-methyl-N-nitrosourea-induced random mutagenesis. Journal of Applied Phycology 2004, 16, (2), 135-144.
- Wen, Z. Y.; Chen, F., Heterotrophic production of eicosapentaenoic acid by microalgae. Biotechnology Advances 2003, 21, (4), 273-294.
- Jones, G. J.; Nichols, P. D.; Johns, R. B.; Smith, J. D., The effect of mercury and cadmium on the fatty-acid and sterol composition of the marine diatom Asterionella glacialis. Phytochemistry 1987, 26, (5), 1343-1348.
- Jha, A., UK announces world's largest algal biofuel project: Carbon Trust launches £26m project to develop transport fuels made from algae by 2020. http://www.guardian.co.uk/environment/2008/oct/23/biofuels-energy/print 2008.
- Tempelman, R. J., Generalized linear mixed models in dairy cattle breeding. Journal of Dairy Science 1998, 81, (5), 1428-1444.
- Dahl, G. E.; Buchanan, B. A.; Tucker, H. A., Photoperiodic effects on dairy cattle: A review. Journal of Dairy Science 2000, 83, (4), 885-893.
- Dahl, G. E.; Petitclerc, D., Management of photoperiod in the dairy herd for improved production and health. Journal of Animal Science 2003, 81, (Suppl. 3), 11-17.
- Stockdale, C. R., Influence of milking frequency on the productivity of dairy cows. Australian Journal of Experimental Agriculture 2006, 46, (6-7), 965-974.
- Hejazi, M. A.; Wijffels, R. H., Milking of microalgae. Trends Biotechnol 2004, 22, (4), 189-94.
- Hejazi, M. A.; Holwerda, E.; Wijffels, R. H., Milking microalga Dunaliella salina for β-carotene production in two-phase bioreactors. Biotechnology and Bioengineering 2004, 85, (5), 475-481.
- Andersen, F. O., Fate of organic carbon added as diatom cells to oxic and anoxic marine sediment microcosms. Marine Ecology-Progress Series 1996, 134, (1-3), 225-233.
- Mock, T.; Kruse, M.; Dieckmann, G. S., A new microcosm to investigate oxygen dynamics at the sea ice water interface. Aquatic Microbial Ecology 2003, 30, (2), 197-205.
- Carter, C. M.; Ross, A. H.; Schiel, D. R.; Howard-Williams, C.; Hayden, B., In situ microcosm experiments on the influence of nitrate and light on phytoplankton community composition. Journal of Experimental Marine Biology and Ecology 2005, 326, (1), 1-13.
- Cozar, A.; Echevarria, F., Size structure of the planktonic community in microcosms with different levels of turbulence. Scientia Marina 2005, 69, (2), 187-197.
- Kress, N.; Thingstad, T. F.; Pitta, P.; Psarra, S.; Tanaka, T.; Zohary, T.; Groom, S.; Herut, B.; Mantoura, R. F. C.; Polychronaki, T.; Rassoulzadegan, F.; Spyres, G., Effect of P and N addition to oligotrophic Eastern Mediterranean waters influenced by near-shore waters: A microcosm experiment. Deep-Sea Research Part Ii-Topical Studies in Oceanography 2005, 52, (22-23), 3054-3073.
- Scarratt, M. G.; Marchetti, A.; Hale, M. S.; Rivkin, R. B.; Michaud, S.; Matthews, P.; Levasseur, M.; Sherry, N.; Merzouk, A.; Li, W. K. W.; Kiyosawa, H., Assessing microbial responses to iron enrichment in the Subarctic Northeast Pacific: Do microcosms reproduce the in situ condition? Deep-Sea Research Part Ii-Topical Studies in Oceanography 2006, 53, (20-22), 2182-2200.
- Heid, H. W.; Keenan, T. W., Intracellular origin and secretion of milk fat globules. Eur J Cell Biol 2005, 84, (2-3), 245-58.
