| Previous Session | Paper 1 | Paper 2 | Paper 3 | Paper 4 | Paper 5 | Paper 6 | Paper 7 | Paper 8 | Paper 9 | Paper 10 | Paper 11 | Paper 12 | Paper 13 | Paper 14 | Paper 15 | Paper 16 | Paper 17 | Paper 18 | Paper 19 | Next Session |
SESSION-4: Limnology of Lakes, Reservoirs, Wetlands
PAPER-7: Heavy Metal Contamination In The Fishes Of
Select High Altitude Water Bodies In The District Nilgiris
Muralidharan S., Jayakumar R., Veerakumar N. and Suresh Babu N.
CONTENTS-
Abstract
Introduction
Materials and Methods
Results and Discussions
Conclusion
Acknowledgement
Bibliography
| Abstract | up | previous | next | last |
The study documents the magnitude of metal contamination in a few species of fishes, namely Scale Carp Cyprinus carpio communis , Mirror Carp Cyprinus carpio specularis , Leather Carp Cyprinus carpio nudus and Rainbow Trout Salmo gairdneri gairdneri in Nilgiris district, Tamil Nadu from five high altitude water bodies, namely Upper Bhavani, Emerald, Pykara, Kuntha and Kamaraj Sagar, and Ooty Lake. An average of 4.35 ppm of copper, 72.4 ppm of zinc and 0.72 ppm of lead wet weight was recorded among all the four species of fishes included in the study. Lead concentration was below detectable level in 91% of the fishes studied. Of all the fishes Cyprinus carpio nudus suffered the maximum metal load (Cu 1.47 ppm; Zn 88.78 ppm Pb 1.47 ppm). The fishes of Emerald recorded the maximum metal load and Kamaraj Sagar Dam the minimum. Among the tissues, kidney had the highest metal load. The study also investigated the daily dietary intake of the metals by humans through fish and compared with the guidelines stipulated by statuary agencies for human safety. The study further recommends for an intensive investigation to document the metal contamination on long-term basis so as to understand the potential impact on fishes and other components of the ecosystem particularly piscivorous birds.
| Introduction | up | previous | next | last |
Heavy metals are some of the most toxic, persistent and widespread contaminants in aquatic systems (Carvalho et.al., 1999) and their impact on various components of the ecosystem particularly fishes is a well documented phenomenon.
Many species of fishes are among the top consumers in the aquatic ecosystems (Dallinger and Kautzky, 1987) and therefore metal concentration in fishes can act as environmental indicators to describe the status of the environment (OECD, 1991; Jorgensen and Pedersen, 1994). Thus measuring the level of heavy metals in fishes will not only reflect the exposure but also give an indication of temporal and spatial extent of metal accumulation as well as an assessment of its potential impact on human health (when consumed) and other organisms (if they have been exposed to elevated levels of metals or if consumed by predators) (Merian, 1991; Bryan and Langston, 1992).
The major objectives of the present study was to assess the magnitude of metal contamination in the fishes of select high altitude water bodies in the district Nilgiris, Tamil Nadu, examine the bioaccumulation pattern of metals among the various tissues and evaluate the suitability for public consumption.
| Materials and Methods | up | previous | next | last |

Nilgiris, popularly called as Blue Mountain or Queen of Hills is about 2.54 lakh hectares in area. It is surrounded by Periyar district in the east, Kerala and Karnataka states in the West and North and Coimbatore district in the south. (Fig.1). The annual average rainfall is about 1778 mm in three rainfall seasons. The maximum temperature ranges from 19 to 32 o C and minimum from 3 to 12 o C. The district although small in extent, is not devoid of environmental problems due to industrial and agricultural activities. There are seven major industries in the district in addition to about 175 tea factories and many small-scale industries. Effluents from the industries join the rivers, namely Bhavani, Coonoor and Moyar, and a number of reservoirs are located in the course of the rivers.
