Introduction
Fossil fuels such as oil, coal and natural gas are the major commercial energy
sources and about 87% of global CO2 emitted due to the anthropogenic activities [1,2]
are contributed by utilization of coal (43%), oil (36%) and natural gas (20%). Earth endows finite source of oil
reserve and its increased consumption in several sectors has led to increased oil production, exerting pressure on
the reserves which is apprehended to peak and no longer suffice the world’s demand with the fast dwindling stock [3]. Rising population with higher consumption levels coupled with a fast pace of
development have spurred higher exploitation of fossil fuels leading to the escalating prices and resultant
greenhouse gases (GHGs) posing problems for planet’s climatic stability [4–6]. It is imperative
to ensure energy security through the sustainable alternative energy sources [7,8]. Globally,
nations are actively addressing the issues concerning greenhouse gases and peak oil crisis through several
mitigation measures such as; energy conservation, fuel substitution, incentives for the use of unconventional and
renewable oil, and policy reforms such as carbon tax [8]. Therefore, the current focus is on
carbon neutral renewable sources, notably photovoltaic, wind, hydrogen, etc. These alternative sources were useful
in addressing the electricity requirement, but the exploration for viable alternatives to oil in order to meet the
requirement of transport sector, etc. is quintessential. Despite the existence of possible solutions such as
renewable resources, energy efficient products (CFLs and LEDs) have not been widely adopted due to market barriers.
Wind power contributes 2.5% of world electricity output and are weather dependent, susceptible to geographic and
climatic changes [9,10]. Dependency on conventional generation coupled with the depleting stock and the enhanced
environmental awareness in the public have been the major constraints faced by the land based energy systems [11,12]. Nuclear power witnessed 2% growth in Europe, but encountered resistance with
respect to disposal of waste, safety during nuclear accident and declining global uranium stocks. Nuclear disaster
at Fukushima Daiichi nuclear power plant in 2011[13], led Germany to rethink its energy policy
[14].
India has been the 3rd largest energy consumer surpassing Russia, China and USA and about 80% of India’s
energy consumption was contributed by imported crude oil [15], which was estimated to be 213.93
Million Metric Ton (MMT) in the year 2016-17. This is attributed to the poorly endowed natural reserve of
hydrocarbon in India, however crude oil production in India is about 36.01 MMT, from the 0.3% oil reserves [15]. India is emerging as the fastest growing economy next to China with the growing
energy demand, burgeoning population (at 1.58% annual) and dwindling stock of fossil fuel in next few decades, it is
challenging to support this growing economy demand[16]. The total CO2 emission in
India accounts for 965.9Tg/yr, with electricity generation (343Tg/yr) and transport (246.23Tg/yr) sectors as the
major contributors [17]. The higher level of CO2 emissions necessitates
implementation of efficient management strategies to mitigate changes in climate [18]. The new
renewable energy resources are being explored to meet the energy demand in all sectors and also research is underway
to address the intermittency problems associated with wind and solar based energy systems [19–21].
In this context, studies have shown that biofuels are emerging as promising alternative to liquid fuels. Realizing
the potential of biomass, different technologies have evolved towards the conversion of biomass into fuels,
popularly known as biofuels [22–24]. Produced from renewable plant sources or other organic
wastes, biofuels have the advantages of cutting down carbon emission and dependency on oil [25].
In India, around 80% of rural energy [26] is met by biomass energy consumption, in the form of
firewood, agriculture residues, cow dung cake and other natural feedstock [24,27,28].
Fig. 1 represents the share of each country in the global bioethanol production, which
highlights that India’s share is only 2% [29] despite burgeoning demand for fossil fuel. This
emphasizes the need for augmentation with the viable indigenous alternative feedstock to minimize fossil fuel
dependence.