- Mather, I. H.; Keenan, T. W., Origin and secretion of milk lipids. Journal of Mammary Gland Biology and Neoplasia 1998, 3, (3), 259-273.
- Deyrup-Olsen, I.; Luchtel, D. L., Secretion of mucous granules and other membrane-bound structures: a look beyond exocytosis. Int Rev Cytol 1998, 183, 95-141.
- Patton, S., Milk secretion at the cellular level: a unique approach to the mechanism of exocytosis. J Dairy Sci 1978, 61, (5), 643-50.
- Boutry, J. L.; Bordes, M.; Février, A.; Barbier, M.; Saliot, A., La diatomée marine Chaetoceros simplex calcitrans Paulsen et son environnement. IV. relations avec le milieu de culture: Étude des hydrocarbures [Marine diatom, Chaetoceros simplex calcitrans Paulsen and its environment .IV. Relation to culture medium: study on hydrocarbons]. Journal of Experimental Marine Biology and Ecology 1977, 28, (1), 41-51.
- Hejazi, M. A.; Kleinegris, D.; Wijffels, R. H., Mechanism of extraction of β-carotene from microalga Dunaliellea salina in two-phase bioreactors. Biotechnology and Bioengineering 2004, 88, (5), 593-600.
- Ginzburg, M.; Ginzburg, B. Z.; Wayne, R., Ultrarapid endocytotic uptake of large molecules in Dunaliella species. Protoplasma 1999, 206, (1-3), 73-86.
- Croteau, R., Oil-filled glands on a leaf. Trends in Plant Science 2001, 6, (9), 439-439.
- Lange, B. M.; Wildung, M. R.; Stauber, E. J.; Sanchez, C.; Pouchnik, D.; Croteau, R., Probing essential oil biosynthesis and secretion by functional evaluation of expressed sequence tags from mint glandular trichomes. Proceedings of the National Academy of Sciences of the United States of America 2000, 97, (6), 2934-2939.
- Sharma, S.; Sangwan, N. S.; Sangwan, R. S., Developmental process of essential oil glandular trichome collapsing in menthol mint. Current Science 2003, 84, (4), 544-550.
- Wagner, G. J.; Wang, E.; Shepherd, R. W., New approaches for studying and exploiting an old protuberance, the plant trichome. Annals of Botany 2004, 93, (1), 3-11.
- Schmid, A. M. M.; Eberwein, R. K.; Hesse, M., Pattern morphogenesis in cell walls of diatoms and pollen grains: A comparison. Protoplasma 1996, 193, (1-4), 144-173.
- Dugdale, T. M.; Willis, A.; Wetherbee, R., Adhesive modular proteins occur in the extracellular mucilage of the motile, pennate diatom Phaeodactylum tricornutum. Biophysical Journal 2006, 90, (8), L58-L60.
- Higgins, M. J.; Crawford, S. A.; Mulvaney, P.; Wetherbee, R., Characterization of the adhesive mucilages secreted by live diatom cells using atomic force microscopy. Protist 2002, 153, (1), 25-38.
- Fernández, F. G.; Pérez, J. A.; Sevilla, J. M.; Camacho, F. G.; Grima, E. M., Modeling of eicosapentaenoic acid (EPA) production from Phaeodactylum tricornutum cultures in tubular photobioreactors. Effects of dilution rate, tube diameter, and solar irradiance. Biotechnol Bioeng 2000, 68, (2), 173-83.
- Raniello, R.; Iannicelli, M. M.; Nappo, M.; Avila, C.; Zupo, V., Production of Cocconeis neothumensis (Bacillariophyceae) biomass in batch cultures and bioreactors for biotechnological applications: light and nutrient requirements. Journal of Applied Phycology 2007, 19, (4), 383-391.
- Schlichting, H.; Gersten, K., Boundary Layer Theory. 8th rev. and enl. ed.; Springer Verlag: 2000; p xxiii/799p.
- Barry, P., Thinning fuel before injection boosts efficiency. Science News 2008, 174, (9), 9.
