The study was undertaken in 32 localities (16 in the upper catchment and 16 in the lower catchment) of the Sharavathi River Basin to assess the phytoplankton composition.
In the upper catchment, 216 species belonging to 59 genera (belonging to Bacillariophyceae, Desmidials, Chlorococcales, Cyanophyceae, Dinophyceae, Euglenophyceae and Chrysophyceae) were recorded. During the sampling, 100, 117 and 110 species of phytoplankton were recorded in collection I, II and III respectively. Species composition was almost uniform in all the three collections, whereas species diversity and species richness varied across stations and collections. Species compositions as well as population of diatoms were more in streams, while that of desmids was more in reservoir water. Various pollution indices applied showed oligotrophic nature of the reservoir waters with slight organic pollution in stream waters.
In the lower catchment, 86 species belonging to 38 genera (belonging to Bacillariophyceae, Desmidials, Chlorococcales, Cyanophyceae, Dinophyceae, Euglenophyceae and Chrysophyceae) were recorded . In I-collection 44, II-collection 47 and in III-collection 45 phytoplankton species were recorded. Diatom species as well as population predominated in almost all the streams. Pollution indices showed oligotrophic nature of down stream region.
Introduction
Population pressure, urbanization, industrialisation and increased agricultural practices have significantly contributed to the pollution and toxicity of aquatic ecosystems. Pollutants bring about a change not only in physical and chemical quality of water but also modify the biotic components resulting in the elimination of some, probably valuable species. Attempts have been made by many workers to decide the trophic status of water bodies based on phytoplankton groups or species. Microscopic suspended algae or phytoplankton occur in different forms such as unicellular, colonial or filamentous, which are mainly photosynthetic in nature.
Phytoplanktons are among the rapid detectors of environmental change. This is because of their quick response to toxins and other chemicals. A marked change in algal community severely affects the species diversity (Biligrami, 1988). Eutrophication or organic pollution of aquatic ecosystem results in replacement of algal groups. It has been observed that many species are sensitive to the nutritional loading but equally good numbered are pollution tolerant.
Certain species of phytoplankton grow luxuriantly in eutrophic waters while some species cannot tolerate waters that are contaminated with organic or chemical wastes. Some of the species that indicate clean waters are Melosira islandica, Cyclotella ocellata and Dinobryon. The pollution indicating plankton includes Nitzschia palea, Microcystis aeruginosa and Aphanizomenon flosaquae. The latter two species have been found to produce toxic blooms and anoxic conditions. Some algae were found to cause noxious blooms in polluted water that tastes bad with intolerable odour. Plankton adapt quickly to the environmental changes because of their short life cycles. Their standing crop and species composition indicate water quality. Plankton influence on factors such as pH, colour, taste and odour. This is mainly because of the small size and great numbers. Often their scant distribution along with their transient nature cannot be totally relied upon for assessing the water quality (APHA, 1985).
In the present work an attempt has been made to assess the distribution pattern of phytoplankton in Sharavathi River Basin. Comparative study of various stations of the reservoir (lacustrine ecosystem) and streams (lotic ecosystem) is unique.
In the present work an attempt has been made to assess the distribution pattern of phytoplankton in Sharavathi River Basin. Comparative study of various stations of the reservoir (lacustrine ecosystem) and streams (lotic ecosystem) is unique.
Objectives -
- To study species composition and variations between the stations of reservoir, up and downstreams.
- To study the population and bloom of phytoplankton among these stations.
- To study species diversity, richness, and dominance.
- To assess the trophic status of the river basin using phytoplankton group, genera and species as a measure.
For this purpose phytoplankton sampling was made on monthly basis for three months during October-December 2002 at the following respective stations.
Sampling sites
| Station |
Upstream |
Downstream |
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16 |
Sharavathi-I
Sharavathi-II
Sharmanavathi
Keshavapur
Haridravathi
Nandiholé
Muppane
Talakalale
Dam outlet
Reservoir centre
Valagere
Yenneholé
Huruliholé
Sampekai
Madenur
Nittur |
Hebbankere
Dabbefalls
Hossagadde
Dabbod
Magodholé
Gazni/hennur
Chandavar
Gudankatehole
Bhaskere
Chandubanu
Haddinabal
Mavinahole
Mahasati-reservoir
Hennehole/watahalla
Upponi/loc 1
Upponi village loc 2 |
Materials and Methods
In each sampling station phytoplankton collection was made by towing a net, made up of bolting silk net No.25, for 5 minutes. Sedimentation of phytoplankton was made in 4% formaldehyde. For identification of phytoplankton algal monographs on Bacillariophyceae by Hustedt (1976) and Algae (Desmidials, Chlorococcales, Cyanophyceae, Euglenophyceae, Dinophyceae, Chrysophyceae) by Prescott (1982) were followed. For counting of phytoplankton drop count method (Trivedy and Goel, 1984) was followed. The results are expressed as organisms per ml (O/mL). For the trophic status study, different indices given by Biligrami (1988) and Palmer (1980) were applied.
Trophic status assessment
In order to apply biological means of determining the trophic status Shannon and Weiner’s species diversity values, Nygaard’s phytoplankton Quotient and Palmer’s pollution indices of phytoplankton were calculated for the three collections of phytoplankton.
Nygaard (1949) has given ratios for plankton communities to decide the trophic status. For oligotrophic lakes, the values for Cyanophycean, Chlorococcales, Diatom, Eugleninae and Compound quotients are 0.0-0.4; 0.0-0.7; 0.0-0.0; 0.0-0.2 and 0.0-1.0 respectively and for eutrophic lakes they are 0.8-3.0; 0.7-3.5; 0.2-3.0; 0.0-0.2 and 2.0-8.75 respectively. The ∞ value indicates the absence of algal quotient representing groups in that collection.
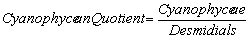
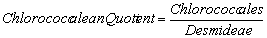
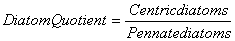
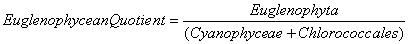
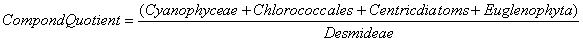
Biligrami’s pollution values
Biligrami (1988) has given the degrees of pollution based on the ranges of Shannon and Weiner’s species diversity. The pollution status based on this are, ‘slight’ (species diversity 3.0 – 4.5), ‘light’ (species diversity 2.0 – 3.0), ‘moderate’ (species diversity 1.0 – 2.0) and ‘heavy’ (species diversity 0.0 – 1.0).
Palmer’s Algal Genera and Algal Species Indices
Palmer (1980) has listed top 8 pollution tolerant genera, the Euglena, Oscillatoria, Chlamydomonas, Scenedesmus, Chlorella, Nitzschia, Navicula and Stigeoclonium and top 9 species Ankistrodesmus falcatus, Euglena viridis, Nitzschia palea, Oscillatoria limnosa, Oscillatoria tenuis, Pandorina morum, Scenedesmus quadricauda, Stegioclonium tenue and Synedra ulna. Further he has given the algal pollution indices developed for use in rating water samples for high or low organic pollution (based on 20 genera and 20 species). In analysis of a water sample, all of the 20 genera and species of algae that are present are recorded separately. An alga is called ‘present’ if there are 50 or more individuals per mL. The pollution index factors of the algae present are then totalled. A score of 20 or more for a sample is taken as evidence of high organic pollution while a score of 15-19 is taken as probable evidence of high organic pollution. Low figures indicate that the organic pollution of the sample is not high.
Results and Discussion
Upstream
Different aquatic ecosystems of Sharavathi River basin showed rich and diverse phytoplankton population (Appendix-I). Phytoplankton in the collections belonged to Bacillariophyceae (diatoms), Desmidials (desmids), Chlorococcales, Cyanophyceae, Dinophyceae, Euglenophyceae and Chrysophyceae. During the study, 216 species belonging to 59 genera were recorded. List of phytoplankton of all the three collections are given in Appendix-II.
Collection – I
During first sampling, 100 species belonging to 37 genera were recorded. Of these 48 species belonged to Bacillariophyceae, 38 to Desmidials, 8 to Chlorococcales, 3 to Cyanophyceae, 2 to Euglenophyceae and one to Dinophyceae. Qualitative dominance of the phytoplankton in this collection was in the order of Bacillariophyceae > Desmidials > Chlorococcales > Cyanophyceae > Euglenophyceae > Dinophyceae. In this collection population of Desmidial member Staurastrum multispiniceps was highest (58,944/mL) in Station-7 (Muppane) of the reservoir. Among streams population of Bacillariophyceae member Synedra ulna was highest (35,136/mL) in Haridravathi main tributary.
Collection – II
In this collection 117 species were recorded from 49 genera. Bacillariophyceae dominated with 49 species followed by Desmidials with 44; Chlorococcales with 14; Cyanophyceae with 5; Chrysophyceae with 3; and Dinophyceae with 2 species. Qualitative dominance was in the order of Bacillariophyceae > Desmidials > Chlorococcales > Cyanophyceae > Chrysophyceae > Dinophyceae. Among streams population of Gomphonema longiceps a Bacillariophycean was highest (21,568/mL) in Nandiholé, while among reservoir waters in Nittur, population of Dinobryon sertularia was highest (4752/mL).