In the present investigation five reservoirs, namely Kuntha (2220-m MSL), Kamraj Sagar Dam (2430 m MSL), Emerald (2555 m MSL), Upper Bhavani (2140 m MSL), and Pykara (2511 m MSL) and Ooty Lake (2160 m MSL) have been selected as study sites and the outcome is expected to project the contamination status of the entire district. While Pykara and Kamaraj Sagar receive some quantity of industrial effluents, Emerald and Kuntha receive agricultural run-off. Untreated sewage and also agricultural run-off contribute to the contamination of Ooty Lake. Upper Bhavani although relatively pristine, does receive run off from the tea estates.
Standard fishing gears were employed to collect fishes from the study sites between 1995 and 1997. Morphometric measurements were recorded at the collection spot and the samples were transported in an icebox to laboratory at SACON. Later the fishes were dissected and tissues, namely brain, heart, liver, kidney and body muscle were taken out, homogenised and about 1 to 1.5 g of sample was weighed out with the help of a top loading balance, Mettler AE240 (0.01g), in clean specimen tubes and stored in deep freezer at -20 0 C till digestion was taken up.
Sample digestion and analysis
The tissues were digested in a Microwave Digestion System (Milestone, MLS 1200 fitted with an Exhaust Module (EM45) using specific mineral acids at standardised time settings (HNO 3 –10ml for 10 min; perchloric acid –1 ml for 5 min; H 2 O 2 - 5 ml for 10 min at 250 W magnetron power). The digested samples were stored in clean polythene vials in refrigerator till the samples were analysed for heavy metals using a double beam Atomic Absorption Spectrophotometer (Perkin-Elmer, Model 3300). The results are expressed in ppm wet weight.
| Results and Discussions | up | previous | next | last |
A total of 76 fishes comprising four species, namely Scale carp Cyprinus carpio communis, Mirror Carp Cyprinus carpio specularis , Leather Carp Cyprinus carpio nudus and Rainbow Trout Salmo gairdneri gairdneri were collected from all the study sites. Samples were analysed for three metals namely copper, lead and zinc.
The level of metal contamination has been viewed at two angles; variation in levels among the various species and study sites included in the study. The results are also consolidated to look at the tissue wise accumulation pattern. Further based on the levels of various metals, the suitability for human consumption has been worked out. Analyses of variance (one-way ANOVA) were attempted to find the differences in metal concentrations among the various organs, species and locations using SPSS 9.0 students' version.
Variation in contamination among species
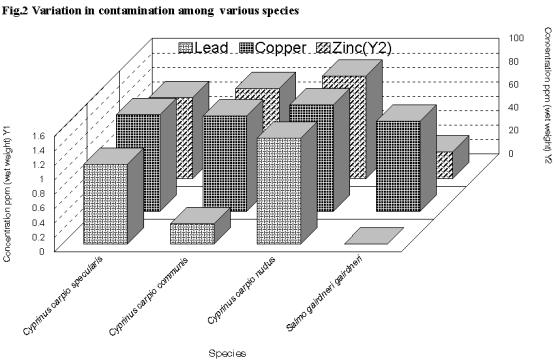
The average mean concentrations (ppm wet weight) of copper, zinc and lead were 4.35 ppm; 72.4 ppm and 0.72 ppm respectively among all the four species of fishes included in the study. Of all the fishes Cyprinus carpio nudus suffered the maximum metal load (Cu 1.47 ppm; Zn 88.78 ppm, Pb 1.47 ppm) and Salmo gairdneri gairdneri the minimum (Cu 0.52 ppm; Zn 22.75 ppm and Pb BDL) (Fig.2). Zinc was present invariably in all species at appreciable concentrations. Lead concentration was below detectable level (BDL) in 91% of the fishes studied. While there are reports on the level of metals in water and their impact on the fish such as hematological alterations ( Ramalingam et.al. 2000), biochemical changes and hampering of locomotion (Hodson et.al. 1978; Shrivastava and Mishra, 1979), there is no information available on the possible impact on fish based on the levels of metals in the tissues. The level of variation among the species did not vary significantly (P>0.001) as the species included in the current study are subsurface dwellers and the contamination level of the food base is likely to have only minor variations.