- Tao, R.; Huang, K.; Tang, H.; Bell, D., Electrorheology leads to efficient combustion. Energy & Fuels 2008, 22, (6), 3785–3788.
- Evans, J. H., Further investigations of the algae of pond margins. Hydrobiologia 1960, 15, (4), 384-294.
- Hoekema, S.; Douma, R. D.; Janssen, M.; Tramper, J.; Wijffels, R. H., Controlling light-use by Rhodobacter capsulatus continuous cultures in a flat-panel photobioreactor. Biotechnology and Bioengineering 2006, 95, (4), 613-626.
- Hoekema, S.; Douma, R. D.; Janssen, M.; Tramper, J.; Wijffels, R. H., Erratum: Controlling light-use by Rhodobacter capsulatus continuous cultures in a flat-panel photobioreactor (vol 95, pg 613, 2006). Biotechnology and Bioengineering 2008, 99, (1), 249-249.
- Carvalho, A. P.; Meireles, L. A.; Malcata, F. X., Microalgal reactors: A review of enclosed system designs and performances. Biotechnology Progress 2006, 22, (6), 1490-1506.
- Shi, D. J.; Hall, D. O., The Azolla-Anabaena association: historical perspective, symbiosis and energy metabolism. Bot. Rev. 1988, 54, (4), 353-386.
- Lee, J. J., Algal symbiosis in larger foraminifera. Symbiosis 2006, 42, (2), 63-75.
- Pienaar, R. N.; Sakai, H.; Horiguchi, T., Description of a new dinoflagellate with a diatom endosymbiont, Durinskia capensis sp. nov. (Peridiniales, Dinophyceae) from South Africa. J Plant Res 2007, 120, (2), 247-58.
- Cerrano, C.; Calcinai, B.; Cucchiari, E.; Di Camillo, C.; Totti, C.; Bavestrello, G., The diversity of relationships between Antarctic sponges and diatoms: the case of Mycale acerata Kirkpatrick, 1907 (Porifera, Demospongiae). Polar Biology 2004, 27, (4), 231-237.
- Inagaki, Y.; Dacks, J. B.; Doolittle, W. F.; Watanabe, K. I.; Ohama, T., Evolutionary relationship between dinoflagellates bearing obligate diatom endosymbionts: insight into tertiary endosymbiosis. Int J Syst Evol Microbiol 2000, 50 Pt 6, 2075-81.
- Janson, S.; Wouters, J.; Bergman, B.; Carpenter, E. J., Host specificity in the Richelia-diatom symbiosis revealed by hetR gene sequence analysis. Environ Microbiol 1999, 1, (5), 431-438.
- Lee, J. J.; Reimer, C. W.; McEnery, M. E., Identification of diatoms isolated as endosymbionts from larger foraminifera from the Gulf of Eilat (Red Sea) and the description of two new species, Fragilaria shiloi sp nov and Navicula reissii sp nov. Botanica Marina 1980, 23, (1), 41-48.
- Peyton, K. A.; Hanisak, M. D.; Lin, J. D., Marine algal symbionts benefit benthic invertebrate embryos deposited in gelatinous egg masses. Journal of Experimental Marine Biology and Ecology 2004, 307, (2), 139-164.
- Hasle, G. R., Navicula endophytica sp. nov., a pennate diatom with an unusual mode of existence. Br. Phycol. Bull. 1968, 3, (3), 475-480.
- Buck, K. R.; Bentham, W. M., A novel symbiosis between a cyanobacterium, Synechococcus sp., An aplastidic protist, Solenicola setigera, and a diatom, Leptocylindrus mediterraneus, in the open ocean. Marine Biology 1998, 132, (3), 349-355.
- Bavestrello, G.; Arillo, A.; Calcinai, B.; Cattaneo-Vietti, R.; Cerrano, C.; Gaino, E.; Penna, A.; Sarà, M., Parasitic diatoms inside antarctic sponges. Biol Bull 2000, 198, (1), 29-33.
