4. RESULTS AND DISCUSSION
4.1 Kathalekan- Glimpses of Ecological History
Western Ghats, one of the richest centers of biodiversity on the earth is also one of the most threatened regions due to high degree of anthropogenic influents. Any plans for conservation, utilization and management of the bio-recourses here have to take in the account the historical background of the region with respect to human impact. According to Dargavel (1988), the history of forests in tropical Asia raises the great question of development and environment in their most acute and urgent form. These ecologically richest forests are also home for forest dwelling communities; there are rapidly growing population, transmigration, forest clearing, destructive logging and environmental degradation. This necessitates appropriate forest management strategies to ensure sustainability of these ecologically fragile forests.
4.1.1 Agricultural colonization and alteration of primary forest: The early human caused vegetation change in the Western Ghats probably date back to a period of agri-pastoralism during the Neolithic or New stone Age in the Deccan (5000-3000 years BP). Forests were systematically cleared during the Megalithic period (3000-2000 years BP) when the west coast was rather intensely settled. Iron implements introduced during this period would help forest clearance, especially for shifting cultivation. Hallur, on the bank of Thungabhadra River, in the Haveri district close to Siddapur-Sirsi taluk of Uttara Kannada witnessed the first iron implement in South India (Chandran, 1997). Palynological study by Caratini et al. (2001) shows that there was a decline in pollen from forest tree species and increase in savanna pollen during the middle of fourth millennium BP. Although this was argued to be the result of climatic charge towards aridity in the Indian sub-continent, Chandran (1997) attributes the increase in savanna plants to the introduction of agriculture in the forest area after slashing and burning of vegetation. In fact Caratini’s Palynological study showed the occurrence of Dipterocarpus as north as the Karwar. The very presence in good number of this hygrophilous tree species in the kan sacred groves such as Karikan and Kathalekan in Honnavar and Siddapur taluk respectively to this day, clearly indicates that it could have been clearance of forest by human and not climatic change that increased savanna species such as grasses. Not only Dipterocarpus even Myristica swamp are associated with some of the sacred kans.
4.1.2 Shifting cultivation in the Western Ghats: Shifting cultivation was one of the earliest forms of agriculture in the Western Ghats. The shifting cultivators seem to have normally occupied a zone below 1000 m (Bourdillon, 1893), perhaps avoiding the colder and wind-swept heights. Thin human population and long fallows often permitted the return of the forest (Cleghorn, 1891 and Bourdillon, 1893). Shifting cultivation in the humid hill tops and slopes had its own implication. The exposed soil of tropical regions is fragile, being prone to severe erosion and loss of fertility. Shifting cultivation was a major form of land use in the Western Ghats including the study area. Francis Buchanan made a study on the traditional land use in the Western Ghats in 1801 by touring in to Malabar and Canara region. At Gokarna in Uttara Kannada he found records of 1450s relating to tax on shifting cultivation. Coastal hills of Uttara Kannada were formed to terraces for cultivation of gingelly and black gram. In the interior hills in the first season after burning the woods, were sown ragi (Eleucine coracana), red gram (Cajanus cajan), and castor (Ricinus communis). Next year on the same ground was raised a crop of shammy (Panicum sumatrense) (Buchanan, 1870). The tribals of Travancore hills planted rice, cowpea, gingelly, tapioca, yams, cucurbits, brinjal, chilly and plantain with cleared forest patches (Bourdillon, 1893). The shifting cultivation evolved as a form of land use to circumvent major problems of tropical agriculture like soil erosion, low nutrient status and pest pressures. Slashing and burning through the millennia could be one of the major reasons for the decline of primary forest and it leading to the formation of large tracts of secondary forests and savanna. ‘Kumri’, ‘hakkal’, etc were the vernacular names for such cultivation practices. By the close of 19th century the British rulers banned such cultivation and since then secondary deciduous forest started turning evergreen as role of fire as an ecological factor diminished considerably (Chandran, 1993).
4.1.3 Conservation by pre-colonial farmers: A variety of regulatory measures has been an integral part of utilization of biological resources by most human societies. Such measures have ranged over quotas as to how much material is harvested, restriction of harvests to certain season or life history stages, restriction on harvesting techniques employed, to complete protection to certain biological communities in areas set aside as refugia (Gadgil and Berkes, 1991). The shifting cultivation on the hill tops and slopes by clearance of the primary forest caused inevitable loss of species rich evergreen forest. It also meant impoverishment of the resource base which would have prompted these early peasants to leave untouched patches of evergreen forests in the vicinity of their settlement. These distinct forest block locally known as kans, are present in hundreds even today in Uttara Kannada and neighboring districts (Chandran and Gadgil, 1993). Regionally these sacred kans are known as Devarakadus (Coorg), Kavu (Kerala), etc. These kan forests were the early centers of folk worship. Activities like extraction of forest products were restricted by local taboos. In some area these taboos continue to this day and the groves represent relics of the erstwhile forest and Kan centered culture continues although in a diminished form.
4.1.4 Significance of kan forest in traditional land use management: The kans probably date back to an ancient era of nature worship by primitive societies and therefore are of high biological significance. In Uttara Kannada of pre British period, kans existed with the ordinary supply forests and cultivation fallows creating greater landscape heterogeneity favourable also for richness of wildlife including corridors, edges and various land features which support the wildlife richness. The traditional land use and resource management systems underwent radical changes in the course of 19th century with the state claiming the common property resource like forests grazing and even shifting cultivation lands (Chandran, 1993). The goods and services provided by kan forests in the traditional land use system were many. The kans acted as local centers of biodiversity, and as a green belt preventing fire, providing good water shed value and also various non timber products of subsistence value. Pepper that was exported once in large scale from Uttara Kannada was mainly a product of kans.
4.1.4.1 Kans- a local center of biodiversity: Kans have been protected through generation on account of sacredness and they functioned literally safety forests. Kanspromoted good biodiversity because they were preserved pieces of primeval nature. Their forming a mosaic with the adjoining forest, and other landscape elements created heterogeneity. Landscape ecology stresses the high positive correlation between landscape heterogeneity and biodiversity. Such a landscape contains an extensive amount of edge habitat with edge species and also animals that use more than one ecosystem in close proximity, say for breeding, feeding and resting (Forman and Gordon, 1986). Spatial extent of Kans ranging from part of a hectare to few hundred hectares and protected from time immemorial, may be considered as the best samples of climax forest of the region. Even the smaller sacred groves often harbour some old and magnificent specimens of the trees and climbers (Chandran and Gadgil, 1993; Gadgil and Vartak (1975). In the kan forest there exists a favourable climatic condition without any considerable changes. This matters for the rich assemblage of flora and fauna in such forest. Many of the individuals are endemic and rare in the area. Wingate (1888), the forest settlement officer of Uttara Kannada, noted that the kans were of “great economic and climatic importance. They favour the existence of springs, and perennial streams, and generally indicate the proximity of valuable spice gardens, which derive from them both shade and moisture”. Among the rare evergreen trees found in kan forests were Dipterocarpus indicus, Mesua ferrea, Vateria indica, Palaquium ellipticum etc. The kan forest is found to be a seed bank for numerous species, ideally helping forest regeneration. These sacred groves also often serve to this day as the last refugia for many arboreal birds and mammals and no doubt for other forest loving animals as well. (Chandran and Gadgil, 1993).
