|
Materials and Methods
Study Area Benthic diatoms were sampled across salinity gradients along the shorelines (intertidal regions) of the Aghanashini estuary extending between 14° 27' 53.64'' N, 74° 29' 26.88'' E - 14° 31’ 18.8” N, 74° 20’ 54.44” E in the Indian west coast. Six different sampling stations were fixed after a reconnaissance survey of the study region and were categorized primarily based on varying salinity gradients as well as the levels of anthropogenic pressures prevalent at each station. Figure 2 maps the locations of sampling stations. Stations S1 and S2 are mesohaline (5 – 18 ppt) regions, S3 and S4 are polyhaline (8 – 25 ppt) regions at upper reaches, and S5 and S6 are polyhaline (25 – 30 ppt) regions at lower reaches of the estuary. The station S1 to S6 also vary in their levels of anthropogenic stresses evident based on the value of population density of each village around Aghanashini estuary. Station S1 has the second-highest population density among other villages with 14 – 18 persons per hectare. Stations S2 and S5 has a population density of approximately 9 – 14 persons per hectare. Stations S2 and S4 have the least population density of 0 – 4 persons per hectare. S6 falls in the range of 4 – 9 persons per hectare. The thematic layers based on population density (No of persons per Ha.) of the villages around the sampling locations are represented in Figure 2.
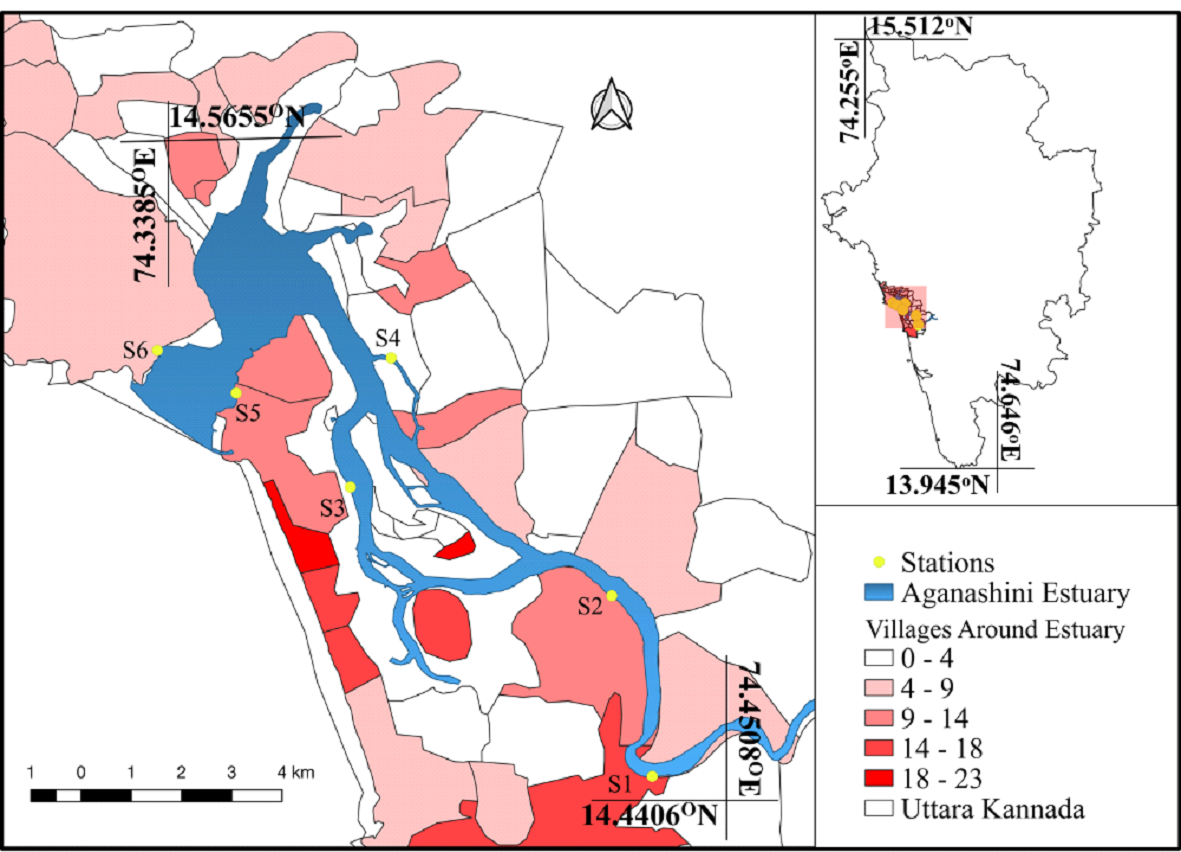
Figure 2: Aghanashini estuary with sampling locations Sampling The water along with diatom samples was collected at select sampling locations in the intertidal regions of the estuary, once in every month, during July 2016 to June 2017 at low tide hours of the day as visibility and better accessibility of landmass, mud-flats, sediments, and stones was possible during low tide as the water recedes towards the downstream during low tide. Water and benthic diatom samples were collected from different substrata. Physico-chemical parameters like air temperature, water temperature, pH, DO and salinity were measured at respective sampling locations. Dissolved oxygen was estimated through Wrinkler’s titration method following APHA 2005 protocol. Nutrient levels (Nitrates (NO3-), Phosphates (PO43-) and silicates (SiO44-) of the water samples was assessed in the laboratory within 4 hours of sampling, following the standard protocols of APHA (2005). Diatom Sample Collection and Preparation Diatom samples were collected at each sampling locations (from substrata such as stones, cobbles, boulders, macrophytes, sedges, shells, halophytes (mangrove plantations), sediments, and sand. Triplicate samples were collected from each substratum. The epilithic (diatoms attached to stones) were collected by scraping using a clean spatula after washing the substrata under the water to remove the silt deposited on hard surfaces. Epiphytic (diatoms attached to plants) were collected mainly from small mangrove plants grown along the shoreline. A portion of sedges and the mangrove stems were cut and placed inside the sampling bottle containing distilled water and were shaken to dislodge the diatom cells attached on the surface of the plants. Epipelic (Diatoms