- Jolley, E. T.; Jones, A. K., Interaction between Navicula muralis Grunow and an associated species of Flavobacterium. British Phycological Journal 1977, 12, (4), 315-328.
- Fogg, G. E., Some speculations on the nature of the pelagic mucilage community of the northern Adriatic Sea. Science of the Total Environment 1995, 165, (1-3), 59-63.
- Okamoto, N.; Nagumo, T.; Tanaka, J.; Inouye, I., An endophytic diatom Gyrosigma coelophilum sp nov (Naviculales, Bacillariophyceae) lives inside the red alga Coelarthrum opuntia (Rhodymeniales, Rhodophyceae). Phycologia 2003, 42, (5), 498-505.
- Booth, W. E., Navicula climacospheniae sp-nov, an endophytic diatom inhabiting the stalk of Climacosphenia moniligera Ehrenberg. Nova Hedwigia 1986, 42, (2-4), 295-300.
- Drum, R. W., Endophytic diatoms in cortex of submerged Salix roots. Journal of Phycology 1967, 3, (Suppl.), 4.
- Biebl, R.; Kusel-Fetzmann, E., Beobachtungen über das Vorkommen von Algen an Thermalstandorten auf Island [Observations on the occurrence of algae at thermal sites in Iceland]. Österr. Bot. Zeitschr. 1966, 113, (3/4), 408-423.
- Jha, M., Hydrobiological studies on Suraj Kund and Chandrama Kund, hot-springs of Rajgir, Bihar, India. Internationale Revue Der Gesamten Hydrobiologie 1992, 77, (3), 435-443.
- Mpawenayo, B.; Cocquyt, C.; Nindorera, A., Diatoms (Bacillariophyta) and other algae from the hot springs of Burundi (Central Africa) in relation with the physical and chemical characteristics of the water. Belgian Journal of Botany 2005, 138, (2), 152-164.
- Brown, P. B.; Wolfe, G. V., Protist genetic diversity in the acidic hydrothermal environments of Lassen Volcanic National Park, USA. Journal of Eukaryotic Microbiology 2006, 53, (6), 420-431.
- Bryanskaya, A. V.; Namsaraev, Z. B.; Kalashnikova, O. M.; Barkhutova, D. D.; Namsaraev, B. B.; Gorlenko, V. M., Biogeochemical processes in algal-bacterial mats of the Urinskii alkaline hot spring. Microbiology 2006, 75, (5), 611-620.
- Jones, B.; Renaut, R. W., Growth of siliceous spicules in acidic hot springs, Waiotapu geothermal area, North Island, New Zealand. Palaios 2006, 21, (5), 406-423.
- Schinteie, R.; Campbell, K. A.; Browne, P. R. L., Microfacies of stromatolitic Sinter from acid-sulphate-chloride springs at Parariki stream, Rotokawa Geothermal Field, New Zealand. Palaeontologia Electronica 2007, 10, (1), http://palaeo-electronica.org/2007_1/sinter/index.html.
- Owen, R. B.; Renaut, R. W.; Jones, B., Geothermal diatoms: a comparative study of floras in hot spring systems of Iceland, New Zealand, and Kenya. Hydrobiologia 2008, 610, 175-192.
- Fairchild, E.; Sheridan, R. P., Physiological investigation of hot spring diatom, Achnanthes exigua Grün. Journal of Phycology 1974, 10, (1), 1-4.
- Zijffers, J. W. F.; Janssen, M.; Tramper, J.; Wijffels, R. H., Design process of an area-efficient photobioreactor. Marine Biotechnology 2008, 10, (4), 404-415.
- Zijffers, J. W. F.; Salim, S.; Janssen, M.; Tramper, J.; Wijffels, R. H., Capturing sunlight into a photobioreactor: Ray tracing simulations of the propagation of light from capture to distribution into the reactor. Chemical Engineering Journal 2008, 145, (2), 316-327.
- MNP Group, Fiber Optics and Algae. http://www.myninjaplease.com/green/?p=696 2007.