4.1.4.2 Watershed value: Sacred groves act as microwater shed in local areas. The sacred groves are always associated with a fresh water ecosystem used to trap and collect the rain water for the local water supply. If they are destroyed or disrupted the water cycle would be interrupted. Heavy canopy and undergrowth along with the litter of the sacred groves is helpful in reducing the impact of rain drops directly falling on the earth surface (Pushpangadan et al 1998). This reduces soil erosion and helps in recharging of ground water table by enhancing filtration. The Government of Bombay (1923) highlighted the water shed value of the kans of Uttara Kannada as “Throughout the area, both in Sirsi and Siddapur, there are few tanks and few deep wells and the people depend much on springs…In the midst of heavy evergreen forest during the dry season the flow of water from any spring continues, even though no rain water not fallen for several months…”
4.1.4.3 Fire proof system: The evergreen forest patches of sacred groves usually form a green fire proof barrier. If there is any incidence of fire in the forest, this evergreen forest act as fire proof system and halts the spread of fire into the other forest patches, human settlement and cultivation.
4.1.4.4 Subsistence: Kan forests once supplemented the major livelihood of local communities who depended on the non wood products for subsistence and sale. Kans harboured till today have plants which are economically and medicinally important. Their produce included edible fruit from trees like Mangifera indica, Artocarpus heterophyllus, A. hirsutus, Garcinia indica, Syzigium cumini, etc. Kans were once important centers of black pepper (Piper nigrum) that was major export commodity. Myristica malabarica, Murraya koenigii, Garcinia cambogea, and G. indica, and honey were important products that people harvested from kans. Toddy tapping from the palm Caryota urens was a major occupation of ‘Kan Divars’. Many medicinal plants and resources such as Pandanus Ochlandra, Calamus etc. widely used for mat weaving were found in the kans.
4.1.5 British colonization and Forest reservation: The British domination of the Western Ghats began early in 19th century. The traditional management of the forest didn’t impress them. Madras Government banned the shifting cultivation in 1860 and in Uttara Kannada the Govt of Bombay banned it late in the 19th century. Only little land area was allotted to the habitual shifting cultivators like Kunbis and Marattis in few places (Chandran, 1997). In the early stage of British forestry, concentration was mainly on deciduous timbers like teak. The ban on shifting cultivation minimized fire as an ecological factor. This promoted the recovery of evergreens and regeneration of teak was reduced under the evergreens. Due to the increased demand for teak wood, the foresters started teak monoculture in the Western Ghats by massive clearance of the evergreens causing intensive vegetational changes in the Western Ghats. (Dixit, 1985; Gadgil and Chandran, 1989). The State domination over the forest resulted in the local community losing the hold over their sacred kans. Under the Indian Forest Act 1878, most kanscame under state control. The people were prohibited from collecting forest products except some dry fuel wood as in eastern part of Sirsi and Siddapur (Govt. of Bombay, 1923). In the higher altitude forest the state policy was to promote commercial cultivations like, coffee, tea, wattle and Eucalyptus. The spurt in commercialization of natural resources and commodity production also attracted an exodus of migrant laborers with overall serious ecological consequence on the biota (Prabhakar, 1994). The forest reservation resulted in many of the kanslosing their special identity and got merged with the secondary forests.
4.1.6 Industrial exploitation of the forest: In the early 1940’s, during the Second World War opportunistic felling of both deciduous and evergreen timbers had happened due to the relaxation of the exploitable girth of many plants (Gadgil and Chandran, 1989). The expansion of railway net work and escalating demand for industrial timber were too taxing on Western Ghats forest. During the 1940’s Dipterocarpus indicus, found sheltered in some of the kans, was supplied to the railways and plywood company (Shanmukhappa, 1977). A working plan for the forests of Sirsi and Siddapur brought under it, 73 kans also totaling an area of over 4000 hectares, for felling of industrial timber (Shanmukhappa, 1966). Gadgil and Chandran (1989) who documented the environmental impact of forest based industries in Uttara Kannada point out the industrial sector became more influential and dominant during 1950’s and selective felling of trees in the evergreen forest reached peak in 1960’s to 1980’s period. The timber exploitation in the evergreen forest severely affects the equilibrium of the forest ecosystem. The selective felling of large trees created many canopy gaps in the forest with tumultuous effect on the evergreen forest ecosystem as a whole, adversely affecting the faunal species also. Logging affect the ground layer vegetation also and promoting weeds and heliophilous commonplace pioneer species. Felling of trees having large canopy cover always created vast opened area. Tolerant deciduous species are more promoted in such open area as the seeds of evergreen species are more sensitive to the sunlight and could not survive in such condition in the intense light. In many cases, large scale logging resulted in the deterioration of microclimatic conditions and also resulted in soil erosion.
4.1.7 Recovery of the forest: Logging in the evergreen forest was banned in 1986 (Gadgil and Guha, 1992), resulting in the reduction of disturbances in the forest, favouring the recovery of forests in the logged area. During the current study, it was noticed that due to the stoppage of industrial logging (about two decades past), the forest are on the road to recovery.
4.1.8 Kathlekan - a sacred grove: Kathalekan is considered to be a kanforest in the central Western Ghats. It is a patch of evergreen forest blessed with rich diversity of flora and fauna and good number of them are endemic to Western Ghats. A preliminary survey of the region reveals some glimpses of the ecological history of the forest. The landscape element of the forest reflects the passage of forest history. The elements documented are dense evergreen forest, grass land, streams and swamps, rocky formation, agricultural and human settlement area, etc. There are many canopy gaps here, which are yet to close fully. On the whole large emergent trees are lesser in number, however there is a profusion of young growth in the forest floor. Young evergreen tree of different species are found competing with each other to come up in the canopy gaps. Savannas on some of the hill slopes (is grid nos.5, 7, 8, and 9) were under shifting cultivation (kumri) and one bears the epithet ‘Seven year kumri (meaning seven year shifting cultivation cycle). Forest from the valleys was found advancing towards summit of these grassland covered hills in Kathlekan and its adjoining areas. Pioneer colonising shrub and trees was found as the forerunners of vegetational re-establishment followed by late successional species and climax species such as Dipterocarpus indicus and Syzygium gardneri. These constitute a good part of Kathalekan, especially in the valleys. Kathalekan was a sacred grove (Kan) because of the following reasons:
- The presence of less modified forest elements such as Myristica swamps and there adjoining areas rich in southern endemic climax species such as Dipterocarpus indicus, Palaquium ellipticum, Semicarpus kathlekanensis, Pinanga dicksonii, Mesua ferrea, Cyathea nilagirica (tree fern), etc.