attached to sediments) and Episammic (diatoms attached to sand) were collected from damp and moist surfaces exposed during low tide by moving the sampling container along the sand/sediment surface. The collected diatom samples were immediately subjected to fixation using 2% Lugol’s Iodine to cease cell multiplication and preserved at 4oC until analysis. Table 1 lists the details of study locations and different substrata considered at each station during the study. Diatom samples collected were pre-treated and processed to remove the organic contents inside the cells using potassium permanganate and hydrochloric acid as per the standard protocols of (Karthick et al., 2013; Kelly et al., 1998; Taylor et al., 2007). Table 1 Different Substrata Considered for Diatom Sample Collection Stations | Diatoms Sampled | Different Substrata considered | S1 | Epilithic | laterite (Cobble) Stones | S2 | Epipelic, Epilithic, and Epiphytic | Sediments, laterite (Boulders) and Sedges | S3 | Epilithic, Epiphytic | Shells, Mangrove plants, sediments | S4 | Epipelic, Epiphytic and Epilithic | Sediments, Mangrove plants, laterite (Boulders) | S5 | Epilithic, Episammic | Rocks, Sand | S6 | Epilithic and Episammic | Rocks, Sand |
The processed diatom samples were observed under high-resolution fluorescence microscope (Model: Olympus BX51 integrated with Olympus camera: TV1X-2) 1000× magnification. The images of diatom frustules were captured by adjusting the dilution of the sample to a maximum of 2 - 5 valves per view using a digital camera fixed to the microscope. Diatoms were identified using standard identification keys (Van Heurck; Karthick et al., 2013; Krammer K and Bertolet H, 1986; Patrick and Reimer, 1966). The identified species were also enumerated as per DARES protocol by counting 400 valves per sample to determine the relative abundances, species diversity, and community composition. Light micrographic images of few diatom species were listed in Figure 3. Statistical Analyses Two-way ANOVA statistical analysis was carried out to estimate the level of significance of seasonal physico-chemical parameters on species richness (S). The data were compiled season-wise (premonsoon, post-monsoon and monsoon) and subjected to agglomerative hierarchical clustering for understanding the dynamics of diatom species’ abundance w.r.t seasons. Non-metric Multi-Dimensional Scaling (MDS) technique was used to understand the species abundance pattern of benthic diatom communities’ season-wise across stations. Agglomerative hierarchical clustering and nMDS analysis were performed using R Studio version 1.1.423. Season wise and station wise diatom diversity (Shannon – Wiener (H’)) and Simpson’s Dominance (D) were computed using Shannon diversity and Simpson’s dominance indices. Overall species richness (SR) and species abundance (SA) with respect to different substrata sampled at each station are illustrated in the form of a pie chart. The species that had shown a relative abundance of >15% and recorded consistently during all three seasons were considered as tolerant species and radar plot was generated using season-wise relative abundances of those tolerant species at each study station.
|