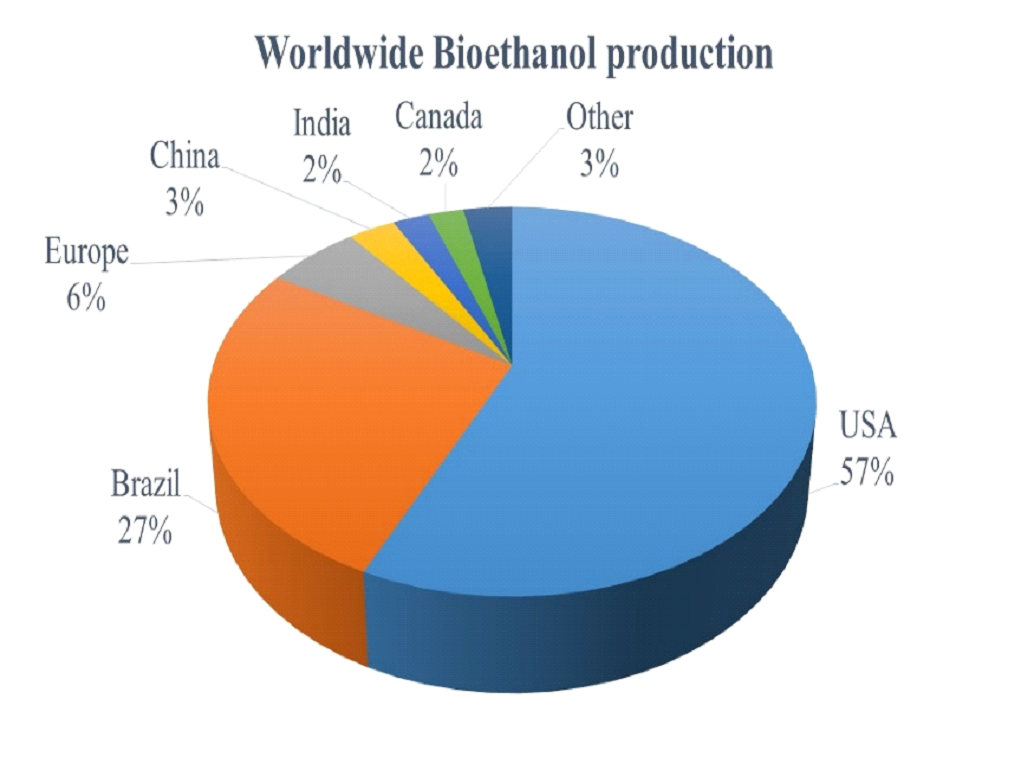 Fig. 1 Fig. 1. Worldwide Bioethanol Production
|
Biofuel from first generation feedstock involved food crops like corn and sugarcane which were exploited for biofuel
production over three decades, but this technique encountered resistance due to the limited stock and competition
with food crops [25]. The inadequacy of first generation feedstock in augmenting the growing
energy demand led to the evolution of second generation feedstock involving lignocellulose biomass (Fig.
2). However, biofuel from second-generation feedstock also failed, due to the difficulty in scaling up and
process technology involved in the cost-intensive delignification process [30]. Due to this,
the cost of production of cellulosic ethanol is two to three fold higher than the price of corn grain ethanol [31]. In the US, it was seen that, the fossil energy required to produce bioethanol from
corn, grain, soybean and wood biomass was more than the energy content of the biofuel, while sufficing only 12% of
gasoline and 6% of the diesel demand. Though first and second generation feedstock are explored for biofuel
production and assessed for carbon sequestration, environmental impacts and production potential only marginally
complies with various other sustainability criteria’s such as; disruption of global food supply, soil erosion,
extensive usage of fertilizers, conversion of ecologically vulnerable wetlands, rainforests, peat lands, savannas
into energy crop lands contributing to several magnitude of CO2 [32,33]. GHGs
footprint of major cities in India [34], recorded aggregation of carbon dioxide equivalent
emission of GHGs in the range of 13,734.59-38,633.2 Gg, with transportation being one of the major sector next to
the energy generation. Emergence of a strong global biofuel feedstock is expected to realize a positive balance
between energy and ecological footprints [35].
Table 1. Yield and Ethanol production of First, Second and Third generation feedstocks
Biofuel |
Crop |
Yield(ton/ha/yr) |
Ethanol (liters/ha/yr) |
First
generation |
Sugarcane |
50-90 |
3,500-8,000 |
Sweet sorghum |
45-80 |
1,750-5,300 |
Sugar beet |
15-50 |
1,350-5,500 |
Fodder Beet |
100-200 |
4,400-9,350 |
Wheat |
1.5-2.1 |
510-714 |
Barley |
1.2-2.5 |
300-625 |
Rice |
2.5-5.0 |
1,075-2,150 |
Irish potatoes |
10-25 |
1,110-2,750 |
Cassava |
10-65 |
1,700-11,050 |
Sweet potatoes |
8-50 |
1,336-8,350 |
Grapes |
10-25 |
1,300-3,250 |
Second
generation |
Nipa palm |
|
2,300-8,000 |
Maize |
1.7-5.4 |
600-1,944 |
Sorghum |
1.0-3.7 |
350-1,295 |
Third
generation |
Algal biomass |
730 |
23400 |
Table 1 illustrates the prospects of algal biomass emerging as an ideal alternative to the
first and second generation [37,38]. Though, algae is being utilized as an energy feedstock
since 1950s [26], the oil crisis of 1970's spurted the research [39].
Algal feedstock do not require prime agricultural land and can be grown in fresh water, wastewater [40]
and saline waters with zero nutrient input and non-interference with the land used for food production [38,41]. Algal biomass have higher photosynthetic efficiency (up to 5%) as compared to
terrestrial biomass (1.8-2.2%) [42], and require for their growth light, carbon dioxide and
nutrients (such as nitrogen, phosphorus, potassium, etc.), which are maintained through continuous flow of water [42]. Algae have a higher yield per unit area compared to terrestrial plants e.g. brown
algae under the cultured condition, yields ~13.1 kg dry weight/m2 over 7 months as compared to sugarcane
yield of ~10 kg dry weight/m2/yr [43]. Algae based on their morphology and size are
grouped into micro and macroalgae [29]. Microalgae accumulate large quantities of neutral
lipids which serves as raw material for biodiesel production [44,45], whereas macroalgae are
carbohydrate rich biomass which are useful for bioethanol production. Large scale cultivation of macroalgae in Korea
reveals an uptake of 8-10 tonne CO2 per hectare [42].
