| |
ENVIS Technical Report: 86, February 2015 |
 |
FISH MORTALITY IN JAKKUR LAKE: CAUSES AND REMEDIAL MEASURES |
 |
Energy and Wetlands Research Group, Centre for Ecological Sciences, Indian Institute of Science, Bangalore – 560012, India.
*Corresponding author: cestvr@ces.iisc.ac.in
|
|
Comparison of Physico Chemical Parameters with Earlier Studies
(Ramachandraet.al, 2014): The variations of physicochemical parameters of lake water are shown in figure 2-5.
Nutrients (Nitrates and Phosphates): The inlet showed an increase in the amount of nutrients with time. The nitrate values have increased almost 4 times as compared to 2013. Also, an increase in the values of phosphate were observed at the inlet. The middle portion of the lake showed 5 times increase in the values of nitrate as compared to 2013. The phosphate values increase around 5 times in the middle portion. Same trend was followed in the outlet of the lake. The 2phosphate and nitrate values increased by 4 times as compared to 2013 values (figure 2).
The results show that there is a continuous increase in the quantity of nutrient inflow into the lake over a period of time. This led to the excess growth of plants i.e., algae and macrophytes. When the plants die and decay, anaerobic conditions prevail with lower oxygen levels, which then turns green or milky white and gives off a strong rotten egg odour. The lack of oxygen is lethal for invertebrates, fish, etc.
Excess plants in a water body can create many problems. An excess in the growth of plants and algae create an unstable amount of dissolved oxygen. During the day, there will be higher levels of dissolved oxygen (in the region of algal bloom), and at night the levels of oxygen decline drastically, creating stressful conditions for fish. If the conditions persist for a long period of time, the stressed fish species will die off.
Dissolved Oxygen (DO): Figure 4 shows the variation in DO in the lake across time period. There was not much variation in the DO values in the inlet. The middle and outlet portion of the lake showed increase in DO compared to earlier studies. This is mainly because of excess of algal and macrophyte growth. An excess in the growth of plants and algae create an unstable amount of dissolved oxygen. During the day, there will be usually be high levels of dissolved oxygen, and at night the levels of oxygen can decrease dramatically. This will create stressful conditions for fish.
COD and BOD: The inlets showed higher BOD and COD values than the earlier investigations. The middle portion of the lake showed higher values than all earlier values. COD was almost 2 times higher than 2013. The outlet of the lake also showed similar trend in the BOD and COD (figure 3). This shows that there is high amount of organic load in the lake. BOD and COD directly affect the amount of dissolved oxygen in lakes, rivers and streams. The greater the BOD and COD, the more rapidly oxygen is depleted in the lake. This means less oxygen is available to higher forms of aquatic life. The consequences of high BOD and COD are the same as those for low dissolved oxygen: aquatic organisms gets stressed, suffocate, and die.
pH: pH was higher (8.8-10.3) throughout compared to previous study (figure 5). The majority of aquatic creatures prefer a pH range of 6.5-9.0. The release of carbonate converted from bicarbonate by plant life can cause pH to climb dramatically (above 9) during periods of rapid photosynthesis by dense phytoplankton (algal) blooms (Wurts and Masser. 2004). A slight change in the pH of water can increase the solubility of phosphorus and other nutrients making them more accessible for plant growth. When the pH of lake becomes highly alkaline, the effect on fish includes death, damage to gills, eyes etc. High pH also increases the toxicity of other substances and increasing the risk of absorption by aquatic life. Example ammonia is 10 times more severe at pH 8 than at pH 7.
Chlorides, Alkalinity and Hardness: The chloride values were high in the inlet compared to earlier studies. The middle and outlet portion did not show much variation (Figure 6). Alkalinity values were higher throughout the lake compared to the earlier analysis. Total hardness was also high during this study than earlier studies. Hardness mitigates metals toxicity, because Ca2+ and Mg2+ help keep fish from absorbing metals such as lead, arsenic, and cadmium into their bloodstream through their gills. The greater the hardness, the harder it is for toxic metals to be absorbed through the gills (Wurts and Robert M. Durborow, 1992).