Collection - III
During this collection 110 species of phytoplankton belonging to 48 genera were recorded. Of these 41 species belonged to Bacillariophyceae, 39 to Desmidials, 16 to Chlorococcales, 9 to Cyanophyceae, 2 species each to Dinophyceae and Chrysophyceae and a single species to Euglenophyceae. Qualitative dominance was in the order of Bacillariophyceae > Desmidials > Chlorococcales > Cyanophyceae > Dinophyceae = Chrysophyceae > Euglenophyceae. Between both the waters of streams and reservoir, population of Navicula viridula was highest in Sharavathi-I, 27,728/mLand Yenneholé, 5,648/mL.
The distribution pattern of phytoplankton was almost similar in all the collections. However, highest species were recorded in collection-II with 117 species and lowest in collection-I with 100 species. During collection-III, 110 species were recorded. From Tables 2.1, 2.2 and 2.3 it is clear that, in general in all the streams (Station 1-6 and 14) Bacillariophyceae (Diatoms) species dominated while in all the waters of reservoir (Station 7-13, 15, and 16) Desmidials predominated during all the collections. From the stationwise list of diatoms (Appendix-II) it is clear that, Gomphonema longiceps, Navicula viridula, Synedra ulna, Surirella ovata and many species of diatoms almost commonly occurred in all the streams. Similarly, species of desmids like Staurastrum limneticum, S. freemanii, S. multispiniceps, Arthrodesmus psilosporus, Triploceros gracile and Xanthedium perissacanthum almost commonly occurred in all the stations of the reservoir during all the three collections. Thus, the distribution pattern of diatoms and desmids indicates that during all the collections species composition was almost similar in streams and reservoir waters.
Cyanophyceae and Chlorophyceae members distributed uniformly in streams and reservoir waters, but Dinophyceae and Euglenophyceae were scantly distributed. Chrysophyceaen members did not occur during collection-I. During collection II and III, they were recorded from reservoir waters with 2 species of Dinobryon.
Bacillariophycean members Anomoeoneis sphaerophora, Gyrosigma attenuatum, G. gracile, Gomphonema lanceolatum, G. longiceps, Navicula viridula, Nitzschia obtusa, N. palea, Pinnularia lundii, P. maharashtrensis, Surirella ovata, Synedra acus and S. ulna were common to all the three collections. Desmidial members common to all the three collections were Arthrodesmus psilosporus, Closterium ehrenbergii, Cosmarium decoratum, Desmidium baileyi, Staurastrum limneticum, S. freemanii, S. multispinceps, S. peristephes, S. tohopekaligense and Triploceros gracile.
Chlorococcalean members Eudorina elegans, Muogeotia punctata, Pediastrum simplex, and Spirogyra rhizobrachialis were common in all the three collections. One Dinophycean member Ceratium hirundinella and one Cyanophycean member Microcystis aeruginosa were common in all the three collections.
Most of the other species of Diatoms, Desmids, Cyanophycean and Chlorococcalean were common to either collection-I and II or I and III or II and III indicating almost similar species composition in all the three collections.
Species Diversity
Tables 42, 43, and 44 reveal the diversity status of phytoplankton during I, II and III-collection. Species diversity is not uniform in any station in any of the collections, mainly due to the non-uniformity in the occurrence of species and their population.
Table 42. Diversity status of phytoplankton in I-Collection.
| Parameter |
Stations |
| 1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
| Total individual |
371 |
390 |
169 |
108 |
3879 |
355 |
10339 |
2770 |
1218 |
3414 |
1845 |
820 |
1298 |
101 |
| Total species |
21 |
13 |
14 |
10 |
12 |
9 |
21 |
13 |
11 |
14 |
18 |
23 |
16 |
5 |
| Species richness |
3.25 |
2.01 |
2.53 |
1.92 |
1.33 |
1.36 |
2.16 |
1.51 |
1.4 |
1.59 |
2.26 |
3.27 |
2.09 |
0.86 |
| Shannon-diversity |
2.44 |
2 |
1.67 |
2.03 |
1.27 |
1.37 |
1.96 |
1.85 |
1.84 |
2.24 |
2.37 |
2.69 |
2.15 |
1.09 |
| Simpson-dominance |
0.11 |
0.19 |
0.33 |
0.16 |
0.39 |
0.31 |
0.2 |
0.22 |
0.21 |
0.12 |
0.12 |
0.09 |
0.17 |
0.47 |
| Simpson-diversity |
0.88 |
0.8 |
0.66 |
0.83 |
0.6 |
0.68 |
0.79 |
0.77 |
0.78 |
0.87 |
0.87 |
0.9 |
0.82 |
0.52 |
Table 43 Diversity status of phytoplankton in II-Collection
| Parameter |
Stations |
| 1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
| Total individual |
187 |
220 |
108 |
50 |
263 |
1552 |
49 |
96 |
79 |
33 |
29 |
437 |
610 |
1303 |
74 |
397 |
| Total species |
17 |
22 |
13 |
14 |
14 |
18 |
15 |
18 |
9 |
18 |
13 |
19 |
16 |
18 |
24 |
20 |
| Species richness |
3.05 |
3.89 |
2.56 |
3.32 |
2.33 |
2.31 |
3.59 |
3.72 |
1.83 |
4.86 |
3.56 |
2.96 |
2.33 |
2.37 |
5.34 |
3.17 |
| Shannon-diversity |
0.86 |
2.29 |
1.99 |
1.97 |
1.79 |
0.66 |
2.43 |
2.11 |
1.08 |
2.75 |
2.42 |
1.97 |
1.19 |
0.93 |
2.85 |
1.16 |
| Simpson-dominance |
0.66 |
0.17 |
0.17 |
0.2 |
0.2 |
0.75 |
0.11 |
0.23 |
0.55 |
0.07 |
0.09 |
0.24 |
0.48 |
0.62 |
0.07 |
0.56 |
| Simpson-diversity |
0.33 |
0.82 |
0.83 |
0.79 |
0.79 |
0.24 |
0.88 |
0.76 |
0.44 |
0.92 |
0.9 |
0.75 |
0.51 |
0.37 |
0.92 |
0.43 |
Table 44 Diversity status of phytoplankton in III-Collection.
| Parameter |
Stations |
| 1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
| Total individual |
1858 |
301 |
204 |
31 |
35 |
166 |
232 |
59 |
52 |
88 |
146 |
585 |
223 |
1133 |
175 |
617 |
| Total species |
19 |
14 |
13 |
12 |
16 |
17 |
21 |
17 |
24 |
15 |
18 |
20 |
14 |
12 |
19 |
22 |
| Species richness |
2.39 |
2.27 |
2.25 |
3.2 |
4.21 |
3.12 |
3.67 |
3.92 |
5.82 |
3.12 |
3.41 |
2.98 |
2.4 |
1.56 |
3.48 |
3.26 |
| Shannon-diversity |
0.41 |
1.37 |
1.94 |
2.01 |
2.59 |
2.17 |
1.57 |
2.45 |
3.07 |
2.21 |
1.69 |
1.57 |
1.66 |
0.15 |
2.21 |
2.46 |
| Simpson-dominance |
0.87 |
0.45 |
0.17 |
0.21 |
0.09 |
0.14 |
0.4 |
0.12 |
0.05 |
0.14 |
0.35 |
0.38 |
0.34 |
0.95 |
0.15 |
0.11 |
| Simpson-diversity |
0.12 |
0.54 |
0.82 |
0.78 |
0.9 |
0.85 |
0.59 |
0.87 |
0.94 |
0.85 |
0.64 |
0.61 |
0.65 |
0.04 |
0.84 |
0.88 |
From Table 42 it is clear that in general, total individuals are low in almost all the streams and high in almost all the waters of reservoir. Among all the stations total individuals are highest in Station-7 (10339) and lowest in Station-14 (101). Total species is high (23) in Station-12 with highest species richness (3.27) and Shannon diversity values (2.69), which is evident from the low Simpson dominance value and high evenness index value in Station-12. On the other hand in Station-14 species richness and Shannon diversity values are low (0.86 and 1.09 respectively) with high Simpson dominance (0.47) and low evenness index value (0.52).
From Table 43 it is clear that in general, in the waters of streams and reservoir total individuals are almost low as compared to collection - I. Total individuals are lowest (49) in Station-7 where it was high during I collection. Highest individuals were recorded in Station-6 (1552). Total species is high (24) in Station-15 with highest species richness (5.34) and Shannon diversity values (2.85), which is evident from the low Simpson dominance and high evenness index values. Total species is lowest (9) in Station-9 with lowest species richness (1.83) and almost lower Shannon diversity value (1.08). However, lowest (0.66) Shannon diversity is in Station-6 with highest Simpson dominance (0.75) and lowest evenness index values (0.24).