1.1 Potential macroalgal feedstock available
Marine macroalgae or seaweeds establish on hard substratum and grow luxuriantly along nutrient rich coastal zone (Fig. 3). One of the richest seaweed resources in the world is in Nova Scotia/Gulf of St.
Lawrence area [46]. Global seaweed distribution can be summarized as : (i) Least flora <200
Spp in latitudes >60o in both hemispheres, (ii) Moderate flora of 600-700 spp. that occur throughout
warm and cold tropical and temperate regions, (iii) Highest flora of 900-1100 spp. occur in four regions Southern
Australia, Mediterranean, Japan and Philippines.
| images |
Fig. 2.Evolution of biofuel production from
feedstocks and technologies |
Fig. 3. Prominent coastal regions of the world rich in seaweed
resources and potential feedstock for bioethanol production |
Seaweed resources and their uses are well established across regions in the world. Red seaweeds are mostly utilized
for extraction of hydrocolloid valuing $585 million [47] and source of food (e.g. Salads)
valuing $5 billion [48] with Asia as its prime market [43]. Cultivation of
macroalgae is a promising option as seventy percent of the Earth surface is covered by water [39,42,49,50],
therefore in order to satisfy these industrial demands, macroalgae are cultivated in large scale, mainly of the
genus Laminaria,, Undaria, Poryphyra, Eucheuma, Enteromorpha and Gracilaria representing 76% of
total macroalgae aquaculture production [51].
In recent years, algal genera of Kappaphycus, Gelidium, Gracilaria, Sargassum, Laminaria and
Ulva, (Fig. 2) are the promising potential feedstock for biofuel production in addition to
the value added products for phycocolloids extraction, human food, cosmetics, fertilizer and other chemicals [52,53]. These algal feedstock have been chosen considering the availability and
assessment of resources around the globe, ease of cultivation and harvesting. However, there is still scope to
assess other potential macroalgal species based on their availability, biochemical composition and prospects for
cultivation.
1.2 Bioethanol production from Macroalgal Feedstock
Bioethanol from algal biomass is a sustainable and eco-friendly option of renewable biofuel production [39]. Macroalgae or seaweed, saltwater thriving algae have proved to be the viable biofuel
feedstock [54] for sustainable biofuel production as it avoids the competition with fresh
water, food crops or cultivable land [39,55]. Seaweeds are multicellular marine macroalgae,
broadly grouped as green, brown and red based on the pigment present in the thallus. Seaweed consists of
carbohydrates (Table 2), which are converted to bioethanol by appropriate microorganisms such
as yeast or bacteria. The common processes involved in ethanol production are (i) pretreatment, (ii) hydrolysis and
(iii) fermentation.
1.2. Bioethanol production from macroalgal feedstock
Bioethanol from algal biomass is a sustainable and eco-friendly option of renewable biofuel production [39]. Macroalgae or seaweed,
saltwater thriving algae have proved to be the viable biofuel feedstock
[54] for sustainable biofuel production as it avoids the competition with
fresh water, food crops or cultivable land [39,55]. Seaweeds are
multicellular marine macroalgae, broadly grouped as green, brown and
red based on the pigment present in the thallus. Seaweed consists of
involved in ethanol production are (i) pretreatment, (ii) hydrolysis and
(iii) fermentation.
1.3 Pretreatment and Hydrolysis for extraction of macroalgal sugar
Different types of biomass contain different amounts of sugars and the complexity of the biomass is reflected between
structural and carbohydrate components [62,63]. Plant biomass is mostly composed of lignin
(13.6-28.1%), cellulose (40.6-51.2%) and hemicellulose (28.5-37.2%) biopolymer [64], which
serves as raw material for production of fuels. However, critical step involved in biofuel production is the
conversion of biomass to sugars [65]. It is therefore important to carefully choose the
pretreatment process based on the biomass and an optimal pretreatment process towards better yield of sugar with the
low energy input [66].
Pretreatment involves physical, chemical and biological (or combinatorial) process to expose the cell constituents
and cell wall materials of feedstock [67]. Physical pretreatment involves reduction in size of
the feedstock to increase the surface area for better transport of acid/base catalysts, enzymes and steam to the
fibers (cellulose) [68]. Chemical pretreatment involves dilute acid, alkaline, ammonia, organo
solvent and other chemicals. Biological pretreatment involves microorganisms like bacteria and fungi (rich in cellulase
enzyme) to degrade the biomass and release the sugars [69]. Integrated pretreatment involves
combination of all the process such as acid catalyzed steam explosion, ammonium fiber explosion (AFEX), acid
pretreated enzyme hydrolysis etc [66].