- Wohlgeschaffen, G. D.; Rao, D. V. S.; Mann, K. H., Vat incubator with immersion core illumination: a new, inexpensive setup for mass phytoplankton culture. Journal of Applied Phycology 1992, 4, (1), 25-29.
- Weiss, P., Soaking up rays, a primitive marine creature has natural-glass fibers that hint at high tech. Science News 2001, 160, (5), 77-79.
- Bismuto, A.; Setaro, A.; Maddalena, P.; De Stefano, L.; De Stefano, M., Marine diatoms as optical chemical sensors: A time-resolved study. Sensors and Actuators B-Chemical 2008, 130, (1), 396-399.
- Mitchell, E., Diatom nanostructures bend light. http://news.bbc.co.uk/1/hi/sci/tech/7608369.stm 2008.
- Engel, E.; Michiardi, A.; Navarro, M.; Lacroix, D.; Planell, J. A., Nanotechnology in regenerative medicine: the materials side. Trends Biotechnol 2008, 26, (1), 39-47.
- Venugopal, J.; Low, S.; Choon, A. T.; Ramakrishna, S., Interaction of cells and nanofiber scaffolds in tissue engineering. J Biomed Mater Res B Appl Biomater 2008, 84, (1), 34-48.
- Guda, T.; Appleford, M.; Oh, S.; Ong, J. L., A cellular perspective to bioceramic scaffolds for bone tissue engineering: the state of the art. Curr Top Med Chem 2008, 8, (4), 290-9.
- Glowacki, J.; Mizuno, S., Collagen scaffolds for tissue engineering. Biopolymers 2008, 89, (5), 338-44.
- Dunn, J. C., Analyses of cell growth in tissue-engineering scaffolds. Regen Med 2008, 3, (3), 421-4.
- Arumuganathar, S.; Jayasinghe, S. N., Living scaffolds (specialized and unspecialized) for regenerative and therapeutic medicine. Biomacromolecules 2008, 9, (3), 759-66.
- Wang, G. J.; Ho, K. H.; Hsu, S. H.; Wang, K. P., Microvessel scaffold with circular microchannels by photoresist melting. Biomedical Microdevices 2007, 9, (5), 657-663.
- Drum, R. W., Light and electron microscope observations on the tube-dwelling diatom Amphipleura rutilans (Trentepohl) Cleve. Journal of Phycology 1969, 5, (1), 21-26.
- Lobban, C. S., Environmental factors, plant responses, and colony growth in relation to tube-dwelling diatom blooms in the Bay of Fundy, Canada, with a review of the biology of tube-dwelling diatoms. Diatom Res. 1989, 4, (1), 89-109.
- Nultsch, W., Phototactic and photokinetic action spectra of the diatom Nitschia comunis. Photochem. Photobiol. 1971, 14, (6), 705-712.
- Nultsch, W., Phototaxis and photokinesis. In Primitive Sensory and Communication Systems: The Taxes and Tropisms of Micro-Organisms and Cells, Carlile, M. J., Ed. Academic Press: New York, 1975; pp 29-90.
- Cohn, S. A.; Weitzell Jr., R. E., Ecological considerations of diatom cell motility: I. Characterization of motility and adhesion in four diatom species. Journal of Phycology 1996, 32, (6), 928-939.
- Bejan, A., Shape and Structure, from Engineering to Nature. Cambridge University Press: Cambridge, 2000.
- Bejan, A., The constructal law of organization in nature: tree-shaped flows and body size. J Exp Biol 2005, 208, (Pt 9), 1677-86.
- De Stefano, M.; De Stefano, L., Nanostructures in diatom frustules: functional morphology of valvocopulae in Cocconeidacean monoraphid taxa. Journal of Nanoscience and Nanotechnology 2005, 5, (1), 15-24.
- Kingston, J. C., Araphid and monoraphid diatoms. In Freshwater Algae of North America. Ecology and Classification, Wehr, J. D.; Sheath, R. G., Eds. Academic Press: 2003; pp 595–636.