- Part of the swamps near to a woodland deity (in grid no 8) is still being worshipped by few families form adjoining villages.
- In spite of the Bangalore road passing through almost the heart of Kathlekan the roadside forest itself has the look of primeval forest, true to its name ‘Kathale-kan’ that means ‘dark sacred grove’.
4.2 Species Composition of the Kan Forest
Species composition is regarded as an identity of every community. Deciphering of the species composition pertaining to a particular community is the crucial procedure in any ecological inventorying. In this endeavor species composition of a kan forest, Kathalekan of 2.25 km2 area was studied. Nine transects with 45 quadrats covering an area of 1.8 ha were laid, which provide a good profile of the forest. This profile unveils the species composition of Kathlekan and ecosystem value specially as a kan forest, a refugia of vast variety of species, mainly Western Ghat endemics, several of them being RET species. The forest may be considered as a relic of the primary forest of the Western Ghats. Sampling was done in both tree layer and ground layer (shrub/herb layer), as mentioned in the methodology. Number of species in tree, shrubs and herb layers encountered during the field survey are listed in Table 4.2.1. Altogether 185 species, 109 trees, 39 shrubs, 12 herbs and 25 of climbers were documented from transects. In the 104 species of tree layer species having GBH ≥30 cm, were 99 species of trees 5 of lianas. Saplings of 80 species of trees dominated the shrub layer. Shrubs constituted only 34 species and 3 were tall herbs. The 17 species of climbers included young lianas and herbaceous climbers. In the study area, out of 100 sp in the herb layer only 11 were herbs. Poverty of herbs in the ground layer in rain forests have been found generally, which can be attributed to the presence of good canopy casting its shade on to forest floor.
Table 4.2.1: No. of species in tree and ground layers of Kathlekan.
| Layer |
Sampled area(ha) |
Total species |
Tree species |
Shrub species |
Herb species |
Climbers / Liana |
| Tree layer |
1.8 |
104 |
99 |
Nil |
Nil |
5 |
| Shrub layer* |
0.225 |
134 |
80 |
34 |
3 |
17 |
| Herb layer** |
0.009 |
100 |
63 |
20 |
11 |
6 |
* includes shrubs and saplings of trees < 30 cm girth and > 1m height, ** Includes juveniles of trees. Shrubs and climbers < 1 m height.
The entire study area was dominated by evergreen tree species with good canopy covering that checks light filtration in to the forest floor substantially. Understandably various microclimatic conditions are changed also favouring survival of mostly climax species. It was only in disturbed areas, canopy gaps and forest edges herbs and shrubs are able to proliferate. The occurrence of many tree species in all the vertical layers indicates that the forest has attained climax state. Examples of such climax tree species are Dipterocarpus indicus, Hopea ponga, Holigarna grahamii, Syzygium Gardneri, Knema attenuata, Palaquium ellipticum etc.
4.2.1 Sampling effort: Species area curve of total sampled area of 1.8 ha (by pooling together nine transect cum quadrats each form a grid of 500×500m) given in Figure 4.2.1. The curve reaches near saturation with the addition of 7th grid sample.
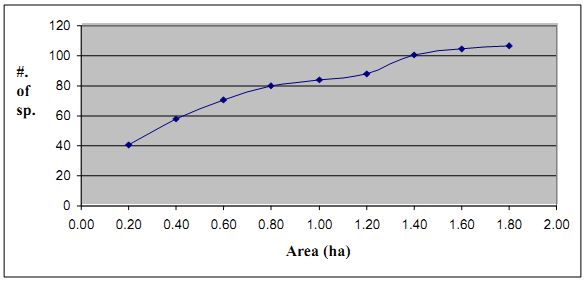
Figure 4.2.1: Species area curve for the trees in 9 grids of Kathalekan
It has been argued that the productivity of the system and structural complexity or heterogeneity determine species richness in the community (Brown 1981). However neither ecosystem productivity nor structural complexities seem sufficient on their own to explain the observed pattern of species richness (Putman, 1994). Slobodkin and Sanders (1969) opined that species richness of any community is a function of severity, variability and predictability of the environment in which it develops. Therefore, diversity tends to increase as the environment becomes more favorable and more predictable (Putman 1994). In the sacred groves it seems that the favorable climatic conditions of the area and protection over a long period of time have played a major role in making these forest patches highly complex and species-rich (Upadhaaya, et al, 2003). Obviously favourable conditions such as good rainfall, undisturbed soil with much moisture content and an excellent litter cover, have also benefited Kathalekan ecosystem.