First generation biomass is starch based and requires no stringent pretreatment conditions to extract sugar, whereas
lignocellulose biomass is complex in structure due to the presence of biopolymer lignin that embeds cellulose in a
matrix resulting in a higher degree of polymerization and crystallization, which is the main factor responsible for
recalcitrance [66,70–72] requiring a high cost for delignification process [73].
Therefore, the process of sugar extraction requires severe pretreatment conditions such as steam explosion at 200
oC [74], at 121 oC [75], AFEX, Sulphite pretreatment
to overcome recalcitrance of lignocellulose (SPORL) [31], pressurized steam liquefaction [76]. It is seen that alkaline based pretreatment is effective in solubilizing significant
portion of lignin from lignocellulose biomass [69]. Lignin was removed from cotton stalk
pretreated using sodium hydroxide at high temperature and 96% fermentable sugars were recovered [77,78].
Around 11.4 MMT cotton plant wastes available in India, can generate 3,533 billion litres of ethanol considering 90%
fermentation efficiency [78]. Removal of 89% lignin and 69.77% hemicellulose in rice husk was
achieved through wet air oxidation pretreatment method [79]. Hydrothermal pretreatment of wheat
straw was carried out and viewed under scanning electron microscope (SEM), which reveal partial de-fibration of the
lignin fibers due to pretreatment, whereas in delignification process lignin appears as layer of globular deposits
exposing the cellulose structure [71].
Compared to this, macroalgae with the large concentration of structural polysaccharides (Table
3) and low lignin contents [80] requires mild and low-cost processes for extraction of
sugars. The most widely used chemical pretreatment method for macroalgal biomass is dilute acid (Table
4), as it solubilizes hemicellulose and exposes cellulose fibers for further enzyme hydrolysis [68]. The energy consumed in acid pre-treatment is comparatively low as compared to other
pre-treatments and higher sugar yields are achieved [69]. Dilute acid concentration for
hydrolysis varies based on the feedstock, listed in Table 3. However, limitation of dilute
acid pretreatment is the formation of Hydroxymethyl furfurals (HMF) and Levulinic acid (LA) resulting from the
degradation of sugars that inhibit the subsequent process (fermentation) in ethanol production [81,82].
These inhibitors are mitigated by neutralization process before fermentation [83,84] or by
employing other sustainable alternatives such as biological pretreatment: enzyme hydrolysis [53,85–94].
Enzyme hydrolysis of cellulose is carried out efficiently by cellulolytic (cellulase) enzyme, which
is comprised of exo-, endo-glucanases and cellobiase (β- D-glucosidase) enzymes [71]. Endoglucanases
cleave cellulose at random sites of β-1, 4-bond and form free reducing ends and short-chain oligosaccharides [84] Exoglucanases cleaves the accessible ends of cellulose molecules to liberate
glucose and cellobiose. β- D-glucosidase hydrolyses soluble cellobiose and other cellodextrin to produce glucose
molecules [95]. Enzyme conversion is substrate specific without any by-product formation. The
process could be enhanced [7], by exposing the cellulose fibres through pre-treatment using
acid. Enzymatic hydrolysis disintegrates the cellulose and hemicellulose into simple sugars [96]. Along with this, depolymerization of xylan (polysaccharide composed of xylose) can
be achieved by dilute-acid pretreatment [88] with about 64% xylose conversion efficiency.
Pretreatment techniques include high thermal liquefaction process (HTLP) [97], alkali
pretreatment, CaO, Ozonolysis, etc. However it was seen that the acid treated biomass was more susceptible for
enzyme attack than HTLP, NaOH, CaO and other pretreatment [87].
Algal cell wall is composed of cellulose Iα (triclinic crystalline form) unlike the cellulose Iβ (monoclinic
crystalline form) in plant cell wall. Cellulose Iα consists of weaker hydrogen bonds resulting from spatial
arrangement of individual cellulose chains, resulting in easy access to endocellulases enzymes during enzyme
hydrolysis [98]. Most common categories of enzymes considered for cell wall depolymerization
are cellulases, hemicellulases and accessory enzymes [99], produced from wood-rot (soft rot)
fungi such as Trichoderma, Penicillium, and Aspergillus [100]. The
production costs of these enzymes are relatively higher.