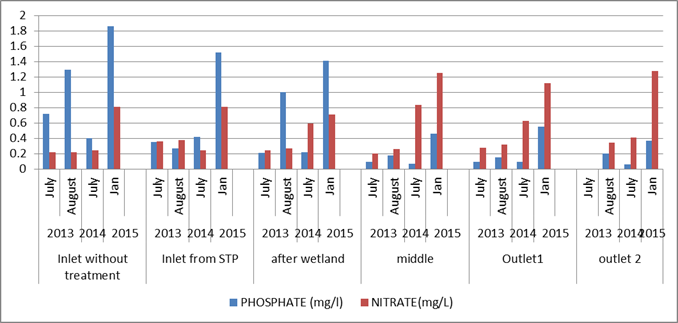
Figure 2: Comparison of Nutrients in the lake across time period

Figure 3: Comparison of COD and BOD in the lake across time period
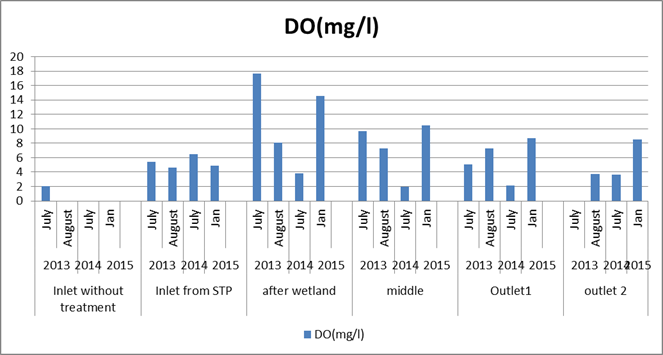
Figure 4: Comparison of DO in the lake across time period
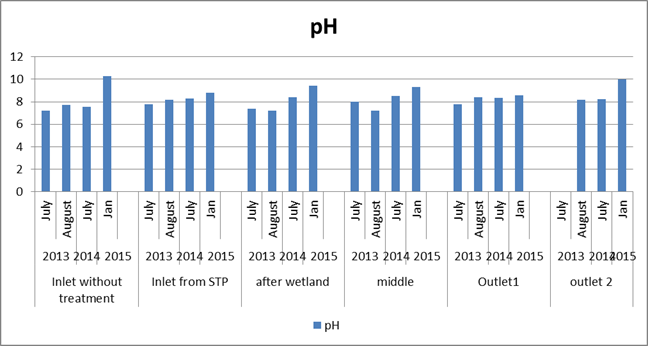
Figure 5: Comparison of pH in the lake across time period
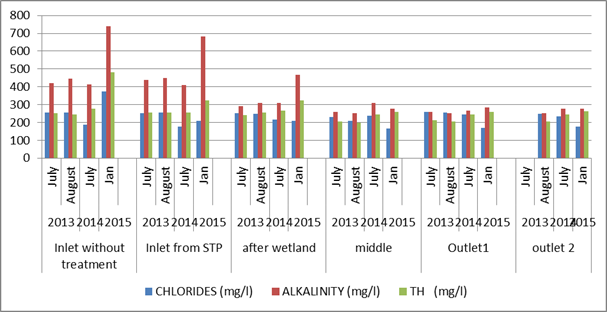
Figure 6: Comparison of chlorides, alkalinity and total hardnessin the lake across time period
|
T.V. Ramachandra
Centre for Sustainable Technologies, Centre for infrastructure, Sustainable Transportation and Urban Planning (CiSTUP), Energy & Wetlands Research Group, Centre for Ecological Sciences, Indian Institute of Science, Bangalore – 560 012, INDIA.
E-mail : cestvr@ces.iisc.ac.in
Tel: 91-080-22933099/23600985,
Fax: 91-080-23601428/23600085
Web: http://ces.iisc.ac.in/energy
Sudarshan P.Bhat
Energy & Wetlands Research Group, Centre for Ecological Sciences, Indian Institute of Science, Bangalore – 560 012, INDIA.
E-mail: asulabha@ces.iisc.ac.in
Sincy V.
Energy & Wetlands Research Group, Centre for Ecological Sciences, Indian Institute of Science, Bangalore – 560 012, INDIA.
E-mail: sincy@ces.iisc.ac.in
Asulabha K S
Energy & Wetlands Research Group, Centre for Ecological Sciences, Indian Institute of Science, Bangalore – 560 012, INDIA.
E-mail: sudarshan@ces.iisc.ac.in
Kruthika Lakkangoudar
Energy & Wetlands Research Group, Centre for Ecological Sciences, Indian Institute of Science, Bangalore – 560 012, INDIA.
E-mail: bharath@ces.iisc.ac.in
Rahaman M.F.
Energy & Wetlands Research Group, Centre for Ecological Sciences, Indian Institute of Science, Bangalore – 560 012, INDIA.
E-mail: bharath@ces.iisc.ac.in
Citation: Ramachandra T V, Sudarshan P. Bhat, Asulabha K S, Sincy V, Kruthika L. and Rahaman M F, 2015.Fish mortality in Jakkur lake: Causes and Remedial Measures, ENVIS Technical Report 86, CES, Indian Institute of Science, Bangalore 560012
| |