Variation in contamination in the fishes among study sites
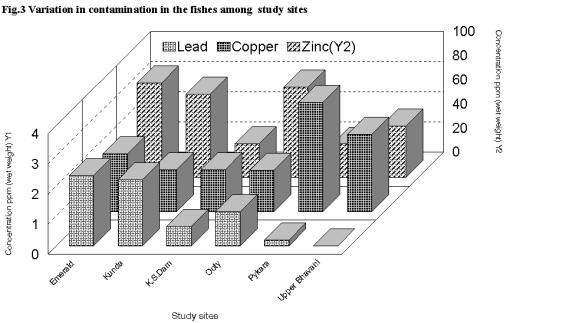
The fishes of Emerald reservoir recorded the maximum metal load (Cu 1.92 ppm; Pb 2.33 ppm; Zn 78.67 ppm) followed by Ooty lake (Cu 1.38 ppm; Pb 1.13 ppm and Zn 75.25 ppm) and Kamaraj Sagar Reservoir (Cu 1.39 ppm; Pb 0.65 ppm; Zn 28.18 ppm) (Fig.3) the minimum. Higher concentration in the fishes of Emerald reservoir could be due to the extensive agricultural operations in the catchment area. Domestic sewerage and agricultural runoff could attribute to the contamination in Ooty Lake. The lead contamination in the fishes of Upper Bhavani was less than the other sites. However, copper (2.56 ppm) and zinc (42.75-ppm) levels were relatively high. This could again be due to the agricultural runoff. The observed variation in the contamination of fishes among the study sites is not significant (P>0.001) indicating that the magnitude of contamination in these water bodies does not vary substantially.
Although there are many studies on the metal contamination in fishes in India (Muralidharan, 1995; Pandey et.al. 1995; Sadhukhan et.al. 1996; Mwachire and Durve 1997; Sultana and Rao, 1998; Jayakumar, 2001; Misra et.al. 2002) and abroad (Kotze et. al., 1999; Al-Yousuf and El-Shahawi, 1999; Kalay et.al. 1999; Al-Saleh and Shinwari, 2002), it is very difficult to compare the magnitude of contamination. It is largely because the species referred are different and so also the level of contamination and sources. Further the accumulation of metal toxicants from the aqueous environment by fishes depend upon the availability and persistence of the contaminants in water (George and Olsson, 1994), the duration and level of exposure (Muralidharan, 1995), dissolved humic substances, alkalinity, pH and hardness of the water (Langston, 1990; Roesijadi and Robinson, 1994; Kotze et. al., 1999), species differences (Kotze et. al., 1999), and physiological mechanisms involved (Al-Yousuf and El-Shahawi, 1999; Kalay et.al., 1999).
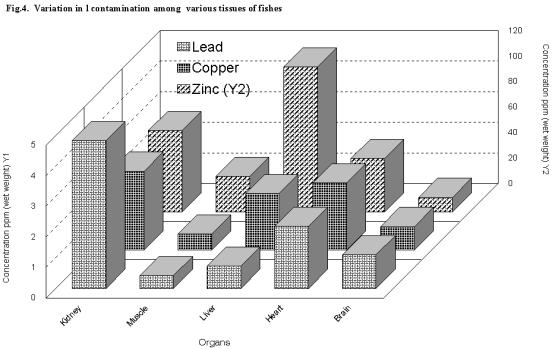
Variation in accumulation among various tissues of fishes
Among all the organs, kidney accumulated the highest concentration of all the metals (Cu 2.59 ppm; Pb 4.83 ppm) except zinc, which was in liver (113.96 ppm). While muscle tissues accumulated the lowest levels of Cu (0.98ppm) and Pb (0.27 ppm), brain tissues accumulated the least level of zinc (11.51ppm) (Fig. 4).