Table 2
Detailed characteristics of different types of Seaweeds |
Characteristics |
Green seaweed |
Red seaweed |
Brown seaweed |
Number of species recorded |
6032a |
7105b |
2039c |
Habitat |
Freshwater and Marine |
Strictly marine |
Strictly marine |
Photosynthetic pigment present |
Chlorophyll a, b, carotene
and Xanthophyll |
Phycoerythrin |
Fucoxanthin |
Photosynthetic rate (µmol CO2 /h) g/dry |
30 to 1786 |
20-1808.7 |
100-500 |
Productivity [dry g/(m2 year)] |
7100 |
3300-11300 |
3300-11300 |
Nature of cell wall |
Cellulose, pectin rarely hemi-cellulose |
Cellulose and pectic material with polysulphate esters |
Cellulose with alginic acid and fucocinic acid |
Sexuality |
Isogamy to oogamy |
Advanced and complex (oogamous) |
Isogamy to oogamy |
No. of flagella and their insertion |
2 or 4, equal anterior, whiplash |
Absent |
Only in reproductive cells, 2 unequal, lateral whiplash and tinsel |
Cell structure |
Eukaryotic |
Eukaryotic |
Eukaryotic |
Phycolibins |
Absent |
Allophycocyanin,
r-Phycoerythrin
r-Phycocyanin |
Absent |
Carotenoids |
α-, β-, γ- carotene |
α-, β- carotene |
α-, β-, ε- carotene |
Xanthophylls |
Lutein
Prasinoxanthin |
Lutein |
Fucoxanthin, Violaxanthin,
Diadinoxanthin, Heteroxanthin,
Vacheriaxanthin |
Carbohydrate (%) |
30-60 |
30-50 |
20-30 |
Protein (%) |
10-20 |
6-15 |
10-15 |
Lipid (%) |
1-3 |
0.5-1.5 |
1-2 |
Ash (%) |
13-22 |
5-15 |
14-28 |
Photosynthetic reserve*
(Stored food) |
Starch |
Floridean starch (intermediate between true starch and dextrin) |
Laminarin and mannitol (hexahydride alcohol) |
Source: [50,54,56–61] |
Table 3
Sugar profile of Macroalgae |
|
Green seaweeds |
Red seaweeds |
Brown seaweeds |
Structural polysaccharide |
Cellulose |
Cellulose, lignin |
Cellulose, Alginate |
Storage polysaccharide |
Starch, Ulvan, Mannan |
Agar, Carrageenan |
Fucoidan, laminarin |
Monosaccharides |
Glucose, Mannose, Rhamnose, Xylose, Galactose |
Glucose, Galactose, Agarose |
Glucose, Galactose, Fucose, Xylose |
Sugar alcohol |
|
|
Mannitol |
Sugar Acid |
Uronic acid, Glucuronic acid |
|
Uronic acid, Mannuronic acid, Glucuronic acid, Alginic acid |
Commercial industrial enzymes are produced from aerobic
fungi
Trichoderma reesei, which produces over 100 g per liter of crude cellulase enzyme with higher
specific activity, achieved by genetic engineered strains
[101]. Most common enzymes employed
for seaweed hydrolysis are commercial enzymes such as Cellulase, Celluclast 1.5 L, Viscozyme L, Novozyme 188,
Termamyl 120 L, β-glucosidase, Multifect, Meicelase, Amyloglucosidase etc operated at pH 4.5-5.5 and temperature
35-55
oC, incubation time varies based on the algal feedstock
[56,71,
,89,90,103–109].
Cellulase producing microbes have been screened and isolated from various sources such as soil from forest and nature
reserves, hot water springs, marine bacteria [90] compost, sewage, animal manure and bovine
rumen [91]. Enzymatic hydrolysis has been done conventionally at <50oC, resulting
in lower sugar yield [95]. Therefore, research is under progress for isolating efficient
cellulolytic enzyme systems from a wide variety of bacteria, fungi, aerobes, anaerobes, mesophiles, thermophiles and
thermo-stable microbes [92,93,96] which can overcome low sugar yield for biofuel production.
Cellulase from thermophilic and psychrophilic microbes are preferred as they are resistant to high and low
temperatures respectively [91]. Thermo-stable enzymes increase solubility of reactants and
products, facilitating easy recovery of end products [96] while reducing hydrolysis time,
decreasing contamination and cost of energy.
Marine fungus Cladosporium sphaerospermum was isolated to extract cellulase enzyme and used to hydrolyze
U. pertusa biomass, which yielded 112 mg/g of reducing sugar at pH 4 and temperature 25 oC for
42 h [94]. Similarly, marine bacteria was isolated from degrading U.lactuca to extract
cellulase enzyme, which is tolerant to high salt concentration and alkaline pH [86].
Polysaccharolytic enzymes extracted from the gut of the abalone Haliotis midae degraded the polysaccharides
laminarin, carboxymethylcellulose (CMC), alginate, agarose and carrageenan [109].
1.2.2 Fermentation of macroalgal sugars
Macroalgal biomass contain different types of polysaccharides, exclusively composed of glucose i.e., glucans. Main
glucans present in green: cellulose and starch; red: cellulose and floridean starch; brown: cellulose and laminarin
[46,50,61]. Non-glucans are sulphated polysaccharides such as agar, carrageenan and alginate.