Table 44 indicates that the total individual value is highest (1858) in Station-1 and lowest (31) in Station-4. Total species is high (24) in Station-9 with highest species richness (5.82) and Shannon diversity (3.07) values. Lowest species richness value is in Station-14 (1.56) with lowest Shannon diversity (0.14), which is evident from the higher Simpson dominance (0.95) and lower evenness index (0.04) values. Vice versa was true with Station-9 where the Shannon diversity is high (3.07) with low Simpson dominance (0.05) and high evenness index values (0.94).
Table 45 reveals the average diversity status of phytoplankton of all the three collections. This table shows the highest population (3540) in Station-7 and lowest (63) in Station-4. Highest number of species is in Station-15 (22) with highest species richness (4.41) and highest Shannon diversity (2.53) values, which are indicated by low Simpson dominance (0.11) and high evenness index values (0.88). Lowest number of species is in Station-14 (12) with lowest species richness (1.59) and Shannon diversity (0.72) and with the highest Simpson-dominance (0.68) and lowest evenness index values (0.31). From Table 7d it is also clear that species richness and species diversity values are almost high in the waters of reservoir as compared to waters of streams. This might be due to the higher number of species (24 species) of Staurastrum, a desmidial member, which might have resulted in higher species diversity value in reservoir waters.
Table 45 Average diversity status of phytoplankton in all the three Collections
| Parameter |
Stations |
| 1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
| Total individual |
805 |
304 |
160 |
63 |
1392 |
691 |
3540 |
975 |
450 |
1178 |
604 |
614 |
710 |
846 |
124 |
507 |
| Total species |
19 |
16 |
13 |
12 |
19 |
15 |
19 |
16 |
15 |
16 |
16 |
21 |
15 |
12 |
22 |
21 |
| Species richness |
2.94 |
2.72 |
2.44 |
2.81 |
2.62 |
2.26 |
3.14 |
3.05 |
3.01 |
3.19 |
3.07 |
3.07 |
2.66 |
1.59 |
4.41 |
3.21 |
| Shannon-diversity |
1.23 |
1.88 |
1.86 |
2 |
1.88 |
1.4 |
1.98 |
2.32 |
1.99 |
2.88 |
2.16 |
2.07 |
1.66 |
0.72 |
2.53 |
1.81 |
| Simpson-dominance |
0.54 |
0.27 |
0.22 |
0.19 |
0.22 |
0.4 |
0.23 |
0.19 |
0.27 |
0.37 |
0.18 |
0.23 |
0.33 |
0.68 |
0.11 |
0.33 |
| Simpson-diversity |
0.44 |
0.72 |
0.77 |
0.8 |
0.76 |
0.59 |
0.75 |
0.8 |
0.72 |
0.88 |
0.8 |
0.75 |
0.66 |
0.31 |
0.88 |
0.65 |
From the Tables 42, 43 and 44 it is clear that the Stations 7 and 1, which harboured highest and lowest total individuals respectively during I collection had almost low and high total individuals during II and III-collection. Similarly during collection – II, Station-6, which harboured highest total individuals, showed lower population during I and III collection. This indicates that the growth and distribution patterns of phytoplankton are not uniform during all the collections. Further as compared to II and III-collections total individuals were high during I-collection. It might be because of the rains during the month of September just prior to I-collection during October, which might have added nutrients to the waters along with run-off water from surrounding catchment areas.
Thus, from the above discussion about species diversity of phytoplankton in various stations of streams and reservoir it is clear that diversity and species richness were not uniform in any stations during all the three collections. However, during I collection total population was highest in reservoir waters as compared to streams. It might be because of the higher nutrient load in stagnant waters of reservoir (due to rain just before I collection), which might have resulted in higher population of Desmidials in these waters.
In general the requirement of dissolved oxygen for the growth of many diatom species is well documented. In the present study, in stream waters higher population of diatoms coincided with the higher dissolved oxygen, as oxygen is generally high in stream (flowing) waters compared to reservoir waters. The studies of Venkateshwarlu (1970) and Sheavly and Marshal (1989) who found that diatoms prefer well-aerated waters that are rich in dissolved oxygen is in support of present observation. Rao (1977) has observed dissolved oxygen favouring different species of diatoms, which is also found true for diatoms in the present study.
On the other hand reservoir waters showed lower species composition as well as population of diatoms. It may be due to their slight stagnant nature where dissolved oxygen content is less as compared to streams. However, in reservoir waters desmid species predominated. Generally, paucity of desmids is seen in the organically polluted waters. Waters supporting luxuriant growth of Desmidials have been found to be chemically distinct from those harbouring other members of algae (Hegde, 1985). The present study is on par with these observations, since desmids predominated in reservoir waters, which might have had lower organic pollution. On the other hand stream waters harboured lower desmid population indicating probable evidence of organic pollution as compared to the waters of reservoir.
Trophic Status
Table 46, 47 and 48 indicate the Nygaard’s phytoplankton quotient values. From Table 6.1 it is clear that almost all the values are very low to represent the eutrophic nature of the water. However, in some Stations (1, 3, 4, 5 and 6), the values were above the values given by Nygaard for oligotrophic waters. In Station-1, Chlorophycean and Compound quotient values, in Station-2 Compound quotient value, in Station-3 and 4 Euglenophycean quotient values, in Station-5 Chlorophycean, Euglenophycean and Compound quotient values and in Station-6 Cyanophycean, Chlorophycean and Compound quotient values exceeded the values given for oligotrophic nature of water. Interestingly all these Stations represents the streams. All the waters of reservoir show the oligotrophic nature as their quotient values are in between the values given by Nygaard for oligotrophic water. Similarly from Table 3b it is clear that, stream waters are slightly eutrophicated, as in Station-1 Cyanophycean, Chlorophycean and Compound quotient values, in Station-3 Chlorophycean and Compound quotient values and in Station-4 and 5 Cyanophycean and Chlorophycean quotient values exceeded the values given for oligotrophic nature of water. Similar to I-collection, in II-collection also in all the reservoir waters the Nygaard’s phytoplankton values are in the range of oligotrophic nature.
Table 46. Nygaard's Phytoplankton Quotient for the I collection
| Family |
Stations |
| 1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
| Cyanophycean Quotient |
∞ |
0.5 |
∞ |
∞ |
∞ |
1 |
0.05 |
0.1 |
∞ |
0.09 |
∞ |
0.08 |
0.08 |
∞ |
| Chlorophycean Quotient |
2 |
∞ |
∞ |
∞ |
1 |
1 |
∞ |
0.1 |
∞ |
∞ |
0.06 |
0.16 |
∞ |
∞ |
| Diatom Quotient |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
| Euglenophycean Quotient |
∞ |
∞ |
1 |
1 |
1 |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
| Compound Quotient |
2 |
1.5 |
0.5 |
1 |
2 |
2 |
0.05 |
0.2 |
∞ |
0.09 |
0.06 |
0.25 |
0.08 |
∞ |
Table 47. Nygaard's Phytoplankton Quotient for the II collection
| Family |
Stations |
| 1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
| Cyanophycean Quotient |
2 |
0.5 |
∞ |
1 |
1 |
0.33 |
0.11 |
0.11 |
0.33 |
0.09 |
0.16 |
0.09 |
0.14 |
0.5 |
0.18 |
0.07 |
| Chlorophycean Quotient |
2 |
2 |
2 |
1 |
1 |
0.33 |
0.11 |
0.11 |
0.66 |
0.9 |
0.16 |
0.09 |
0.14 |
2 |
0.18 |
0.07 |
| Diatom Quotient |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
| Euglenophycean Quotient |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
| Compound Quotient |
4 |
1.5 |
3 |
2 |
2 |
0.66 |
0.22 |
0.22 |
1 |
0.18 |
0.18 |
0.33 |
0.28 |
2.5 |
0.36 |
0.15 |
Table 48. Nygaard's Phytoplankton Quotient for the III collection
| Family |
Stations |
| 1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
| Cyanophycean Quotient |
1 |
1 |
0.5 |
1 |
2 |
0.2 |
0.076 |
0.12 |
0.062 |
0.166 |
0.1 |
0.18 |
0.25 |
0.2 |
0.1 |
0.33 |
| Chlorophycean Quotient |
1 |
1 |
0.5 |
3 |
2 |
0.2 |
0.076 |
0.375 |
0.062 |
0.33 |
0.1 |
0.09 |
0.5 |
0.2 |
0.1 |
0.66 |
| Diatom Quotient |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
| Euglenophycean Quotient |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
∞ |
| Compound Quotient |
2 |
2 |
1 |
4 |
4 |
0.4 |
0.153 |
0.5 |
0.125 |
0.5 |
0.2 |
0.27 |
0.75 |
0.4 |
0.2 |
1 |
Table 48 almost confirms the findings of I and II–collections, as the stream waters in III-collection are also eutrophic in nature. In Station-1 Cyanophycean, in Station-2 Chlorophycean, in Station-4 Cyanophycean, Chlorophycean and Compound quotient values and in Station-5 Cyanophycean, Chlorophycean and Compound quotient values have exceeded above the oligotrophic values. On the other hand waters of the reservoir are in between the values given for oligotrophic waters. Thus, it is clear from Nygaard’s pollution index that stream waters are slightly eutrophic in nature as compared to reservoir waters. Generally, waters of streams with rapid flow carry organic matter from the soil. In the present study, stream waters might have carried the organic matter from the soil and decomposed dried leaves of surrounding trees and resulted in the slight eutrophic nature of the waters. It is quite natural that in reservoir waters, the organic matter brought from runoff water during rains will settle down to the bottom in the winter season. This might be the reason for the lower organic pollution and oligotrophic nature of the reservoir waters as the collections of phytoplankton were made during the winter season.