- Boyle, J. A.; Pickett-Heaps, J. D.; Czarnecki, D. B., Valve morphogenesis in the pennate diatom Achnanthes coarctata. J. Phycol. 1984, 20, 563-573.
- Goldman, J. C.; McGillicuddy, D. J., Effect of large marine diatoms growing at low light on episodic new production. Limnology and Oceanography 2003, 48, (3), 1176-1182.
- Karsten, U.; Schumann, R.; Rothe, S.; Jung, I.; Medlin, L., Temperature and light requirements for growth of two diatom species (Bacillariophyceae) isolated from an Arctic macroalga. Polar Biology 2006, 29, (6), 476-486.
- Tolomio, C., Approche expérimentale pour l’étude Des diatomées Des sédiments de la Kagune de Venise, Italie[Experimental approach for the analysis of diatoms in Venice Lagoon sediment, Italy]. Diatom Research 2004, 19, (1), 81-101.
- Friedman, A. L.; Alberte, R. S., Biogenesis and light regulation of the major light harvesting chlorophyll-protein of diatoms. Plant Physiol 1986, 80, (1), 43-51.
- Bartual, A.; Gálvez, L. A., Growth and biochemical composition of the diatom Phaeodactylum tricornutum at different pH and inorganic carbon levels under saturating and subsaturating light regimes. Botanica Marina 2002, 45, (6), 491-501.
- Lohr, M.; Wilhelm, C., Algae displaying the diadinoxanthin cycle also possess the violaxanthin cycle. Proc Natl Acad Sci U S A 1999, 96, (15), 8784-9.
- Coesel, S.; Obornik, M.; Varela, J.; Falciatore, A.; Bowler, C., Evolutionary origins and functions of the carotenoid biosynthetic pathway in marine diatoms. PLoS ONE 2008, 3, (8), e2896.
- Lee, R. F.; Baker, J., Ethane and ethylene production in an estuarine river––formation from the decomposition of polyunsaturated fatty acids. Marine Chemistry 1992, 38, (1-2), 25-36.
- Muñoz, J.; Mudge, S. M.; Sandoval, A., Effects of ionic strength on the production of short chain volatile hydrocarbons by Dunaliella salina (Teodoresco). Chemosphere 2004, 54, (8), 1267-1271.
- Bonsang, B.; Al Aarbaoui, A.; Sciare, J., Diurnal variation of non-methane hydrocarbons in the subantarctic atmosphere. Environmental Chemistry 2008, 5, (1), 16-23.
- Broadgate, W. J.; Liss, P. S.; Penkett, S. A., Seasonal emissions of isoprene and other reactive hydrocarbon gases from the ocean. Geophys. Res. Lett. 1997, 24, (21), 2675-2678.
- Kim, J. D.; Lee, C. G., Systemic optimization of microalgae for bioactive compound production. Biotechnology and Bioprocess Engineering 2005, 10, (5), 418-424.
- Vidoudez, C.; Pohnert, G., Growth phase-specific release of polyunsaturated aldehydes by the diatom Skeletonema marinoi. Journal of Plankton Research 2008, 30, (11), 1305-1313.
- Ribalet, F.; Wichard, T.; Pohnert, G.; Ianora, A.; Miralto, A.; Casotti, R., Age and nutrient limitation enhance polyunsaturated aldehyde production in marine diatoms. Phytochemistry 2007, 68, (15), 2059-67.
- Wichard, T.; Poulet, S. A.; Halsband-Lenk, C.; Albaina, A.; Harris, R.; Liu, D.; Pohnert, G., Survey of the chemical defence potential of diatoms: screening of fifty one species for α,β,γ,δ-unsaturated aldehydes. J Chem Ecol 2005, 31, (4), 949-58.
- Pohnert, G.; Boland, W., The oxylipin chemistry of attraction and defense in brown algae and diatoms. Nat Prod Rep 2002, 19, (1), 108-22.