In order to obtain higher ethanol, hydrolysis of glucan as well as non-glucan with the fermentation of the resulting
sugars is essential [60]. Sugar released from the pretreatment process has been fermented using
microorganisms such as yeast, bacteria, and fungi, which ferment these sugars to produce ethanol as a by-product [41,110]. Saccharomyces cerevisiae is the commonly used yeast microorganism for
fermentation as it readily ferments glucose [111]. However, pretreatment releases mixed sugars
namely; glucose, galactose, mannitol, rhamnose and xylose. Due to the lack of xylose transport system,
S.cerevisiae is not capable of utilizing xylose [112]. Its uptake takes place through
glucose transport system and is regulated by the concentration of glucose. At only low concentration of glucose,
xylose is consumed by the yeast [113]. As a result, studies related to isolation of wild yeast
strains from various sources is done that can ferment both hexose and pentose sugars yielding higher ethanol.
Bacteria, yeast and fungi are explored for xylose fermenting organisms, and mostly preferred organisms are bacteria
and yeast as fungi are too slow for competitive industrial process [7].
Single or combination of strains are being attempted for utilization of sugars. Laminaran and mannitol obtained from
L. hyperborea were subjected to fermentation using one bacterium (Zymobacter palmae T109) and
three yeast strains (Pichia angophorae, Pacchysolen tannophilus and Kluyveromyces marxianus). It was seen
that only P. angophorae is capable of fermenting laminaran and mannitol at higher oxygen
transfer rate to produce 0.43 g ethanol/g substrate [114]. Utilization of mannitol by Zymobacter
palmae resulted in the production of 0.37 g ethanol/g mannitol [115], however
mannitol was utilized at lower oxygen rate in fermentation media. Mannitol was effectively fermented by
E.coli KO11 for production of 0.41 g ethanol/g mannitol [116]. Similarly, glucuronic
acid fermentation was attempted using Pachysolen tannophilus and E.coli.
Bioethanol production from all forms of macroalgal biomass; wet, dried and residues (after extraction of
hydrocolloid) was attempted. Residues after extraction of hydrocolloids are rich in cellulose, which have been
utilized for bioethanol production. Floating residue of
Table 4
Bioethanol production from macroalgal biomass |
Green Seaweeds |
Pretreatment
conditions |
Enzyme hydrolysis
conditions |
Yeast/Bacterial strain and Fermentation process |
Reducing Sugar g/L |
Ethanol yield g/g |
Theoretical yield (%) |
Reference |
E.intestinalis |
Hydrothermal process
(75mM for 90min) |
Celluclast 1.5 L and Viscozyme L
(55oC, 120 rpm for 54h) |
Saccharomyces cerevisiae KCTC 1126
(pH 5.5, 30oC, 220 rpm for 12h) |
40.4 |
0.21 |
41.74 |
[120] |
U.fasciata |
H2SO4
(0.1% at 100oC for 1h) |
Cellulase 22119
(Sodium acetate buffer pH 4.8 at 45oC for 36h) |
Saccharomyces cerevisiae
(109 CFU/ml 28oC, 120rpm for 48 h) |
20.6 |
0.45 |
88.24 |
[89] |
U.lactuca |
|
Cellulase isolated from Cladosporium sphaerospermum
(pH 4, 25oC, 42h) |
Saccharomyces cerevisiae MTCC180
(28oC for 12h) |
112mg/g |
0.47 |
92.16 |
[94] |
U.pertusa |
Citric acid buffer
(0.1M sterilized using autoclave) |
Meicelase
(combined saccharification)
(pH 5.5, 50oC, 100rpm for 120h) |
Saccharomyces cerevisiae IAM4178
( 30oC for 36h) |
59.1 |
0.47 |
91.24 |
[70] |
HTLP+Enzyme
(150oC, 15 min) |
Cellulase & Amyloglucosidase
(pH 4.8, 50oC, 150rpm for 24h) |
Saccharomyces cerevisiae ATCC24858
(pH 5.5, 150rpm, 30oC for 24h) |
26 |
0.48 |
93.51 |
[97] |
Red Seaweeds |
G. elegans |
|
Meicelase
( pH 5.5 at 50oC for 120h) |
Saccharomyces cerevisiae IAM4178
( 30oC for 36h) |
49 |
0.38 |
73.63 |
[70] |
G. amnasii |
H2SO4
(56-168mM ,45-240min) |
Enzyme Viscozyme L (0.024 FBG/ml) |
Scheffersomyces stipitis
(pH 5.5, 30oC, 200rpm) |
43.5 |
0.47 |
92.40 |
[108] |
|
H2SO4
(2%, 150oC for 4h) |
|
Brettanomyces custersii KCCM11490
(pH 4.8-5.5, 27-30oC) |