4.2.2 Familywise species distribution: Table 4.2.2 lists family wise number of species found in the sampled area. The 185 species found during sampling (excluding fully unidentified species) belong to 51 families and about 135 genera of flowering plants, (Table 4.2.2)
Table 4.2.2: Family wise species distribution
| Sl.No |
Family |
No. of genera |
No. of species |
| 1 |
Euphorbiaceae |
16 |
21 |
| 2 |
Lauraceae |
8 |
12 |
| 3 |
Rubiaceae |
8 |
12 |
| 4 |
Myrtaceae |
1 |
7 |
| 5 |
Annonaceae |
5 |
6 |
| 6 |
Celastraceae |
5 |
6 |
| 7 |
Rutaceae |
6 |
6 |
| 8 |
Clusiaceae |
3 |
5 |
| 9 |
Ebenaceae |
1 |
5 |
| 10 |
Flacourtiaceae |
3 |
5 |
| 11 |
Myristicaceae |
3 |
5 |
| 12 |
Zingiberaceae |
4 |
5 |
| 13 |
Anacardiaceae |
3 |
4 |
| 14 |
Arecaceae |
4 |
5 |
| 15 |
Meliaceae |
2 |
4 |
| 16 |
Rhamnaceae |
4 |
4 |
| 17 |
Acanthaceae |
3 |
3 |
| 18 |
Moraceae |
2 |
3 |
| 19 |
Poaceae |
3 |
3 |
| 20 |
Smilacaceae |
2 |
3 |
| 21 |
Araceae |
2 |
2 |
| 22 |
Combretaceae |
2 |
2 |
| 23 |
Dioscoriaceae |
2 |
2 |
| 24 |
Dipterocarpaceae |
2 |
2 |
| 25 |
Icacinaceae |
2 |
2 |
| 26 |
Loganaceae |
2 |
2 |
| 27 |
Oleaceae |
2 |
2 |
| 28 |
Sapindaceae |
2 |
2 |
| 29 |
Sapotaceae |
2 |
2 |
| 30 |
Verbenaceae |
2 |
2 |
| 31 |
Agavaceae |
1 |
1 |
| 32 |
Ancistrocladaceae |
1 |
1 |
| 33 |
Apocynaceae |
1 |
1 |
| 34 |
Asclepiadaceae |
1 |
1 |
| 35 |
Burseraceae |
1 |
1 |
| 36 |
Capparaceae |
1 |
1 |
| 37 |
Connaraceae |
1 |
1 |
| 38 |
Cornaceae |
1 |
1 |
| 39 |
Cyperaceae |
1 |
1 |
| 40 |
Datiscaceae |
1 |
1 |
| 41 |
Dichapetalaceae |
1 |
1 |
| 42 |
Elaeocarpaceae |
1 |
1 |
| 43 |
Fabaceae |
1 |
1 |
| 44 |
Faboidae |
1 |
1 |
| 45 |
Gnetaceae |
1 |
1 |
| 46 |
Lecythidaceae |
1 |
1 |
| 47 |
Leeaceae |
1 |
1 |
| 48 |
Lythraceae |
1 |
1 |
| 49 |
Malvaceae |
1 |
1 |
| 50 |
Melastomaceae |
1 |
1 |
| 51 |
Menispermaceae |
1 |
1 |
| 52 |
Pandanaceae |
1 |
1 |
| 53 |
Piperaceae |
1 |
1 |
| 54 |
Rhizophoraceae |
1 |
1 |
| 55 |
Sterculiaceae |
1 |
1 |
| 56 |
Symplocaceae |
1 |
1 |
| 57 |
Tiliaceae |
1 |
1 |
| 58 |
Ulmaceae |
1 |
1 |
| 59 |
Vitaceae |
1 |
1 |
Euphorbiaceae dominated with 16 genera and 21 species having representation among trees, shrubs and herbs. It was followed by Rubiaceae and Lauraceae each having 8 genera and 12 species each. Rutaceae (6 genera, 6 species), Celastraceae and Annonaceae (5 genera and 6 species), Zingiberaceae and Arecaceae (4 genera and 5 species), Rhamnaceae (4 genera and 4 species each) are the other notable families enclosing more individuals. There are 28 families represented only by single species. However the genus Syzygium of Myrtaceae were found with 7 species.
4.2.3 Tree composition: As mentioned earlier within the study area 109 tree species (including 10 unidentified species) were recorded. The tree species recorded here belonged to 34 families with 71 genera and 99 species (about 10 unidentified excluded) .Among the families more number of genera was recorded in Euphorbiaceae, with 10 genera followed by Lauraceae (8). Anacardiaceae, Annonaceae, Celastraceae, Clusiaceae, Flacourtiaceae, Myristicaceae, and Rubiaceae having (3 each). Euphorbiaceae was leading in species no. too and Lauraceae with (12) followed by Myrtaceae (7), Ebenaceae, Myristicaceae and Clusiaceae (5 each), Anacardiaceae, Celastraceae, Meliaceae had 4 species each and some families were represented by only one species.
Among them species like Hopea ponga, Dipterocarpus indicus, Holigarna grahamii, Knema attenuata, Syzigium sp, Aglaia roxburghiana have more frequent distribution in almost every grid studied, followed by Olea dioica, Syzygium gardneri Dimocarpus longan, Calophyllum tomentosum, Garcinia talbotii, Palaquium ellipticum, Actinodaphne hookeri, Diospyros candolleana, Litsea sp. etc. Regarding the no. of individuals per species Hopea ponga is coming in first place with 208 individuals in the sampled area, followed by Dipterocarpus indicus (120), Olea dioica (98), Knema attenuata (87), Holigarna grahamii (67), Aglaia roxburghiana (52), Syzygium gardneri (45), Dimocarpus longan (43). One of the striking features noticed here is the gregarious occurrence of Dipterocarpus indicus, a climax species. This indicates the primary nature of the forest. In the present study in Kathalekan, 120 Dipterocarpus trees were recorded from the totaled sampled area of 1.8 ha. Kathalekan is the northern limit of occurrence of this species in addition to Karikan in to the adjoining Honnavar taluk. In Karikan, a sacred forest, close to the shrine of the forest deity the same species occurs in abundance almost like a primeval forest. Chandran, (1993) recorded in a one ha plot of Karikan, 151 trees, of which 18 were reported to be 200 years old.
4.2.4 Shrub composition: The shrubs belonged to 39 species and 22 families. Euphorbiaceae dominated here too with 5 genera and 6 species. It was followed by Rubiacaeae with 3 genera and 7 species, then Rutaceae, Rhamnaceae, Celastraceae and Icacinaceae having 2 genera and 2 species each and others having one each. Dichapetalum gelonioides with 160 individuals was the most populous shrub and was found in all the grids. The gregarious occurrence Pinanga dicksonii a slender endemic under growth palm is one of the noticeable features of Kathlekan shrub layer. The abundance of this species in most of the grids indicates rich moisture content in the soil. Psychotria flavida, Memecylon terminale, Glycosmis pentaphylla, etc. are the other species having more abundance. The shrubs like Nothapodytes foetida, Microtopis wallichiana, Hibiscus furcatus, etc were represented by only one individual each in the sampled area.
4.2.5 Herb composition: Dense evergreen forests are known for poverty of herbs. This study vindicates same scenario. Only 12 species of herbs were recorded from the forest floor. These belonged to 7 families and 10 genera. Among the families Zingiberaceae dominated with 3 genera and 4 species followed by Acanthaceae with 2 genera and 2 species. Cyperaceae, Rubiacaeae, Araceae Poaceae and Agavaceae, had single genera and single species only. Alpinia malaccensis, Boesenbergia pulcherrima, Cyrtococcum oxyphyllum, Dracaena terniflora, Justicia simplex, Lagenandra ovata, Ophiorrhiza hirsutula, Rungia pectinata were among the species. However forest edges receiving more light had greater number of herbs. In the short duration of this study the edge herbs and seasonal herbs could not be recorded. No special studies were also made of epiphytes.