- Wichard, T.; Poulet, S. A.; Boulesteix, A. L.; Ledoux, J. B.; Lebreton, B.; Marchetti, J.; Pohnert, G., Influence of diatoms on copepod reproduction. II. Uncorrelated effects of diatom-derived α,β,γ,δ-unsaturated aldehydes and polyunsaturated fatty acids on Calanus helgolandicus in the field. Progress in Oceanography 2008, 77, (1), 30-44.
- Ianora, A.; Casotti, R.; Bastianini, M.; Brunet, C.; d'Ippolito, G.; Acri, F.; Fontana, A.; Cutignano, A.; Turner, J. T.; Miralto, A., Low reproductive success for copepods during a bloom of the non-aldehyde-producing diatom Cerataulina pelagica in the North Adriatic Sea. Marine Ecology-an Evolutionary Perspective 2008, 29, (3), 399-410.
- Poulet, S. A.; Escribano, R.; Hidalgo, P.; Cueff, A.; Wichard, T.; Aguilera, V.; Vargas, C. A.; Pohnert, G., Collapse of Calanus chilensis reproduction in a marine environment with high diatom concentration. Journal of Experimental Marine Biology and Ecology 2007, 352, (1), 187-199.
- Fontana, A.; d'Ippolito, G.; Cutignano, A.; Miralto, A.; Ianora, A.; Romano, G.; Cimino, G., Chemistry of oxylipin pathways in marine diatoms. Pure and Applied Chemistry 2007, 79, (4), 481-490.
- van Tamelen, E. E.; Webber, B. D., Hydrogenative deoxygenation of organic compounds: direct conversion of amides to alkanes. Proceedings of the National Academy of Sciences of the United States of America-Physical Sciences 1981, 78, (3), 1321-1322.
- van Tamelen, E. E.; Gladysz, J. A., Direct conversion of aldehydes, esters, and 1,2-oxides to alkanes with carbon skeleton preservation. Journal of the American Chemical Society 1974, 96, (16), 5290-5291.
- Mojaat, M.; Foucault, A.; Pruvost, J.; Legrand, J., Optimal selection of organic solvents for biocompatible extraction of β-carotene from Dunaliella salina. J Biotechnol 2008, 133, (4), 433-41.
- Antić, M. P.; Jovančićević, B. S.; Ilić, M.; Vrvić, M. M.; Schwarzbauer, J., Petroleum pollutant degradation by surface water microorganisms. Environmental Science and Pollution Research 2006, 13, (5), 320-327.
- Villareal, T. A., Positive buoyancy in the oceanic diatom Rhizosolenia debyana Peragallo, H. Deep-Sea Research Part a-Oceanographic Research Papers 1988, 35, (6), 1037-1045.
- Moore, J. K.; Villareal, T. A., Buoyancy and growth characteristics of three positively buoyant marine diatoms. Marine Ecology-Progress Series 1996, 132, (1-3), 203-213.
- Eppley, R. W., Sinking rates of ocean phytoplankton - a citation classic commentary on sinking rates of marine-phytoplankton measured with a fluorometer, by Eppley, R.W., Holmes, R.W., and Strickland, J.D.H. Current Contents/Agriculture Biology & Environmental Sciences 1990, (5), 18-18.
- Eppley, R. W.; Holmes, R. W.; Strickland, J. D. H., Sinking rates of marine phytoplankton measured with a fluorometer. J. Exptl. Marine Biol. Ecol. 1968, 1, (2), 191-208.
- Rai, A. N.; Söderbäck, E.; Bergman, B., Cyanobacterium-plant symbioses. New Phytologist 2000, 147, (3), 449-481.
- Carpenter, E. J.; Janson, S., Intracellular cyanobacterial symbionts in the marine diatom Climacodium frauenfeldianum (Bacillariophyceae). J. Phycol. 2000, 36, (3), 540-544.
- Carpenter, E. J.; Montoya, J. P.; Burns, J.; Mulholland, F.; Subramaniam, A.; Capone, D. G., Extensive blooms of N2-fixing diatom/cyanobacterial association in tropical Atlantic Ocean. Marine Ecology Progress Series 1999, 185, 273-283.