From Table 49 it is clear that in general species diversity values of almost all the stations are in the range of moderate or light pollution level. As per the pollution ranges given by Biligrami (1988), waters of Stations 1, 2, 4, 10, 11, 12 and 13 during I-collection, waters of Stations 2, 7, 8, 10, 11 and 15 during II-collection and waters of Stations 4, 5, 6, 8, 9, 10, 15 and 16 during III-collection show light pollution level with species diversity ranging between 2.0 – 3.0. While waters of Stations 3, 5, 6, 7, 8, 9 and 14 during I-collection, waters of Stations 3, 4, 5, 9, 12, 13 and 16 during II-collection and waters of Stations 2, 3, 7, 11, 12 and 13 during III-collection show moderate pollution level (Species diversity ranges between 1.0-2.0).
Table 49. Shannon–Weiner’s diversity values.
| Stations |
Phytoplankton collections |
| I |
II |
III |
| 1 |
2.44 |
0.86 |
0.41 |
| 2 |
2 |
2.29 |
1.37 |
| 3 |
1.67 |
1.99 |
1.94 |
| 4 |
2.03 |
1.97 |
2.01 |
| 5 |
1.27 |
1.79 |
2.59 |
| 6 |
1.37 |
0.66 |
2.17 |
| 7 |
1.96 |
2.43 |
1.57 |
| 8 |
1.85 |
2.11 |
2.45 |
| 9 |
1.84 |
1.08 |
3.07 |
| 10 |
2.24 |
2.75 |
2.21 |
| 11 |
2.37 |
2.42 |
1.69 |
| 12 |
2.69 |
1.97 |
1.57 |
| 13 |
2.15 |
1.19 |
1.66 |
| 14 |
1.09 |
0.93 |
0.14 |
| 15 |
- |
2.85 |
2.21 |
| 16 |
- |
1.16 |
2.46 |
In II and III-collections stream waters show heavy pollution load in some stations. In II-collection waters of Stations 1, 6 and 14 and in III-collection waters of Stations 1 and 14 had heavy pollution load with the species diversity ranging between 0.0-1.0. Only the waters of Station-9 in III-collection had slight pollution level with the species diversity 3.07.
From the average species diversity values it is clear that almost all the waters of streams show moderate pollution level, while almost all the reservoir waters show light pollution level.
Thus, from the above discussion it is clear that waters of only Station-3 and 10 show uniformity i.e., moderate and light pollution level from I-collection to/and III-collection. Remaining waters during different collections show either light or moderate pollution level. Thus, the pollution level was not uniform in almost all the stations. It is in between the light and moderate pollution level with heavy pollution load in few stations of streams.
Tables 50, 51 and 52 reveal the Palmer’s genera index values. From Table 5a it is clear that all the stations with a score of less than 10 except Stations 5 and 13 are said to be less polluted. Stations 5 and 13 with score of 12 and 13 come nearer to the point of suspected pollution. Similarly, II collection and III-collection with scores less than 10 are indicating low organic pollution in all the stations. Thus, Palmer’s pollution index values of all the three collections are not exceeding the score given by Palmer (1960) for the high organic pollution or the probable evidence of organic pollution. Thus, the waters of all the stations during I, II and III-collections showed low organic pollution.
Table 50. Palmer's pollution index of algal genera in I-Collection
| Genera |
Stations |
| 1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
| Gomphonema |
1 |
1 |
1 |
1 |
1 |
- |
- |
- |
1 |
1 |
1 |
1 |
1 |
1 |
| Navicula |
3 |
3 |
3 |
3 |
- |
3 |
3 |
- |
- |
3 |
- |
3 |
- |
3 |
| Nitzschia |
3 |
3 |
- |
3 |
3 |
3 |
3 |
- |
- |
- |
- |
3 |
- |
3 |
| Synedra |
2 |
2 |
2 |
2 |
2 |
2 |
- |
- |
2 |
- |
2 |
2 |
- |
- |
| Closterium |
- |
- |
- |
- |
1 |
- |
- |
- |
- |
- |
- |
1 |
- |
- |
| Euglena |
- |
- |
- |
- |
5 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
| Microcystis |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
1 |
1 |
- |
| Ankistrodesmus |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
2 |
- |
- |
| Total Score |
9 |
9 |
6 |
9 |
12 |
8 |
6 |
0 |
3 |
4 |
3 |
13 |
2 |
7 |
Table 51. Palmer's pollution index of algal genera in II-Collection.
| Genera |
Stations |
| 1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
| Gomphonema |
1 |
1 |
1 |
1 |
1 |
- |
- |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
- |
| Navicula |
3 |
- |
3 |
- |
- |
3 |
3 |
3 |
3 |
- |
- |
- |
- |
3 |
3 |
3 |
| Nitzschia |
- |
3 |
- |
- |
3 |
3 |
- |
- |
- |
- |
- |
- |
- |
3 |
- |
- |
| Synedra |
- |
2 |
2 |
2 |
2 |
2 |
- |
2 |
- |
- |
- |
- |
- |
2 |
- |
- |
| Closterium |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
1 |
- |
- |
- |
| Microcystis |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
1 |
- |
- |
- |
- |
| Ankistrodesmus |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
2 |
- |
2 |
- |
- |
| Scenedesmus |
- |
- |
- |
- |
- |
- |
- |
4 |
- |
- |
- |
4 |
- |
- |
- |
- |
| Melosira |
- |
- |
- |
- |
- |
- |
1 |
- |
1 |
- |
1 |
- |
1 |
- |
- |
1 |
| Total Score |
4 |
6 |
6 |
3 |
6 |
8 |
4 |
10 |
5 |
1 |
2 |
8 |
3 |
11 |
4 |
4 |
Table 52. Palmer's pollution index of algal genera in III-Collection.
| Genera |
Stations |
| 1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
| Gomphonema |
1 |
1 |
1 |
1 |
1 |
1 |
- |
- |
1 |
1 |
- |
- |
- |
- |
1 |
1 |
| Navicula |
3 |
3 |
3 |
- |
3 |
3 |
- |
- |
- |
3 |
3 |
- |
3 |
3 |
3 |
3 |
| Nitzschia |
3 |
- |
3 |
3 |
- |
3 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
3 |
| Synedra |
2 |
2 |
2 |
|
2 |
2 |
- |
|
- |
- |
- |
- |
- |
2 |
- |
2 |
| Closterium |
- |
- |
- |
- |
- |
1 |
- |
- |
- |
- |
- |
- |
1 |
1 |
- |
- |
| Microcystis |
- |
- |
- |
- |
- |
1 |
- |
- |
- |
- |
- |
1 |
- |
- |
- |
- |
| Pandorina |
- |
- |
- |
1 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
| Melosira |
- |
- |
- |
- |
- |
- |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
- |
1 |
1 |
| Total Score |
9 |
6 |
9 |
5 |
6 |
11 |
1 |
1 |
2 |
5 |
4 |
2 |
5 |
6 |
5 |
10 |
Out of the 20-algal species reported by Palmer, Synedra ulna and Nitzschia palea occurred in some of the stations of I and III-collections. In II-collection along with these two species Ankistrodesmus falcatus also occurred in some stations. Some other species like Pandorina morum and Scenedesmus quadricauda, even though occurred in some of the stations, are discarded due to their lower number (less than 50 per ml.).
Tables 53, 54 and 55 reveal the Palmer’s species index values. From these Tables it is clear that the total score of none of the stations of all the three collections exceeded the total score given by Palmer for high organic pollution or even probable high organic pollution. This indicates that waters of all the stations during sampling had low organic pollution.
Table 53. Palmer's pollution index of algal species in I-Collection.
| Genera |
Stations |
| 1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
| Synedra ulna |
3 |
3 |
3 |
3 |
3 |
3 |
- |
- |
3 |
- |
- |
- |
- |
- |
| Nitzschia palea |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
5 |
| Total Score |
3 |
3 |
3 |
3 |
3 |
3 |
- |
- |
3 |
- |
- |
- |
- |
5 |
Table 54. Palmer's pollution index of algal species in II-Collection.
| Genera |
Stations |
| 1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
| Ankistrodesmus falcatus |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
3 |
- |
3 |
- |
- |
| Synedra ulna |
- |
3 |
3 |
- |
3 |
3 |
- |
3 |
- |
- |
- |
- |
- |
3 |
- |
- |
| Nitzschia palea |
- |
5 |
- |
- |
- |
5 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
| Total Score |
- |
8 |
3 |
- |
3 |
8 |
- |
3 |
- |
- |
- |
3 |
- |
6 |
- |
- |
Table 55. Palmer's pollution index of algal species in III-Collection.
| Genera |
Stations |
| 1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
| Synedra ulna |
3 |
- |
3 |
- |
3 |
3 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
3 |
| Nitzschia palea |
- |
- |
- |
- |
- |
5 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
5 |
| Total Score |
3 |
0 |
3 |
0 |
3 |
8 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
8 |
From the species composition and growth of phytoplankton in various streams and reservoirs it is clear that waters of both streams and reservoir were below the level of high organic pollution. High pollution indicating organisms were very less in these aquatic ecosystems and the score of those present did not show the range of Palmer’s total score of high organic pollution.