4.2.6 Lianas and herbaceous climbers: Lianas and climbers are the plants which rooted in ground but for climbing they need a mechanical support of neighboring tree for climbing. Woody climbers are generally described as lianas. They have an important role in the scheming of the forest structure. Except few (5) unidentified species, the lianas and climbers present in the study area is represented by total of 20 species belong to 20 families including Annonaceae, Menispermaceae, Rutaceae, Ancistrocladaceae, Gnetaceae, Loganaceae, Rhamnaceae, Piperaceae, Arecaceae etc, among them Rutaceae is predominating with 2 genera and 2 species followed by Smilacaceae and Arecaceae with one genera and two species and all other families having one genera and one species each., Wild pepper (Piper spp), Pothos are commonly associated with forest trees. They act as indicator species because they are very sensitive to canopy opening. Pepper was one of the important species obtained ones mainly from the Kans of the Uttara Kannada. Wild pepper was taken care of in the kans by local villagers during pre British period (Chandran and Gadgil 1993). Calamus thwaitesii and Calamus sp. also have good number of individuals. Although this spiny climber indicates the disturbances in the forest by human interferences or cattle grazing (Pascal, 1988), moderate presence is characteristic of the undisturbed evergreen. Ancistrocladus heyneanus, Ventilago madraspatana, Combretum latifolium, Smilax zeylanica, Strychnos sp. etc are other important climbers noticed. The climbers occur abundantly in disturbed area and especially in canopy openings.
4.2.7 Species heterogeneity (diversity, dominance and evenness) among trees: Species heterogeneity gives a measure of community organization related to how the relative abundance varies among the different species in the community. This is estimated on the basis of diversity, dominance and evenness of the species in a community. For the diversity measurement most commonly used Shannon index (H’) is adopted and Simpson index is used for dominance and for evenness Pielou index is also calculated. High value of H’ indicates a large number of species with similar abundances, a low value indicates domination by few species. Lower value of Simpson’s dominance indicates the higher diversity and high evenness in the distribution. In these instances the Pielou index is higher. The grid wise result is presented in the Table 4.2.3 along with pooled result for the entire study area.
Table 4.2.3: Diversity, dominance and evenness indices for trees
| Sl.No |
Grid |
Total individual |
Total species |
Shannon diversity |
Simpson’s Dominance |
Pielou evenness |
| 1 |
GI |
143 |
41 |
3.39 |
0.043 |
0.91 |
| 2 |
G2 |
173 |
39 |
3.18 |
0.060 |
0.87 |
| 3 |
G3 |
104 |
37 |
3.42 |
0.038 |
0.95 |
| 4 |
G4 |
129 |
38 |
3.12 |
0.069 |
0.86 |
| 5 |
G5 |
158 |
39 |
3.16 |
0.065 |
0.86 |
| 6 |
G6 |
165 |
23 |
2.39 |
0.140 |
0.76 |
| 7 |
G7 |
141 |
44 |
3.36 |
0.05 |
0.89 |
| 8 |
G8 |
113 |
34 |
2.91 |
0.10 |
0.82 |
| 9 |
G9 |
186 |
18 |
1.77 |
0.318 |
0.61 |
| 10 |
Pooled |
1312 |
104 |
3.58 |
0.05 |
0.77 |
4.7.1 Species diversity for trees: The Shannon index of diversity for the area was 3.58, which is fairly good for the forest. The result indicates the existence of good diversity in the forest. These results are found to be in accordance with the diversity of various other Kan forests in the Sirsi Forest division, of Uttara Kannada, (Devar,2008).The diversity in the sacred groves of Kerala (3.1-3.6) estimated by Rajendraprasad (1995) also matches with that of Kathlekan.
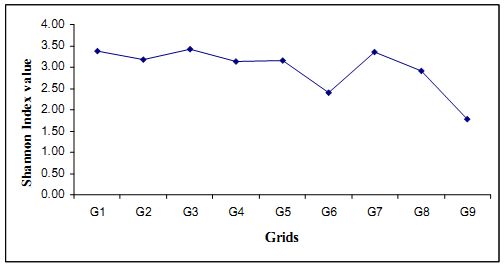
Fig 4.2.2: Shannon index for the 9 grids
From the Fig (4.2.2) it is clear that diversity is varying from the value of 1.77 to 3.42 across transects. The highest diversity is present in Grid 3 and the Grid 9 having lower diversity followed by Grid 6. In other grids diversity is more or less comparable. This can explained with Dominance and Evenness of species.
4.7.2 Species dominance and evenness: Dominance and diversity are inversely proportional to each other. It points out the consistency of a species in a community. For both these parameters the values lie between 0-1. In general, Simpson Diversity estimated in Kathlekan is 0.05 with evenness (Pielou index) value of 0.77. This result reflects that species are distributed uniformly with very least dominance. This matters for the persistence of high diversity in the forest. Figure 4.2.3 illustrates a grid wise analysis of these parameters.
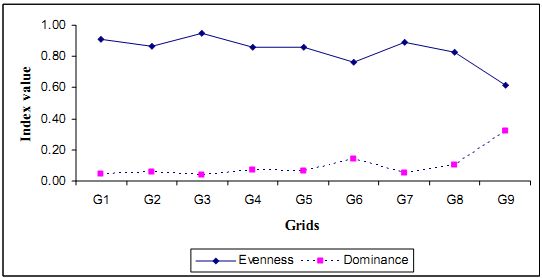
Fig. 4.2.3: Evenness and Dominance in the tree community of Kathlekan
Figure 4.2.3 depicts the dominance and evenness of the vegetation. Grid 3 is found to be more diverse and species are distributed more evenly with less dominance. This part having rocky formation, leading to the deep gorge of Sharavathi River, has micro-heterogeneity and therefore good number of species and more evenness Compared to other area this is not much explored. The evenness sharply declines in the grid 6 (less species richness - 23 species) due to domination of few species. Even though there is a higher abundance of species, grid 9 has least diversity. This grid also merges with the deep gorge of a river. Gregarious occurrence of Hopea ponga, which is more adapted to the edges having good mechanical resistance against wind because of its fibrous wood. In effect, the flourishing of such species in these areas provides shelter to more sensitive species. Olea dioica follows closely in number having more abundance here.
4.3 Landscape Elements and Vegetation
Documenting the flora of a region provides the complete range of plant species there. The vegetation consists of assemblage of plant species forming a green mantle, an almost continuous and conspicuous plant cover over the land surface with the deserts and rocky surfaces being exceptions. The units of vegetation are the individual plant species composition, structure, physiognomy, spatial patterns, temporal patterns are the variable properties of the vegetation. (Chandran, 1993). Vegetation structure for ecological purposes is considered at a number of levels. Physiognomy (for example height), floristic (species richness and composition), stratification (layering of different types of plants on height characteristics), life forms (trees, shrubs, etc) distribution and abundance of species are some of the structural variables usually considered (Puri et al, 1983; Causton, 1988; Mac Nally.1989).