Earlier works have ascertained a fact that accumulation of metals, namely cadmium and lead in kidney is more due to the induction in the synthesis of metallothionin (Sastry and Shukla, 1993; Lionett et.al., 2001). The accumulation of lead in kidney as seen in the present study probably is an indication of synthesis of these metalloproteins and metalloenzymes. Further the variation in the levels of accumulation in different organs in the fishes could be attributed to the differences in the physiological role of each organ. Regulatory ability, behavior and feeding habits are other factors that could influence the accumulation pattern among organs (Kotze et.al., 1999).
Suitability for human consumption
Over the last decade considerable attention has been drawn to evaluate the status of heavy metal contamination in fishes, as they form one of the major food source to humans. This is expected to throw some light on the problem species, the average daily intake of heavy metals through fish by humans, and also the likely ill effect on human beings and fish themselves. Since muscle forms major part of the fish body that is consumed by human beings, the average daily intake of metals through fishes has been calculated based on the contamination level of muscle tissue. Assuming that a person consumes, 250g of fish per week, the average daily intake of metals through fishes could be as follows:
Table 1. Average daily intake (mg) of heavy metals through fish
S.No. |
Fish |
Copper |
Lead |
Zinc |
1. |
C.C. specularis |
0.10 |
0.04 |
2.57 |
2. |
C.C. communis |
0.09 |
0.01 |
2.78 |
3. |
C.C. nudus |
0.13 |
0.02 |
7.38 |
4. |
S.gairneri gairdneri |
0.13 |
- |
0.81 |
- Not calculated
Among all the fishes C.C.nudus contained the maximum load of metals and it is one among the four species, which is commercially exploited (Table 1). Also it appears to be preferred by the piscivorous birds as evidenced by the presence of this species in the gut of a few species of fish-eating birds studied in this district. In many developed countries, limits of metal concentrations in fishes have been set in order to safeguard public health. Saudi Arabia has set a maximum limit of lead, cadmium and arsenic in fish and shell fish of 2, 0.5 and 1 µg/g respectively (SASO, 1997, Al-Saleh and Shinwari, 2002). The European commission (EC) has proposed limits for cadmium (0.05µg/g) and lead (0.5 µg/g) in fishes (Commission of the European communities, 1997). According to WHO/FAO the tolerable daily intake of copper, lead, zinc, cadmium and chromium is 1.3mg, 0.50mg, 10-15mg, 0.05mg and 0.4mg respectively through food and water. Misra et.al., (2002) has recorded Copper (0.37 to 1.02 µg/g), lead (0.39 to 1.457 µg/g) and zinc (0.64 to 1.02µg/g) in the abdomen portion of Grass Carp collected in Upper Lake, Bhopal, India, and said that the levels are within the permissible limits. It may be noted that the levels recorded in the current study fall below the levels reported by Misra et.al., (2002). The levels of lead input to human beings calculated in the present study are comparably low with levels calculated for Channa punctatus of Koeladaeo National Park, Bharatpur, Rajasthan, India (Muralidharan, 1995). The acceptable limits of heavy metals, namely copper, zinc and lead in marine fishes for human consumption are 10, 150, 1.5 ppm wet weight respectively (Nair et.al., 1997). Although the levels reported in the present study fall with the stipulated levels prescribed by any statuary agency, it has to be viewed with concern as the vulnerability would vary according to the per capital consumption. Further duration of consumption is also to be considered since the consumer is vulnerable under chronic exposure. The study carried out by Mason et.al., (1982) considered a range of 5.79-48.2 ppm of zinc as not harmful to piscivorous animals. Accordingly the levels of zinc reported in the present study are unlikely to be harmful to piscivorous birds, which do feed in very large numbers in the r surrounding water bodies . However, information on other metals is not available.
| Conclusion | up | previous | next | last |
The accumulation of heavy metals varied among species and reservoirs. The fishes of Emerald recorded the maximum metal load in the current study. Among all the organs, kidney had the highest load and among the species Cyprinus carpio nudus contained the maximum metal load. The levels reported in the present study are not very high and fall with the stipulated levels prescribed for human consumption. However, they have to be looked at with concern.