By applying various pollution indices, it is clear that in general waters of both streams and reservoir are oligotrophic in nature, as there is no high organic pollution load in these waters. However, there is slight difference in the results of different pollution indices. Nygaard’s pollution index showed slight eutrophic quality for stream waters and oligotrophic for reservoir waters. While pollution index based on Shannon diversity showed no difference between streams and reservoir waters on the basis of oligotrophic and eutrophic natures. Results of this index indicated the slight eutrophication and oligotrophication in both the waters of streams and reservoir. Palmer’s genera as well as species pollution index showed no heavy load of organic pollution in any of the waters of both the streams and reservoir. According to Palmer (1980) Melosira islandica and species of Dinobryon are clean water indicators. The occurrence of Melosira islandica in stream waters and Dinobryon calciformis and D. sertularia in reservoir waters clearly indicate that both the waters are clean. Thus, there is no heavy organic pollution load in any of the waters of both streams as well as reservoir of Sharavathi River basin.
As all the stations of streams and reservoir are away from disturbances from cities and industries, presently there is no heavy organic pollution in these water-bodies. However, in future if there would be any pollutants like domestic and industrial wastes, there is a threat to the indigenous phytoplankton. Phytoplanktons are primary producers, on which many higher-level organisms like zooplankton and other aquatic higher animals are directly or indirectly dependent. So these contaminations may change their environment and affect the food chain. Due to this the organisms, which were in equilibrium with habitat earlier, will be unable to cope up with the changed environment and may disappear slowly.
Downstream
Analysis of phytoplankton sampling in Sharavathi downstream revealed that all the three collections were represented by the members belonging to Bacillariophyceae (Diatoms), Desmidials (Desmids), Chlorococcales, Cyanophyceae (Blue-green algae), Dinophyceae, Euglenophyceae and Chrysophyceae. A total of 86 species belonging to 38 genera were recorded during the study period.
Collection - I
During first sampling a total of 44 species belonging to 23 genera were recorded. Of these 23 belonged to Bacillariophyceae, 12 to Desmidials, 4 to Chlorococcales, 3 to Cyanophyceae, and one each to Euglenophyceae and Chrysophyceae. Qualitative dominance of the phytoplankton in this collection was in the order of Bacillariophyceae > Desmidials > Chlorococcales > Cyanophyceae > Euglenophyceae = Chrysophyceae. In this collection population of Bacillariophyceaen member Navicula viridula was highest (1664 O/mL) in Stream-6 (Gazni-Hennur stream).
Collection - II
A total of 47 species were recorded in this collection. These belonged to 27 genera. Bacillariophyceae dominated with 25 species followed by Desmidials with 10, Chlorococcales with 8, Cyanophyceae with 2, Euglenophyceae and Dinophyceae with a single species each. Qualitative dominance was in the order of Bacillariophyceae > Desmidials > Chlorococcales > Cyanophyceae > Euglenophyceae = Dinophyceae. During this collection population of Synedra acus a Bacillariophycean member was highest (1984 O/mL) in Stream-3 (Hosagadde stream).
Collection - III
During this collection 45 sps. of phytoplankton belonging to 29 genera were recorded. Of these 20 species belonged to Bacillariophyceae, 14 to Desmidials, 5 to Chlorococcales, 4 to Cyanophyceae, and a single species each to Dinophyceae, Chrysophyceae and Euglenophyceae. Qualitative dominance was in the order of Bacillariophyceae > Desmidials > Chlorococcales > Cyanophyceae > Dinophyceae = Chrysophyceae = Euglenophyceae. In this collection population of Synedra ulna, a Bacillariophycean member was highest (1472 O/mL) in Stream-2 (Dabbefalls).
The distribution pattern of phytoplankton was almost similar in all the collections. However highest species were recorded in II-collection with 47 species and lowest in I-collection with 44 species. During III- collection 45 species were recorded. In general in all the streams Bacillariophyceae (diatoms) species dominated except the Stream –13 (Mahasati- reservoir main river) where desmids dominated during all the collections. Bacillariophyceae members like Gomphonema longiceps, Eunotia praerupta, Melosira islandica, Synedra ulna and S. acus were almost commonly occurred in all the streams of all the collections. Desmidial members like Desmidium suboccidentale, Staurastrum freemanii and S. multispiniceps were also almost common. However their species composition as well as number were drastically low compared to diatoms.
Species of Cyanophyceae and Chlorophyceae distributed uniformly, while distribution pattern of Euglenophyceae was scanty. Chrysophyceaen and Dinophycean members did not occur during II & I-collection respectively. During other collections only one Chrysophyceaen and two Dinophycean members occurred.
Bacillariophycean members Eunotia praerupta, Gomphonema longiceps, G. viderbhense, Navicula laeta, N. viridula, N. cuspidata, Nitzschia palea, Melosira islandica, Pinnularia lundii, Synedra acus and S. ulna were common to all the three collections. Desmidial members common to all the three collections were Desmidium suboccidentale, Closterium ehrenbergii, Staurastrum freemanii and S. multispinceps.
Chlorococcalean members Ankistrodesmus falcatus, Muogeotia punctata, Spirogyra gratiana were common in all the three collections. Two Cyanophycean members Merismopedia glauca and Microcystis aeruginosa and one Euglenophycean member Euglena acus were common in all the three collections.
Most of the other species of Diatoms, Desmids, Cyanophycean and Chlorococcalean were common to either I and II-collection or I and III-collection or II and III-collection indicating almost similar species composition in all the three collections.
Bacillariophyceae (diatoms) members were high in all the streams except in Stream-13 (Mahasati-reservoir main river), where desmidials dominated. However none of the phytoplankton representing either Bacillariophyceae or desmidials formed bloom during any of the collections. Their individual population was not enough to form a scum on the surface to give distinct colouration to the water. However Navicula viridual inStream-6 (Gazni-Hennur stream), Synedra acus in Stream-3 (Hosagadde stream) and Synedra ulna in Stream-2 (Dabbefalls stream) dominated in I, II and III collection respectively. Population of Cyanophyceae and Chrorophyceae was very low. Dinophyceae, Euglenophyceae and Chrysophyceae occurrence was scanty with negligible population.
In present study ominance of diatoms in streams could be attributed to the well-oxygenated water as they were lotic. Higher desmid population in Stream-13 (Mahasati-reservoir-main river) could be due to addition from Linganamakki reservoir which might have had higher desmid population, as observed during upstream study of Sharavati river (October-December 2001).
Species diversity
Tables 56, 57 and 58 reveal the diversity status of phytoplankton. From the Table 56 it is clear that in general during I collection, species diversity is high (2.35) in Stream-15 (Upponi location-main river) and low (1.34) in Stream-2 (Dabbefalls stream). Among all the streams total individual is highest (5328) in Stream-6 (Gazani-Hennur stream) and lowest (768) in Stream-12 (Mavinhole stream). Total species is high (15) in Stream-15 (Upponi) with highest species rich ness (1.85) and Shannon diversity (2.35) values which are evident from the low Simpson dominance value (0.12) and high evenness index value (0.87).
Collection-II (Table 57)had low total individuals compared to collection-I. Total individual is lowest (256) in Station-6 (Gazani/Hennur stream) where it was high during I-collection. Highest individuals (6980) were recorded in Stream-7 (Chandavar stream). Species richness is high (1.44) in stream-14 (Hennehole/watahalla) with highest Shannon diversity values (2.16), which are evident from the low Simpson dominance (0.13) and high evenness index values (0.86). While species richness is lowest (0.49) in Station-11 (Haddinbal stream) with almost lowest Shannon diversity value (1.23). However, lowest Shannon diversity value (1.08) is in Stream-3 (Hassagadde stream) with highest (0.50) Simpson dominance and lowest (0.49) evenness index values.
Table (58) indicates that the total individual value is highest (2944) in Stream-13 (Mahasati –reservoir main river) and lowest (224) in Stream-8 (Gudankatehole stream). Total species is high (13) in Stream-5 (Magodhole stream) with highest species richness (1.56) and Shannon diversity (1.94) values. Lowest species richness value (0.67) is in Stream-3 (Hossgadde stream) with lowest Shannon diversity (1.20), which is evident from the higher Simpson dominance (0.39) and lower (0.60) evenness index values. From Table 56, 57 & 58 it is clear that the Stream-6 (Gazani/hennur stream) and Stream-12 (Mavinhole stream) which harboured highest and lowest total individual respectively during I-collection had almost low and moderate total individuals during II and III-collection. Similarly during II collection, Stream-7 (Chandavar stream), which harboured highest total individuals showed lower population during collection-I and IIFurther as compared to II and III collections total individuals were high during I-collection. It might be because of the rains during the month of September just prior to the I-collection, which might have added nutrients to the waters along with run-off water from surrounding catchment areas.