4.3.1 Landscape elements: A landscape is a panoramic view that one can get form a high place and usually is a composite of various units called elements. These elements of landscape may incorporate water bodies as well. Landscape ecology stresses the high correlation between landscape heterogeneity and biodiversity (Forman and Gordon, 1986). Figure 3.2 portrays the elements that compose the landscape of Kathalekan, which include:
- Evergreen forest: Most of Kathalekan is dominated by evergreen species.
- Streams and swamps: The landscape is traversed by a network of streams. Some of the streams are perennial (as in grids 1, 2, 4, 5, and 7). Seasonal streams are found in all grids. Streams that run through flat valleys turn sluggish, and swampy. Such swamps are found in grids 2, 5, 7 and 8. The streams are overtopped by the evergreen forests itself. The stream beds and edges have species like Homonoia riparia, Phyllanthus spp., Blachia denudata, Ochlandra spp., Pandanus canarana, Pinanga dicksonii, Elaeocarpus tuberculatus, Calamus spp., Arenga wightii, etc. Thallus like growth of Podostemonaceae is found firmly adhering stream rocks. The swamps dominated specially by the members of Myristicaceae are described separately in chapter 4.5. Apparently some of the extensive areas under swamps have been reclaimed for agriculture in grids 4 and 8. Bunding of streams and diversion of their ways to agricultural area are threatening factors for characteristic swamp species
- Savanna and rocky formations: Savannas in Indian subcontinent are believed to be formations derived from woodland ecosystem through variety of human interventions (Gadgil and Meher-Homji, 1986). Savannization in Kathalekan was apparently due to kumri cultivation (a form of shifting cultivation in the hill tops and slopes) in the past by agri-pastoralists. Forests were slashed and burned in patches and abandoned after cultivating for one or two years. After a fallow period of varied no. of years the Kumri cycle was repeated. The British stopped this cultivation on Uttara Kannada by close of 19th century giving a chance for forest recovery (Chandran, 2003). In Kathalekan savanna in grid 2 and 4 even now has known to the locals as 7 years Kumri area. The slashing and burning wipe out the soil fertility and drastically change the microclimatic condition. Repeated burning of the forest can create grasslands. A vegetational study of savanna in Kathalekan is given in this chapter.
- Agriculture / horticulture: Valleys with perennial water sources have always been targeted in the Western Ghats for cultivation. At present in Kathalekan two such cultivation areas have been located. The large one (Grid 4) has an area of about 2 ha. and the smaller one in grid 8, has an area of 1 ha. The crops grown are mainly arecanut, banana, pepper, vegetable, paddy and some fruit trees.
- Road: The Honnavar – Bangalore road passes through Grids 1, 4 and 8. The road has increased the forest edge and paved the way for many light loving edge species such as Carvia callosa, Strobilanthus sp., Callicarpa, Lea indica, Ficus sp., Glochidion sp., Actinodaphne hookeri, Calycopteris floribounda, Calamus sp., Combretum etc,. Many grasses and herbs are also characteristic of the roadside vegetatation. The weed Chromolaena odorata is found to grow prolifically in many places.
4.3.1 Evergreenness of the Forest: The forest of Kathalekan is dominated by evergreen trees. Almost 97.3% of total trees recorded are evergreens and hence this forest is considered as high evergreen forest. This forest was maintained as a kanforest, since time immemorial. The word ‘kan’ appended to the forest (Kathalekan means ‘dark forest’ in Kannada). In later times the forest came under the State forest department and lost its sacred grove status. Nevertheless some of the local people still worship the deity of the kan in a portion of a Myristica swamp. Despite selection felling of trees during 1940-1985 periods the kancontinues to be of heavy evergreen in composition. Figure 4.3.1 shows that that the grid 3 has lesser percentage of evergreen trees (91%) compared to other grids, due to the presence of deciduous trees like Terminalia paniculata, Tetrameles nudiflora, Careya arborea etc. Nevertheless, their number is negligible in the entire forest. Deciduous species include Terminalia paniculata, Tetrameles nudiflora, Careya arborea, Lagerstroemia microcarpa etc.
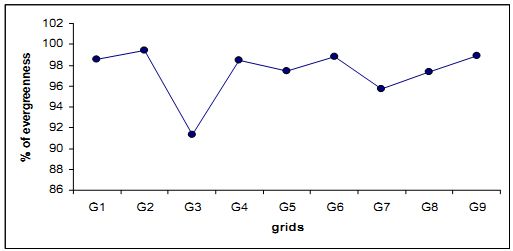
Fig. 4.3.1: Percentage of evergreenness in different grids
4.3.2 Structural features of the forest: Mainly trees determine the architecture and microclimatic conditions of the forest and hence changes in tree community may strongly affect the other ecological processes (Didham et al, 1996). It is very hard to get a natural vertical stratification of the forest patch. The human interferences that happened in the past in the form of selection felling during the period of 1940-1985 changed the genuine nature of the forest and its vertical stratification. Tallest trees having huge girth were extracted causing decline of emergent trees and creating large canopy gaps. This is the reason for the lower distribution of trees in the height more than 30 m. Emergent species having good timber value like Dipterocarpus indicus, Calophyllum tomentosum, Palaquium ellipticum, Artocarpus hirsuta, Mesua ferrea etc. had suffered a lot during especially post independence period (Gadgil and Chandran, 1989). The logging in the evergreen forest of India was banned in 1986 (Gadgil and Guha, 1992) and hence these evergreen forests are passing through a recovery phase. It is observed that those logged trees have reasonable regeneration now. Shifting cultivation in the forest also played a major role in the deterioration of the forest structure. Cutting and burning for the cultivation purpose wiped out much of the original vegetation mainly in the elevated part of the forest. Repeated burning reduces soil fertility increases soil compactness and erosion. This makes forest recovery difficult mainly in savanna lands. The savannized lands are fit for few fire resistant deciduous trees only.
In order to depict vertical stratification of the forest, the whole vegetation is assigned to different strata based on height classes. Each of the species within the community has large measure of its structural and functional individualism and has more or less different ecological amplitude (Singh and Joshi, 1979). Based on the height class distribution of tree species, three stratas have been identified in different range of height among the tree species. The ground layer vegetation constituted by shrubs, herbs and juvenile form of tree species, form the base of the stratification.
Top strata in the forest is composed of emergent trees having a height of 20 m and above. The second strata is constituted by medium height (10-20 m). Trees having the height below 10 m are included in the third strata and a very few trees have height less than 5m.