42.2 |
0.38 |
74.51 |
[126] |
G. verrucosa |
H2SO4
(1.5%, at 80oC for 2 h) |
|
|
87 |
0.43 |
84.29 |
[60] |
373mM H2SO4 |
Celluclast 1.5L and Viscozyme L
(pH 5, 45oC, 150rpm for 72h) |
Saccharomyces cerevisiae KCTC1126
(pH 5, 30oC, 150rpm for 114h) |
20.4 |
0.48 |
94 |
[110] |
Gracilaria sp. |
H2SO4
(0.1N,121oC for 30min) |
Commercial enzyme
(pH 4.5, 50oC) |
Saccharomyces cerevisiae
( 30oC for 48h) |
11.46 |
0.42 |
82.80 |
[132] |
K. alvarezii |
Soaked in 1.6 L distilled water for 30 min and boiled at 90oC for 1 h |
Celluloclast 1.5 L & Novozyme
(pH 5, 50oC, 150 rpm for 24h) |
Saccharomyces cerevisiae
(pH 5,35oC, 130 rpm for 6h) |
79.2 |
0.25 (SHF) |
49 |
[88] |
0.27
(SSF) |
52.9 |
H2SO4
(0.2M, 130oC for 15min) |
|
Commercial brewer’s yeast
(30oC 120 rpm pH 5 for 72 h) |
20.4 |
0.21 |
41.18 |
[133] |
H2SO4
(0.9N, 100oC for 1h)
5 cycles |
|
Saccharomyces cerevisiae NCIM
(5% v/v, 30oC 150 rpm, pH 6.4-6.8 for 48 h) |
51.9 |
0.42 |
82.36 |
[117] |
P. palmata |
Acid hydrolysis |
|
|
21.84 |
0.173 |
33.92 |
[133] |
Brown Seaweeds |
A. crassifolia |
Citric acid buffer
(0.1M sterilized using autoclave) |
Meicelase
(5g/l at 50°C for 120 h) |
Saccharomyces cerevisiae IAM 4178
(30oC for 36h) |
66.3 |
0.38 |
75 |
[70] |
L. hyperborea |
Extracted in water at 65°C |
|
Pichia angophorae |
30 |
0.43 |
84.31 |
[114] |
|
Extracted in water 121oC for 20min |
|
Zymobacter palmae
(pH 6, 30oC) |
3.8 (mannitol) |
0.38 |
74.51 |
[115] |
S. sagamianum |
|
|
Pichia stipitis
(pH 5, 200rpm) |
19.8 |
0.35 |
69.32 |
[125] |
S. janponica |
H2SO4
(1mM, 121oC, for 120min) |
Cellulase and cellobiase
(pH 4.8, 50oC, 150rpm for 48 h) |
Saccharomyces cerevisiae
(pH 6.5, 30oC for 36 h) |
34 |
0.41 |
80.74 |
[103] |
Acid hydrolysis
(0.1N, 121oC for 15min) |
Celluclast 1.5L, Viscozyme L, Novoprime 959, Novoprime 969 or AMG 300L
(50oC, 150rpm for 24h) |
E.coli KO11
(30oC for 24 h) |
30.54 |
0.41 |
80.39 |
[116] |
H2SO4
(40mM , 121oC for 60min) |
Novozyme
(Termamyl 120L) |
Pichia angophorae KCTC 17574
(5% 30oC at 200 rpm, 136h) |
45.6 |
0.16 |
33.3 |
[52] |
Shredding and enzymatic
(23oC for 30min) |
|
Ethanol Red yeast
(32oC) |
35 |
0.45 |
88.24 |
[126] |
U. pinnatifida |
Dilute acid
(5% H2SO4, 120oC for 24h) |
Celluloclast 1.5L & Novozyme188
(pH 4.6, 45oC) |
E.coli
(pH 7,170 rpm, 37oC for 12h) |
20 |
0.144 |
28.2 |
[107] |
L. japonica was subjected to acid pretreatment
followed by enzyme hydrolysis, an ethanol yield of 14 g/L was obtained from 34 g/L of reducing sugar achieving 41.2%
conversion efficiency
[103]. Similarly,
K. alvarezii dried residues after extraction
of sap were utilized for production of bioethanol
[117]. Wet biomass of
G. amansii was
used as bioethanol feedstock,
Brettanomyces custersii KCTC 18154P strain
was utilized for
fermentation of the hydrolysate due to the ability of the strain in exhibiting co-fermentability. Utilization of raw
or wet macroalgal biomass is not feasible for bioethanol production due to high viscosity of the medium for
fermentation
[118]. In green seaweeds, studies have focused on conversion of cellulose and
starch to bioethanol. Whereas conversion of other sulphated polysaccharides such as Ulvan to produce ethanol is yet
to be explored
[60]. Non availability of natural strains capable of fermenting alginate, a
major polysaccharide of brown algae
[60], makes it difficult to achieve higher ethanol
production.
Fermentation is carried out in two process, Separate Hydrolysis and Fermentation (SHF) and Simultaneous
Saccharification and Fermentation (SSF) [119]. SHF involves hydrolysis and fermentation
performed sequentially, whereas SSF involves performing simultaneous hydrolysis and fermentation [74].
Saccharina japonica, Undaria pinnatifida and Poryphyra were subjected to SSF using Pichia
angophorae KCTC strain and obtained 7.7 g/L of ethanol [52]. SHF process is
faster but presence of inhibitors resulting from acid pretreatment has significant impact on yeast microorganisms.