Thus from the above discussion about species diversity of phytoplankton in various stations of streams it is clear that diversity and species richness were not uniform in any streams during all the three collections. However during I-collection total population was slightly highest in almost all the streams. This could be due to the increased population of Gomphonema longiceps and Synedra ulna, whose populations were more during this collection.
Table 56: Diversity status of Phytoplankton in I collection
| Parameters |
Stations |
| 1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
| Total individual O/ml |
- |
3552 |
- |
- |
- |
5328 |
1936 |
- |
2784 |
- |
1376 |
768 |
1728 |
- |
1887 |
- |
| Total Species |
- |
9 |
- |
- |
- |
8 |
13 |
- |
12 |
- |
8 |
9 |
13 |
- |
15 |
- |
| Species richness |
- |
0.97 |
- |
- |
- |
0.81 |
1.58 |
- |
1.38 |
- |
0.96 |
1.20 |
1.60 |
- |
1.85 |
- |
| Shannon Weiner’s diversity |
- |
1.34 |
- |
- |
- |
1.50 |
2.27 |
- |
1.88 |
- |
1.70 |
1.76 |
1.95 |
- |
2.35 |
- |
| Simpson’s dominance |
- |
0.36 |
- |
- |
- |
0.27 |
0.12 |
- |
0.22 |
- |
0.23 |
0.23 |
0.24 |
- |
0.12 |
- |
| Simpson’s diversity |
- |
0.63 |
- |
- |
- |
0.72 |
0.87 |
- |
0.77 |
- |
0.76 |
0.76 |
0.75 |
- |
0.87 |
- |
Table 57: Diversity status of Phytoplankton in II collection
| Parameters |
Stations |
| 1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
| Total individual O/ml |
1824 |
960 |
2880 |
176 |
880 |
256 |
6980 |
384 |
2496 |
3616 |
416 |
1024 |
- |
512 |
1792 |
2592 |
| Total Species |
9 |
9 |
10 |
5 |
7 |
5 |
11 |
7 |
11 |
10 |
4 |
9 |
- |
10 |
8 |
11 |
| Species richness |
1.06 |
1.16 |
1.12 |
0.77 |
0.88 |
0.72 |
1.12 |
1.00 |
1.27 |
1.09 |
0.49 |
1.15 |
- |
1.44 |
0.93 |
1.27 |
| Shannon Wiener’s diversity |
1.64 |
1.51 |
1.08 |
1.36 |
1.52 |
1.49 |
1.98 |
1.58 |
1.75 |
1.32 |
1.23 |
1.59 |
- |
2.16 |
1.35 |
1.60 |
| Simpson’s dominance |
0.25 |
0.33 |
0.50 |
0.30 |
0.28 |
0.27 |
0.17 |
0.29 |
0.26 |
0.36 |
0.32 |
0.30 |
- |
0.13 |
0.36 |
0.31 |
| Simpson’s diversity |
0.74 |
0.66 |
0.49 |
0.69 |
0.71 |
0.75 |
0.82 |
0.70 |
0.73 |
0.63 |
0.67 |
0.69 |
- |
0.86 |
0.63 |
0.68 |
Table 58: Diversity status of Phytoplankton in III collection
| Parameters |
Stations |
| 1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
| Total individuals O/ml |
- |
2624 |
1728 |
576 |
2176 |
2016 |
544 |
224 |
832 |
800 |
448 |
2080 |
2944 |
2816 |
2080 |
1824 |
| Total Species |
- |
7 |
6 |
7 |
13 |
9 |
6 |
6 |
9 |
8 |
8 |
7 |
11 |
7 |
9 |
8 |
| Species richness |
- |
0.76 |
0.67 |
0.94 |
1.56 |
1.05 |
0.79 |
0.92 |
1.18 |
1.04 |
1.14 |
0.78 |
1.25 |
0.75 |
1.04 |
0.93 |
| Shannon Wiener’s diversity |
- |
1.21 |
1.20 |
1.72 |
1.94 |
1.48 |
1.48 |
1.74 |
1.72 |
1.68 |
1.90 |
1.67 |
1.64 |
1.54 |
1.65 |
1.41 |
| Simpson’s dominance |
- |
0.38 |
0.39 |
0.20 |
0.19 |
0.31 |
0.27 |
0.18 |
0.25 |
0.22 |
0.17 |
0.22 |
0.24 |
0.24 |
0.24 |
0.31 |
| Simpson’s diversity |
- |
0.61 |
0.60 |
0.79 |
0.80 |
0.68 |
0.72 |
0.81 |
0.74 |
0.77 |
0.82 |
0.77 |
0.75 |
0.75 |
0.75 |
0.68 |
Trophic status
Species diversity values of almost all the stations are in the range of moderate or light pollution level. As per the Biligrami’s pollution ranges, waters of Stream-7 and 15 during I-collection and waters of Stream-14 during II-collection show the light pollution level with species diversity range between 2.0-3.0. While during I-collection waters of stream-2, 6, 9, 11, 12 & 13 and during II-collection waters of Stream-1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 15 and 16 and during III collection waters of all the streams show the moderate pollution level (Species diversity range between 1.0-2.0).
Thus waters of almost all the streams showed uniformity i,e moderate pollution from I-collection to III-collection. While the waters of only three streams (Stream-7, 14 and 15) during different collections showed either light or moderate pollution level. Thus the pollution level was uniform in almost all the streams. It was in moderate level through out the study period. However none of the waters of any streams during any collection showed the heavy pollution load indicating pollution level is low in down stream region.
Conclusion
The biological examination of the stream and reservoir ecosystems of Sharavathi River basin showed a rich and diverse phytoplankton population. Desmids predominated in reservoir waters while diatoms in streams. Species diversity is not uniform either in streams or reservoir waters.
From the Biligrami’s and Palmer’s pollution indices it is clear that, in general waters of all the streams of down stream region are oligotrophic in nature as there is no high organic pollution load in these waters. However Biligrami’s (Shannon and Weaver’s pollution index) showed waters of most of the streams had moderate pollution level. According to Palmer (1980) Melosira islandica is a clean water indicator. The occurrence of M. islandica in these waters and least occurrence of Palmer’s pollution indicating phytoplankton clearly indicate that there is no heavy pollution load in any of the waters of these streams of down stream region.
Both upstream and downstream regions of Sharavathi River showed low organic pollution. This could be due to least domestic and industrial pollutants in this region. Compared to upstream region, down stream region is less polluted as far as phytoplankton abundance is concerned. Phytoplankton number and species composition were very less in down stream region as compared to upstream region. According to Palmer (1980), higher organic pollution directly relates with higher eutrophication and phytoplankton population. This observation holds good with present study as the phytoplankton population was not so high, in any of the streams, to form the eutrophic condition indicating lower organic pollution (eutrophication). Further, Wielgolaski (1975) opines that, in freshwater most dinoflagellates are sensitive to eutrophication. Thus, in present study, occurrence of only two Dinophyceaen members, with very low number, clearly indicates the lesser organic pollution in this region.
From various pollution indices, it is clear that the waters of reservoir are in oligotrophic nature, even though the streams showed slight organic pollution. The study emphasises the requirement of proper conservation of phytoplankton-the primary producers, on which most of the higher aquatic organisms are dependent.
REFERENCES
- American Public Health Association (APHA), American Water Works Association (AWWA) and Water Pollution Control Federation (WPCF), 1985. Standard Methods for the Examination of Water and Wastewater. Sixteenth Edition, Ed. by Arnold E.Greenberg, R. Rhodes Trussell and Lenore S. Clesceri, Washington DC.
- Biligrami K. S., 1988. Biological monitoring of Rivers, Problems and Prospects in India. Aquat. Ectovicol. (Proc. Indo. Dutch. Symp. Eds. de kruijf et. al) 1988: 245-50.
- Hegde, G. R. 1985. On the succession of algae in a temple tank at Dharwar, Karnataka State, India. Geobios, 12(6): 261-263.
- Hustedt, F., 1976. Bacillariophyta (Diatomaeae). Otto Koeltz Science Publishers W-Germany.
- Nygaard, G. 1949. Hydrobiological studies of some Danish ponds and lakes II. The quotient hypothesis and some new or little known phytoplankton organisms. K. Danske Vie dersk. Selsk skr., 7(1):1-293.
- Palmer, C. M., 1980. Algae and water pollution. Castel House Publishers Ltd., England: 123pp.
- Prescott G. W., 1982. Algae of the Western Great lakes area. Otto Koeltz Science Publishers W-Germany.