The study of Pascal (1988) in the kan patches in the Karnataka distinguished two types of formations i.e., Diospyros spp -Dysoxylum malabaricum-Persea macrantha type which corresponds to the Sorab region and another one the Dipterocarpus indicus-Persea macrantha type of the hill ranges situated to the west of Shimoga which is considered as a Kan forest. Kathalekan it found to be Hopea ponga-Dipterocarpus indicus-Holigarna grahamii type of formation. These species are more frequently distributed and having high importance value index (Table 4.3.1). The occurrence of Dipterocarpus indicus shows the primeval nature of the forest, as this species is absent in most of the secondary forests in Uttara Kannada and northern forests (Chandran, 1997). Its occurrence in Uttara Kannada is mainly restricted to the primary evergreen forest, mainly the kans (Kathalekan and Karikan ( in Honnavar). The spread of Hopea ponga in the neighboring district of Dakshina Kannada in the last few years is striking. This evergreen species which may exceed 30 m under favourable conditions occupies regions disturbed by recent exploitations and there was a noticeable increase in its area (Pascal, 1988). The three species mentioned in the type of formation are also included in RET (IUCN) category. This draws greater attention to the forest from conservation point of view. Kathalekan is also home to Myristica swamp and newly discovered critically endangered tree Semecarpus kathalekanensis (Dasappa and Swaminath, 2000).
- Strata 1-Emergent trees: The emergent trees form uppermost layer in a forest and in Kathalekan most of them have adequate regeneration highlighting climax status. Major emergent species formed here are Dipterocarpus indicus, Calophyllum tomentosum, Holigarna grahamii, Syzygium travancoricum, Mesua ferrea, Palaquium ellipticum, Artocarpus hirsutus, Ficus nervosa, Syzygium gardneri, Canarium strictum, Lophopetalum wightianum, Diospyros crumenata, etc, Among them Syzigium gardneri reaches the maximum height of 40 m. Selective felling badly affected these type of trees because most of them have good timber value.
- Strata-2-Canopy trees: The plants under this category provide good canopy cover to the forest. Canopy cover is more or less continuous except in some logged area and in those exposed spot secondary species is emerging. Trees having height within the range of 10-20 m are considered as canopy trees. The species include Symplocos racemosa, Syzigium spp., Actinodaphne hookeri, Ixora brachiata, Mangifera indica, Aglaia anamallayana Dimocarpus longan, Phobae catheae, Syzigium cumini, Antidesma menasu, Litsea spp., Garcinia talboti, Dimorphocalyx spp., Nothopegia colebroockiana etc. Emergent trees in this height range also provide dense canopy cover to the forest.
- Strata-3- trees: These are the smallest trees which make up the third layer in a vertical stratification profile of a forest. Trees having height below 10 m are included in this category. Trees which are habitually coming under this category are very few in number but in most samples this strata is filled by regenerating forms of the larger trees. The species like Ervatamia heyneana, Mitrephora heyneana, Meiogyne pannosa, Syzigium laetum, Syzigium macrophylla, and Dimorphocalyx lawianus are the frequently distributed trees within theses range.
- Shrubs/Herbs: The microclimatic condition of the forest floor is decisive in the composition of ground layer. Light intensity is a critical factor in the rain forest regime. In the dense canopied forest like Kathalekan, the light intensity is quite low favouring only shade loving shrubs and herbs and the numbers of which are limited. Grasses are nearly absent. This study uncovers a total of 38 species of shrubs and 11 species of herbs from the sampled area. There of course could some more species in the sampled area. However the juvenile forms of the trees are the major contributors to this layer. In the shrubs layer are Agrostistachis indicus, Atalantia racemosa, Blachia denudata, Canthium rheedii, Dichapetalum gelonioides, Memecylon terminale, Ochlandra sp, Pinanga dicksonii, Psychotria flavida. Scutia myrtina and Strobilanthus heynianus are the common shrubs. Pinanga dicksonii, a slender endemic palm that grows gregariously in association with streams, swamps and wet shaded soils. Ochlandra sp. also found Strobilanthus heyneanus is very common in moist semi shaded areas. It is an under growth of the forest edges advancing into savannized hill tops. Alpinia malaccensis, Cyrtococcum oxyphyllum, Dracaena terniflora, Justicia simplex, Ophiorrhiza hirsutula, Lagenandra ovata, and Boesenbergia pulcherrima are the main herbs of the forest floor.
- Climbers and Lianas: Climbers and lianas also have an integral role in constituting complex evergreen forest structure. They vary from slender herbaceous ones to large lianas which are literally climbing trees. Lianas like Ancistrocladus heyneanus, Combretum latifolium, Gnetum scandens, etc. are notable among them.
- Palms in the undergrowth: Tropical forests are well known for palms. In Kathalekan few species of palms which occur are Pinanga dicksonii, Arenga wighitii, Caryota urens . and some species of Calamus. Gregarious growth of Pinanga in this forest indicates the high moisture content of the soil.
4.3.3 Species with root modification: Roots of many rain forest trees are known for their diverse morphology to suit their heterogeneous environment. Stilt root, pneumatophores, knee roots, floating roots flying buttresses and buttresses are the common adaptations. Buttress formation and serpentine roots are found in trees which may be considered as adaptation to wind but it is not always found in every tall tree like Dipterocarpus indicus (Pascal, 1988). Stilt roots furnish additional anchorage in soft soils. E.g., Myristica fatua, Myristica malabarica, Syzygium gardneri. Gymnacranthera canarica, a tree of Myristica swamp produces loop like pneumatophores full of large lenticels. Semecarpus kathalekanensis, a newly discovered critically endangered endemic tree produces knee like protrusions, studded with lenticels, which help in gaseous exchange in swampy condition. Serpentine roots of Lophopetalum wightianum spread over the surface of soft swampy soil and provide additional anchorage and help in root respiration. Syzygium gardneri, Ficus nervosa, Elaeocarpus tuberculatus, Tetrameles nudiflora, etc. produce large buttresses from the base of their trunks to provide additional mechanical support.