- Coolen, M. J. L.; Volkman, J. K.; Abbas, B.; Muyzer, G.; Schouten, S.; Sinninghe Damsté, J. S., Identification of organic matter sources in sulfidic late Holocene Antarctic fjord sediments from fossil rDNA sequence analysis. Paleoceanography 2007, 22, (2).
- Jarvis, E. E.; Dunahay, T. G.; Roessler, P. G.; Zeiler, K. G.; Brown, L. M., Genetic engineering of microalgae for biodiesel production. Abstracts of Papers of the American Chemical Society 1994, 207, 187-BIOT.
- Roessler, P. G.; Bleibaum, J. L.; Thompson, G. A.; Ohlrogge, J. B., Characteristics of the gene that encodes acetyl-CoA carboxylase in the diatom Cyclotella cryptica. Ann N Y Acad Sci 1994, 721, 250-256.
- Rosenberg, J. N.; Oyler, G. A.; Wilkinson, L.; Betenbaugh, M. J., A green light for engineered algae: redirecting metabolism to fuel a biotechnology revolution. Curr Opin Biotechnol 2008.
- Gordon, R., Computer controlled evolution of diatoms: design for a compustat. Nova Hedwigia 1996, 112, (Festschrift for Prof. T.V. Desikachary), 213-216.
- Ruddiman, W. E., How did humans first alter global climate? Scientific American 2005, 292, (3), 46-53.
- Heinloth, K., Die Energiefrage: Bedarf und Potentiale, Nutzen, Risiken und Kosten. Vieweg+Teubner Verlag: 2003.
- Martinot, E., Renewables 2007 Global Status Report. Worldwatch Institute: Washington, D.C., 2008.
- Schmalensee, R.; Stoker, T. M.; Judson, R. A., World carbon dioxide emissions: 1950-2050. Review of Economics and Statistics 1998, 80, (1), 15-27.
- Soares, M., The ocean our future. Cambridge University Press: Cambridge, England, 1998; p 248 p.
- World Coal Institute, Coal Facts 2008. http://www.worldcoal.org/pages/content/index.asp?PageID=188 2008.
- Dyer, G., End of oil coming faster than we think. Winnipeg Free Press 2008, (December 23), A11.
- Shafiee, S.; Topal, E., An econometrics view of worldwide fossil fuel consumption and the role of US. Energy Policy 2008, 36, (2), 775-786.
- Pickens, T. B., America is addicted to foreign oil. http://www.pickensplan.com/pdf/ThePlan_0710_01.pdf 2008.
- Meng, Q. Y.; Bentley, R. W., Global oil peaking: Responding to the case for 'abundant supplies of oil'. Energy 2008, 33, (8), 1179-1184.
- Mowat, F., Sea of Slaughter. McClelland and Stewart-Bantam: Toronto, 1984.
- Daly, H. E.; Cobb Jr., J. B.; Cobb, C. W., For the Common Good: Redirecting the Economy Toward Community, the Environment, and a Sustainable Future. 2nd ed.; Beacon Press: Boston, 1994; p viii, 534 p.
- Thorpe, M.; Mitra, S., Growing economic interdependence of China and the gulf cooperation council. China & World Economy 2008, 16, (2), 109-126.
- Boussafir, M.; Lallier-Vergès, E., Accumulation of organic matter in the Kimmeridge Clay formation (KCF): an update fossilisation model for marine petroleum source-rocks. Marine and Petroleum Geology 1997, 14, (1), 75-83.
- McKenna, H., Anatomy of a leaf. http://commons.wikimedia.org/wiki/File:Leaf_anatomy.svg 2006.
- Stauth, D.;; Rorrer, G.Media Release: Ancient Diatoms Lead to New Technology for Solar Energy. http://oregonstate.edu/dept/ncs/newsarch/2009/Apr09/diatoms.html, 2009,