Further, the physiological mechanisms, species difference, physico -chemical properties of the surrounding water, availability and absorption of the metal are also to be considered while considering the toxicity. Levels recorded in the present study can be considered as background concentrations as no significant impact is reported to have occurred at these levels elsewhere. It is also feared that if the exposure levels are continuous, certainly resultant physiological damages could result. Hence, the study further recommends for an intensive investigation to document the metal contamination on long-term basis so as to understand the potential impact on fishes and other components of the ecosystem particularly piscivorous birds.
| Acknowledgement | up | previous | next | last |
The authors thank the Ministry of Environmental and Forest, Govt. of India for funding. Authors are grateful to the Tamil Nadu Forest and Fisheries Department for their cooperation. Sincere gratitude is due to Dr. VS Vijayan, Director, SACON for his constant encouragement.
| Bibliography | up | previous | next | last |
Al–Saleh I and N Shinwari (2002). Preliminary report on the levels of elements in four fish species from the Arabian Gulf of Saudi Arabia. Chemosphere 48, 749-755.
Al-Yousouf M H and M S El-Shahawi, (1999). Trace metals in Lethirus lentjan fish from the Arabian Gulf (Ras Al-Khaimah, United Arab Emirates): Metal accumulation in kidney and heart tissues. Bull. Environ. Res. 17: 472-479.
Bryan G, Langston WI, (1992). Bioavailability, accumulation and effects of heavy metals in sediments with special reference to united kingdom estuaries: a review. Environ Pollut 76: 89-113.
Canli M, AY O, Kalay M, (1998). Levels of heavy metals (Cd, Pb, Cu, Cr and Ni) in tissues of Cyprinus carpio, Barbus capito and Chondrostoma regium from the Seyhan River, Turkey. Turkish J. Zoology 22: 149-157.
Carvalho, R.A., M.C. Benfield and P.H. Santschi. (1999). Comparative bioaccumulation studies of colloidally complexed and free-ionic heavy metals in juvenile brown shrimp Penaius astecus (Crustacea: Decapoda: Penaeidae). Limnol.Oceanogr. 44(2):403-414.
Commission of the European Communites. (1997). Draft Commission Regulation Setting Maximum limits for certain Contaminants in Foodstuffs. Doc III/5125/95/Rev.3
Dallinger R, and Kautzky (1987). The importance of Contaminated food for the uptake of heavy metals by Rainbow trout ( Salmo gairdneri ): A field study. Oecologia (Berlin) 67: 82-89.
FAO/WHO, Feed and Agricultural Organization/World Health organization (1972) Evaluation of certain food additives and of contaminants Hg, Pb and Cd.16th report, Rome 84p.
George S G and P E Olsson (1994). Metallothioneins and indicators of trace metal pollution. In : Biomonitoring of coastal waters and estuaries, Kramer, K.J.M (ed.), CRC press, Boca, Balton, EL.
Hodson PV, Blunt Br and DJ Spry (1978). Chronic toxicity of water –borne and dietary lead to rainbow trout (Salmo gairdneri) in Lake Ontario water. Water Research, 12, 869-878.
Jayakumar, (2001). Levels of Heavy metals in commercial fresh water fishes of Coimbatore. M.Phil. Dissertation Thesis submitted to Bharathiar University.
Jorgensen L.A., and Pedersen B, (1994). Trace metals in fish used for time trend analysis and as environmental indicators. Mar. Pollut. Bull. 28(4), 235-243.
Kalay M, Ayo and Carli M,(1999). Heavy metal Concentration in fish tissues from the Northeast Mediterranean sea. Bull. Environ. Contam. Toxicol. 63: 673-681.
Kotze P, H Hdu Preez and JHJ Van Vuren, (1999). Bioaccumulation of Cu and Zn in Oreochromis mossambicus and Clarias gariepinus from the Olifants River Mpumalanya, South Africa. Water SA Vol. 25, No 1, pp. 99-110.
Langston, W.J. (1990). Toxic effects of metals and the incidence of marine ecosystems. In: Furness, R.W., Rainbow, P.S. (eds.) Heavy metals in the marine Environment. CRC Press, New York, pp 256.