SSF is preferred over SHF as the sugars released are readily metabolized by yeast microorganisms, which results in a
faster ethanol production rate and lower capital costs. SSF has a drawback due to the difference in temperature
optima of cellulase (50oC) and fermenting microorganism (35oC). SHF and SSF
of Enteromorpha intestinalis or Ulva (Enteromorpha) intestinalis produced 8.6 g/L and 7.6 g/L with
30.5% and 29.6% fermentation efficiency respectively. Conversion of ethanol to acetic acid by yeast and suboptimal
temperature of 30 oC than the optimum temperature of 55 oC for enzyme activity was attributed
to the lower ethanol yield in SSF [120].
Higher temperature shortens the exponential phase of the yeast cell [121] affecting the ethanol
production. However, this has been overcome through thermotolerant yeast strains or cell immobilization technique
which allows higher processing temperatures [120–123]. Thermotolerant yeast
species such as Candida tropicalis and Kluyveromyces marxianus (38-45oC) are mainly
utilized to produce bioethanol from lignocellulosic biomass [123,124].
Bioethanol production from macroalgae utilized commercial yeast strains such as S. cerevisiae KCTC 1126 [110,111], MTCC 180 [60], IAM 4178 [70], ATCC 24858
[97], KCTC 17574 [52], Pichia stipitis [125], Pichia angophorae [114], Scheffersomyces
stipitis [108], Brettanomyces custersii KCCM 11490 [126], Ethanol red yeast [36] and bacterial strains such as Zymobacter
palmae [115] and Escherichia coli SJL2526 [107]. Fermentation of macroalgal polysaccharides is carried out at pH 4.5-6.8
and temperature 25-30 oC and the incubation time is largely strain dependent. The yeast growth rate is
dependent on temperature and fermentation time [131]. However, exponential phase of yeasts are shortened at large
temperatures and pH < 4, requiring longer incubation for higher ethanol production, as reported in S.cerevisiae
BY4742 [64]. Shorter fermentation time causes inadequate growth of microorganisms resulting in inefficient
fermentation [132].
In order to optimize ethanol yield and improve substrate utilization range [129], studies
focused on immobilization of yeast cells [121,130]. Immobilized yeast cells have enhanced the
ethanol productivity and reusable for 15 cycles with bacterial cellulose-alginate sponge [121].
Free and immobilized strains were used for molasses fermentation. Free cells were unable to ferment at temperatures
greater than 38 oC, compared to immobilized yeast. Immobilized yeast strains exhibited both psychrophilic
and thermo-tolerant characteristics, suitable for fermentation in a wide range of temperatures [131]
and increased ethanol yield and higher cellular stability, while reducing downstream processing expenses [132]. Fermentation of U.lactuca biomass done using immobilized Saccharomyces
cerevisiae strain, yielded ethanol (concentration of 12 g/g of sugar) with conversion efficiency of 47.1%
[130]. Table 4 summarizes ethanol yield from the three types of
macroalgae along with the process conditions and strains utilized for hydrolysis (pretreatment) and fermentation.
Fermentation of red seaweed Gracilaria using free yeast cells yielded 0.41 g/g of ethanol and immobilized
yeast cells yielded 0.42 g/g achieving 80 and 82.8% fermentation efficiency [132].
Studies emphasize on production of bioethanol from readily available carbohydrates of brown and red seaweeds, but
utilization of red and brown seaweeds such as Kappaphycus, Gelidium, Gracilaria, Sargassum, and Laminaria
have the likelihood to override the existing multi-billion dollar hydrocolloid industry [89].
This can be addressed in two ways: (i) utilization of cellulosic rich residue after hydrocolloid extraction, (ii)
exploration of green seaweeds which are abundantly recorded from various estuaries and abandoned aquaculture ponds
across maritime states in India [134]. Green seaweeds exhibit characteristics of a potential
feedstock for biofuel production by their cosmopolitan distribution, wide environmental tolerance, higher growth
rates and year around productivity [135]. In India, seaweeds are seldom consumed as a food
source, and the suitability for biofuel production is still underexplored as several species accumulate different
levels of carbohydrate. Seaweeds contain low amounts of polysaccharides composed of glucose, highlighting the need
for ethanol production from carbohydrates including sulfated polysaccharides, sugar acids and sugar alcohols. Not
all the reported microorganisms are capable of fermenting these sugars and a major limitation is lack of tractable
microorganisms that can efficiently ferment all sugars extracted from seaweed into ethanol. Isolation of yeast
strains to ferment both pentose (C5) and hexose (C6) sugars are vital for achieving high ethanol yield. In this
backdrop, the current study explores bioethanol prospects from viable feedstock habituated in the west coast of
India, which involves:
- screening and prioritizing potential macroalgal feedstock for bioethanol production based on the biochemical
composition;
- comparative performance analysis of chemical and biological pretreatment method for extraction of sugar from
macroalgal biomass; and
- bioethanol potential assessment of green seaweeds and comparative analysis of ethanol yield across macroalgal
species.