- Rao, V. S. 1977. An ecological study of three fresh water ponds of Hyderabad-India IV-The phytoplanktons (Diatoms, Eugleninae and Myxophyceae). Hydrobiologia, 47(2): 319-337.
- Sheavly, S. B. and H. G. Marshall., (1989). Phytoplankton and water quality relationships within the Euphotic zone of lake Trashmore, Virginia: A Borrow p: t Lake: Castanea, 54(3): 153-163.
- Trivedy, R. K. and P. K. Goel., 1984. Chemical and Biological methods for water pollution studies. Environmental pub. Karad, 215pp.
- Venkateshwarlu, V., 1970. An ecological study of the algae of the river Moosi Hyderabad (India) with special reference to water pollution IV. Periodicity of some common species of algae. Hydrobiologia, 35 : 45-64.
- Wielgolaski, F. E., 1975. Biological indicators on pollution, Urban Ecology:
1(1): 63-79
List of Phytoplankton in I, II and III-Collections of the upper catchment
| Desmidials |
Collections |
| I |
II |
III |
| Arthrodesmus constrictus G. M Smith var.longispinus Gronbl. |
|
+ |
+ |
| A. curvatus Turn.var.latus var.nov |
+ |
|
|
| A. psilosporus (Nodrdst. & Lofg.) De Toni Formae |
+ |
+ |
+ |
| Bambusina brebissoni Kuetz. |
+ |
+ |
|
| Closterium calosporum Wittr |
|
+ |
|
| C. ehrenbergii Menegh |
+ |
+ |
+ |
| C. lagoense Nordst.var.crassius Gutw |
|
|
+ |
| C. kuetzingii Breb var. vittatum Nordst |
|
+ |
+ |
| C. porrectum Nordst |
+ |
|
|
| C. ralfsii Breb var.hybridrum Rab |
|
+ |
+ |
| C. setaceum Ehrbg. |
|
|
+ |
| Cosmarium askenasyi Schm.fa.latum Scott & Presc |
|
|
+ |
| C. contractum Kirchn |
|
+ |
+ |
| C. decoratum West & West |
+ |
+ |
+ |
| C. dubium Borge |
+ |
|
|
| C. ordinatum (Borges) West & West var.borgei Scott Gronbl. |
+ |
|
|
| C. lundellii Delp var circulare (Reinch) Krieg |
+ |
|
|
| C. lundellii Delp |
|
|
+ |
| C. margaritatum (Lund) Roy & Biss var sublatum (Nordst.) Krieg |
|
+ |
|
| C. pseudoconnatum Nordst |
+ |
|
|
| C. punctulatum Breb.var.sub punctulatum (Nordst.) Borges |
+ |
|
|
| C. retusiforme (Wille) Gutw. Fa |
+ |
|
|
| C. scabrum Turn |
|
+ |
|
| C. sexangulare Lund Fa |
|
+ |
|
| C. spinuliferum West & West |
|
+ |
+ |
| C. subturgidum (Turn.) Schm. Fa. minus Schm |
|
+ |
+ |
| C. tumidium Lund |
|
+ |
|
| Desmidium baileyi (Ralfs) Nordst fa.longiprocessum fa.nov |
+ |
+ |
+ |
| D. baileyi (Ralfs) Nordst fa. tetragonum Nordst |
|
+ |
|
| D. bengalicum Turn |
+ |
|
+ |
| D. bengalicum Turn fa.quadratum fa. nov. |
|
+ |
|
| D. quadratum Nordst |
|
+ |
|
| D. suboccidentale Scott & Presc. |
+ |
|
+ |
| Euastrum acanthophorum Turn. |
|
+ |
|
| E. ansatum Ehr. v. Triporum |
+ |
|
|
| E. elegans (Breb) Kutz. fa. |
|
|
+ |
| E. gnathophorum West & West var.bulbosum var-nov |
|
+ |
+ |
| E. luelkemuelleri Ducell var. carniolicum (Lutkem.) |
+ |
|
|
| E. sinuosum Lenorm. var. parallelum Krieg |
+ |
|
|
| E. spinulosum Delp. Fa |
|
|
+ |
| Gonatozygon aculeatum Hastings |
|
+ |
+ |
| Hyalotheca dissiliens (Smith) Breb. var. hains Wolle |
+ |
|
+ |
| Micrasterias foliacea Bail var. quadrinflata var-nov |
|
+ |
|
| M. mahabuleshwarensis Hobs.var.chauliodon var-nov |
+ |
|
|
| M. quadridentata (Nordst.) Gronbl.fa, indonesinsis fa.nov |
|
|
+ |
| M. mahabuleshwarensis Hobs var, surculifera lagerh |
|
+ |
|
| Netrium digitus (Ehrbg.) Itzigs & Rothe |
|
|
+ |
| Pleurotaenium ehrenbergi (Breb.) De Bary v. undulatum Schaarschm |
|
+ |
+ |
| Sphaerozosma granulatum Roy & Biss |
+ |
|
|
| Spondylosium nitens (Wall.) Arch.fa.majus Turn |
|
+ |
|
| S. planum (Wolle.) West & West |
+ |
|
|
| Onychonema laeve Nordst. var. latum West & West |
|
|
+ |
| Staurastrum anceps Her. |
|
+ |
|
| S. anceps Ehr. v. hyalina Brun. et.Perag |
|
|
+ |
| S. cerastes Lund. var. coronatum Krieg. fa. inflatus Scott.& Presc. |
+ |
|
|
| S. cerates Lund var pulchrum Scott & Gronbl. fa |
|
+ |
|
| S. euprepes sp.nov. |
+ |
|
|
| S. emaciatum sp.nov. |
|
+ |
|
| S. gralile Ralfs fa. Kriegeri fa .nov |
|
+ |
+ |
| S. freemanii West & West var.nudiceps Scott & Presc. |
+ |
+ |
+ |
| S. indentatum West & West Formae |
+ |
|
|
| S. limneticum Schm. Var. burmense West & West |
+ |
+ |
+ |
| S. longibrachiatum (Borge) Gutw |
+ |
|
|
| S. menggalense sp. Nov |
|
+ |
|
| S. multispiniceps sp.nov. |
+ |
+ |
+ |
| S. peristephes sp.nov |
+ |
+ |
+ |
| S. prionotum sp.nov |
|
+ |
+ |
| S. rosei Playf. var. stemmatum var.nov |
|
+ |
|
| S. sexangulare Lund var.productum Nordst |
+ |
|
|
| S. sebaldi Reinsch var.ornatum Nordst |
|
+ |
+ |
| S. sebaldi Reinsch var.ventriverrucosum var .nov |
|
|
+ |
| S. tauphorum West & West |
+ |
+ |
|
| S. thienemannii Krieg fa. triradiatum fa.nov |
|
+ |
+ |
| S. tohopekaligense Wolle var. insigne West & West Formae |
+ |
+ |
+ |
| S. wildmanii Gutw. |
|
+ |
|
| S. zonatum Borges var.majus.var. nov. |
+ |
|
|
| Triploceros gracile Bail fa. curvatum fa.nov. |
+ |
+ |
+ |
| T. gracile Bail fa. Undulatum Scott & Presc. |
|
|
+ |
| Xanthedium antilopaeum (Breb.) Kutz.var.laeve longispinum fa.nov. |
|
|
+ |
| X. freemanii West & West fa |
+ |
|
|
| X. hastiferum Turn. Var. javanicum (Nordst.) Turn. fa. Planum Turn |
+ |
|
|
| X. perissacanthum Scott. & Presc. Var. minus. Var-nov. |
+ |
+ |
|
Complete list of Phytoplankton in I, II and III-Collections
| Chlorococcales |
Collections |
| I |
II |
III |
| Ankistrodeimus falcatus (Corda) Ralfs |
|
+ |
+ |
| A. spiralis (Turner) Lemmermann |
+ |
|
+ |
| Gomphosphaeria aponina var. delicatula virieux |
|
+ |
|
| Coelastrum microporum Naegeli |
+ |
|
|
| Eudorina elegans Ehrenberg |
+ |
+ |
+ |
| Kirchnerilla lunaris (Krich.) Moebius |
+ |
|
|
| K. obesa (W.West) Schmidle |
|
+ |
+ |
| Muogeotia punctata Wittrock |
+ |
+ |
+ |
| Oocystis submarina Lagerheim |
|
+ |
+ |
| Pandorina morum (Muell.) Bory |
|
+ |
+ |
| Pediastrum duplex var rugulosum Raciborski |
+ |
|
|
| P. simplex Meyen |
+ |
+ |
+ |
| Pleudorina californica Shaw |
|
|