Lionetto, M.G., Giordano, M.E., Caricato, R., Pascariello, M.F., Marinosci, L., and Schetti+no, T. (2001). Biomonitoring of heavy metal contamination along the Salento coast (Italy) by metallothionein evaluation in Mytilus galloprovincialis and Mulls barbatus . Aquatic Conserv.: Mar. Fresh w. Ecosyst. 11: 305-310.
Mason C F, S M Macdonald and V J Aspden (1982). Metals in freshwater fishes in the United Kingdom 1980-1981. A report to the Vincent Wildlife Trust.
Merian E, (1991). Metals and their Compounds in the Environments. Occurrence, Analysis and Biological Relevance. ISBN 0-895 73-562-8 (VCH New York).
Misra S M, K Borana, S Pani, A Bajpai and AK Bajpai (2002) Assessment of Heavy metal concentration in Grass carp ( Ctenopharyngodon idella ). Poll Res.21 (1): 69-71.
Muralidharan S. (1995). Heavy metal contamination in and around the aquatic environs of Keoladeo National Park, Bharatpur,Ph.D.,thesis University of Rajasthan.
Mwachire E.C. and Durve V.S (1997). Heavy metal status of the reservoir Bari near Udaipur (Rajasthan) and the accumulation of the metals in the fish organs. Poll. Res. 16 (2): 67-74.
Nair M, Balachandran K K, Sankaranayan V N and T Joseph (1997). Heavy metals in fishes from Coastal waters of Cochin, South West Coast of India. Indian J. Mar. Sci. 26: 98-100.
Organisation for Economic Corporation and Development (OECD), (1991). “The state of the Environments”. Paris.
Pandey B.K., U.K. Sarkar, M.L. Bhoemik and S.D.Tripathi, (1995). Accumulation of heavy metals in soil, water, aquatic weed and fish samples of sewage fed ponds. J. Environ. Biol. 16(2): 97-103.
Ramalingam V, V Vimaladevi, R Naramdaraji and P Prabakaran (2000). Effect of lead on haemotological and biochemical changes in fresh water fish Cirrhinus mrigala. Poll.Res. 19(1): 81-84.
Roesijadi G, Robinson WE, (1994). Metal regulation in aquatic animals: Mechanisms of uptake, accumulation and release. In: Mallins and Ostrander (ed) Aquatic Toxicology, Lewis Publishers, p 539.
Sadhukhan P.C., Ghosh S., Ghosh D.K., Chaudhuri J. and Mandal A, (1996). Accumulation of Mercury in Edible fish from Wetlands of Calcutta. Indian J. Environ Hlth. Vol.38, No.4, 261-268.
SASO, Saudi Arabian Standards Organization, (1997) Maximum limits of contaminating metallic elements in foods Riyadh, Saudi Arabia.
Sastry, K.V. and Vineeta Shukla. ( 1993) . Uptake and distribution of cadmium in tissues of Channa punctatus. J. Environ .Biol., 14(2):137-142.
Shrivastava AK and S Mishra (1979). Blood dysciasia in a teleost Colisa fasciatus after acute exposure to sublethal concentrations of lead. Journal of Fisheries Research Board of Canada, 14, 199.
Sultana R. and Rao D.P, (1998). Bioaccumulation Patterns of Zinc, Copper, Lead and Cadmium in Grey mullet, Mugil Cephalus from Harbour waters of Vishakapatnam, India. Bull. Environ. Contam.Toxicol. 60: 949-955(6).
U S Environmental Protection Agency (EPA) (1978). Metal bioaccumulation in fishes and aquatic invertebrates. G R Philips and R C Russo, EPA - 600/3/-78-103. Washington D C 116
| Address: | up | previous |
SACON, Coimbatore – 641 108, Tamil Nadu. India Phone: 0422 – 657102 - 108 Fax: 0422 – 657088 E-mail: ecot_mur@yahoo.com , murli_sacon@hotmail.com