+ |
| Scenedesmus acuminatus (Lag) Chodat |
|
|
+ |
| S. bijuga (Turp.) Lagerheim |
|
+ |
+ |
| S. dimorphus (Turp.) Kuetzing |
|
+ |
|
| S. opoliensis var contacta Prescott |
|
|
+ |
| S. quadricauda (Turp.) de Brebisson |
|
+ |
+ |
| Spirogyra gratiana Transeau |
|
+ |
+ |
| S. rhizobrachialis Jao |
+ |
+ |
+ |
| Stigeoclonium staganatile (Hazen) Collins |
|
|
+ |
| Zygnena pectinatum (Vauch.) C.A. Agardh |
|
+ |
|
Complete list of Phytoplankton in I, II and III-Collections
| Cyanophyceae |
Collections |
| I |
II |
III |
| Aphanocapsa rivularis (Carm) Rabenhorst |
|
+ |
+ |
| Chroococcus limneticus var.elegans G. M. Smith |
+ |
|
+ |
| C. turgidus (kuetz.) Naegeli |
|
+ |
+ |
| Coelosphaerium dubium Grunow |
|
+ |
+ |
| Gomphosphaeria aponina var. cordiformis Wolle |
|
|
+ |
| G. lacustris Chodat |
|
|
+ |
| Merismopedia elegans var.major G.M.Smith |
+ |
|
|
| M. glauca (Ehrbg.) Naegeli |
|
+ |
+ |
| Microcystis aeruginosa Kuetz, emend, Elenkin |
+ |
+ |
+ |
| Oscillatoria anguina (Borg.) Gomont |
|
|
+ |
| |
| Dinophyceae |
| Ceratium hirundinella (O.F. Muell) Dujardin |
+ |
+ |
+ |
| Peridinium cinctum (Muell) Ehrenberg |
|
+ |
+ |
| |
| Euglenophyceae |
| Euglena acus Ehrenberg |
+ |
|
+ |
| E. acus var. rigida Huebner |
+ |
|
|
| |
| Chrysophyceae |
| Dinobryon calciformis Bachmann |
|
+ |
+ |
| D. divergens Imhof |
|
+ |
|
| D. sertularia Ehrbg. |
|
+ |
+ |
APPENDIX II Complete list of Phytoplankton in I, II and III-Collections of the Sharavathi downstream region
| Bacillariophyceae (Diatoms) |
Collections |
| I |
II |
III |
| Anomoeoneis lanceolata Gandhi |
+ |
|
|
| A. sphaerophora (Kuetz.) Ptitzer. |
+ |
+ |
+ |
| Caloneis silicula (Ehr.) Cleve v. intermadia Mayer |
|
+ |
|
| C. bengalensis Grun |
|
+ |
|
| Cymbella chandolensis Gandhi |
|
+ |
|
| C. lata Grun v. nagpurensis v. nov. |
+ |
|
|
| C. laevis Naeg |
|
+ |
|
| C. leptoceros (Ehr.) Grum v. rostellata Hustedt f.India Gandhi |
|
+ |
|
| C. osmanabadensis sp.nov |
+ |
+ |
|
| C. powaiana Gandhi |
|
+ |
+ |
| C. ventricosa Kuetz. |
|
+ |
+ |
| C. vidarbhensis sp. Nov |
|
+ |
|
| Eunotia praerupta Ehr. |
+ |
+ |
|
| Frustulia jogensis Gandhi |
|
+ |
|
| Gomphonema gracile Ehr. v. major Grun |
|
|
+ |
| G. intricatum Kuetz. |
|
+ |
|
| G. intricatum Kuetz.v. virbio (Ehr.) Cleve |
+ |
|
|
| G. lanceolatum Her |
+ |
+ |
+ |
| G. longiceps Ehr. v. subclavata Grun |
+ |
+ |
+ |
| G. speculoides Gandhi v. major Gandhi |
|
|
+ |
| G. sphaerophorum Her |
|
+ |
|
| Gyrosigma acuminatum (Kuetz.) Rabh |
+ |
|
|
| G. attenuatum (Kuetz.) Rabh.(Nordst & Lofg.) De Toni |
|
|
+ |
| G. attenuatum (Kuetz.) Rabh |
+ |
+ |
+ |
| G. distortum (W. Smith) Cleve v. porkeri Harrison |
|
|
+ |
| G. gracile Ehr. v. naviculoides (W. Smith) Grun |
|
+ |
|
| G. gracile Ehr. v. intricatiforme Mayer |
+ |
+ |
+ |
| G. kuetzingii (Grun.) Cleve |
+ |
|
+ |
| G. sclalporoides (Rabh.) Cleve |
+ |
|
|
| G. spenceri (W. Smith ) Cleve. v. nodiferum (Grun.) A. Cl |
|
+ |
|
| Hantzschia linearis (O. Muell) A. Cl |
|
|
+ |
| H. voigtii Gandhi |
|
+ |
|
| Navicula cari Ehr. |
|
+ |
+ |
| N. cari Ehr. v. angusta Grun |
|
+ |
|
| N. crysptocephala Kuetz.v.exilis Grun |
+ |
|
|
| N. gastrum Ehr. |
+ |
|
|
| N. gracilis Her |
+ |
|
|
| N. cuspidata Kuetz. |
|
|
+ |
| N. cuspidata Kuetz.f.brevirostrata Gandhi |
+ |
+ |
|
| N. cuspidata Kuetz. v. major Meister |
|
+ |
|
| N. cuspidata Kuetz. v. amigua (Ehr.) Cleve |
|
|
+ |
| N. laeta A. Mayer |
+ |
|
+ |
| N. lanceolata Grun |
+ |
|
|
| N. mutica Kuetz. v. linearis Gonz. et. Gandhi |
+ |
|
|
| N. pygmaea Kuetz. v. indica Skv |
+ |
|
|
| N. reinhardtii Grun. f. gracilis Grun |
+ |
|
|
| N. rostellata Kuetz. |
|
+ |
|
| N. subdapaliformis Gandhi |
+ |
|
|
| N. tusculoides A. Cl. v. mayeri A. Cl. |
|
+ |
|
| N. viridula Kuetz. V. capitata Mayer |
+ |
+ |
+ |
| N. viridula Kuetz. |
+ |
|
|
| Bacillariophyceae (Diatoms) |
Collections |
| I |
II |
III |
| Neidium dubium (Ehr.) Cleve |
|
+ |
|
| N. indicum Gonzalves et Gandhi v. capitata Gonz.&Gandhi |
+ |
|
|
| Nitzschia apiculata (Greg.) Grun |
+ |
|
|
| N. closterium W. Smith |
+ |
+ |
|
| N. lorenziana Grun v. subtilis Grun |
+ |
|
+ |
| N. kuetzingiana Hilse |
+ |
|
|
| N. obtusa W. Smith v. scalpelliformis Grun |
+ |
+ |
+ |
| N. palea (Kuetz.) W. Smith |
+ |
+ |
+ |
| N. philippinarum Hustedt |
+ |
|
+ |
| N. radiosa Kuetz. |
|
+ |
+ |
| N. regula Hustedt v. fennica A. Cl. |
+ |
|
|
| N. sublinearis Hustedt |
+ |
+ |
|
| N. tryblionella Hantzsch v. victoriae Grun |
|
|
+ |
| Melosira islandica O. Muell v. helvetica O. Muell |
|
+ |
+ |
| M. granulata (Ehr.) Ralfs. v. mazzanensis Meister |
|
|
+ |
| Mastogoloea baltica Grun |
|
+ |
|
| Pinnularia brevicostata Cleve v. indica Gandhi |
|
|
+ |
| P. divergense W. Smith v. capitata Mills |
|
+ |
+ |
| P. gracioloides Hustedt |
|
|
+ |
| P. interrupta W. Smith |
+ |
|
|
| P. legumen Ehr. v. florentinu (Grun) Cleve |
|
|
+ |
| P. lundii Hustedt |
+ |
+ |
+ |
| P. kolhapurensis Gandhi |
|
|
+ |
| P. platycephala (Ehr.) Cleve |
|
+ |
|
| P. maharashtrensis sp. nov. |
+ |
+ |
+ |
| P. stomatophoroides Mayer v. ornata A. Cl. f. erlangensis Mayer |
|
+ |
|
| P. streptoraphe Cleve |
|
|
+ |
| P. vidarbhensis sp. nov. |
|
|
+ |
| Stauroneis anceps Ehr. |
|
|
+ |
| S. anceps Ehr. f. linearis (Ehr.) Cleve |
+ |
|
|
| S. kirtikari sp. nov. |
|
+ |
|
| S. phoenicenteron Ehr. |
|
+ |
|
| Surirella capronii Breb |
|
+ |
|
| S. capronioides Gandhi |
|
+ |
|
| S. linearis W. Smith |
|
+ |
|
| S. ovata Kuetz v. pinnata (W. Smith) Hustedt |
+ |
|
|
| S. ovata Kuetz v. salina (W. Smith) Hustedt |
+ |
+ |
+ |
| S. robusta Ehr. |
+ |
|
+ |
| S. tenera Greg. v. nervosa A. S. |
+ |
|
|
| Synedra acus Kuetz. |
+ |
+ |
+ |
| S. acus Kuetz. v. acula (Kuetz.) V. H. |
+ |
|
|
| S. ulna (Nitz.) Ehr. |
+ |
|
|
| S. ulna (Nitz.) Ehr. v. danica Kuetz. Grun. |
+ |
+ |
+ |