4.3.4 Height distribution: Figure 4.3.2 depicts height classes of trees (sampled in nine transects). Individuals positioned in lower class as well as in upper class are comparatively very low. Very few individual were found in the placed in the 0-5 m category composed of some small trees like Ervatamia heyneana, Ixora brachiata and juveniles of the larger trees. Maximum individuals (466) are in 10-14 m range. A declining trend is seen in the succeeding ranges. There are 421 individuals in 15-19m range followed by 224 and 99trees in height classes of 20-24 m and 25-29 m respectively. Only 8 individuals had height exceeding 35 m. Syzygium gardneri was the tallest among all. The extraction of timber during 1940-1985 periods apparently had telling effect on especially larger trees
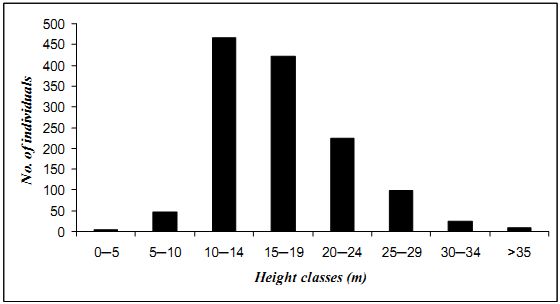
Fig. 4.3.2 Height classes of trees from 9 transects
4.3.5 Tree species density: Total trees density of Kathlekan is found to be 724 trees/ha and a total of 751 stems/ ha. Hopea ponga has an estimated density of 116 trees/ha. followed by Dipterocarpus indicus (66.7), Olea dioica (54.4), Knema attenuata (48.3), Holigarna grahamii (37.2), Aglaia roxburghiana (28.9), Syzygium gardneri (25), and Dimocarpus longan (23.9). Species like Cinnamomum macrocarpum, Ficus callosa, Walsura trifolia, etc having least density (only 2 individual in the sampled area) followed by Randia rhugosa, Tetrameles nudiflora, Murraya paniculata, etc having one individual in the sampled area.
4.3.6 Basal area: Kathlekan forest has an average basal area of 39.16 m2/ha. (considered only trees having GBH ≥ 30 cm). Species wise basal area ranges from 0.0072 m2/ ha to 7.27 m2/ha. Hopea ponga has the highest basal area of 7.27m2/ha followed by Olea dioica (6.83m2/ha), Dipterocarpus indicus (6.76 m2/ha), Syzygium gardneri (6.2 m2/ha), Holigarna grahamii (3.60m2/ha) Calophyllum tomentosum (3.34m2/ha) and least basal area was recorded for Randia rhugosa (0.07 m2/ha). The total basal area for 11 unidentified species is 0.75 m2/ha and for lianas (≥ 30 cm GBH) it was 0.11 m2/ha.
The mean basal area of 39.16 m2/ha. of Kathalekan is closer to the basal area of Kan forest with moderate rain fall range of Uttara Kannada (42.94 m2/ha), in Devar’s (2008) study. A kan forest of Sorab in neighboring Shimoga district studied by Pascal (1988) showed a high basal area of 70.1 m2/ha. having a density of 663 trees/ha. It is due to the high density of big trees. Such abnormality in high basal area for central Western Ghats region could be exceptional or due to extrapolation from small sample size. Larger trees contribute disproportionately to the basal area (Pomeroy, 2003). In Kathalekan, of the estimated 751 stems/ha., most of the trees were in lower girth class so there is relatively less basal area. Industrial logging in the past obviously extracted several large trees in every hectare (Gadgil and Chandran, 1989). Hopea ponga account for highest basal area due to higher number of individuals. Although Syzygium gardneri, is lesser in density, had a more basal area because of larger size. In the low land and mid elevation evergreen forest of the Western Ghats of India (Pascal, 1988; Chandrasekhara and Ramakrishnan, 1994) the range of basal area is from 32.67m2/ha. and 83.83 m2/ha. Chandrasekhara and Sankar (1997) reported 51 tree species from Iringole kavu in Kerala with Hopea ponga and Artocarpus hirsutus as the dominant ones. This sacred grove had a basal area of 37.37 m2/ha with 3341 stems/ha. Detailed study by Chandran (1993) various plots of Uttara Kannada district showed that in a plot of Karikan, a well preserved Kan forest of Honnavar taluk the basal area was 62.81 m2/ha. In a somewhat degraded part of Karikan the basal area was only 40.48 m2/ha. This clearly indicates how disturbance such as logging can adversely affect on the vegetation structure. According to his study in Kathalekan the basal area of the forest of that time was found to be 34.77 m2/ha, in the current study it is 39.2 m2/ha, showing a green signal of forest recovery, as a result of stoppage of timber extraction from mid 1980’s.
4.3.7 Relative frequency: Of the 88 identified tree species of Kathalekan (≥ 30 cm), Aglaia roxburghiana, Dipterocarpus indicus, Holigarna grahamii, Hopea ponga, Knema attenuata, and Syzigium sp. were most frequently distributed each with 2.94 % of relative frequency. These species were represented in all the grids. It shows the ecological amplitude of these species and pervasiveness of suitable soil and climatic conditions within the forest. Dimocarpus longan Diospyros candolleana and Olea dioica, with 2.69% and Actinodaphne hookeri, Nothopegia colebrookeana, Persea macrantha with 2.29% of RF follow closely. Species rare in the forest such as Tetrameles nudiflora, Lepisanthes deficiens, Elaeocarpus tuberculatus, Carallia brachiata, etc have relative frequency 0.33 %.( Table 4.3.1)
Table 4.3.1: Vegetation structure of tree species in Kathalekan.
| Sl.No |
Species |
BA (m2) |
RD (Ind/ha) |
RB (m2) |
RF (%) |
IVI (%) |
| 1 |
Hopea ponga |
7.290 |
16.028 |
10.355 |
2.941 |
29.32 |
| 2 |
Dipterocarpus indicus |
6.776 |
9.202 |
9.626 |
2.941 |
21.77 |
| 3 |
Olea dioica |
6.830 |
7.515 |
9.703 |
2.614 |
19.83 |
| 4 |
Syzygium gardneri |
6.202 |
3.451 |
8.811 |
1.961 |
14.22 |
| 5 |
Holigarna grahamii |
3.607 |
5.138 |
5.124 |
2.941 |
13.20 |
| 6 |
Knema attenuata |
2.242 |
6.672 |
3.184 |
2.941 |
12.80 |
| 7 |
Aglaia roxburghiana |
2.749 |
3.988 |
3.905 |
2.941 |
10.83 |
| 8 |
Dimocarpus longan |
3.083 |
3.298 |
4.379 |
2.614 |
10.29 |
| 9 |
Calophyllum tomentosum |
3.342 |
1.534 |
4.747 |
1.961 |
8.24 |
| 10 |
Syzigium sp. |
0.909 |
2.301 |
1.291 |
2.941 |
6.53 |
| 11 |
Garcinia talbotii |
1.781 |
1.994 |
2.529 |
1.961 |
6.48 |
| 12 |
Palaquium ellipticum |
1.700 |
1.994 |
2.414 |
1.634 |
6.04 |
| 13 |
Diospyros candolleana |
0.576 |
2.071 |
0.818 |
2.614 |
5.50 |
| 14 |
Litsea sp. |
0.759 |
1.610 |
1.079 |
1.961 |
4.65 |
| 15 |
Persia macrantha |
1.006 |
0.920 |
1.429 |
2.288 |
4.64 |
| 16 |
Actinodaphne hookeri |
0.677 |
1.380 |
0.962 |
2.288 |
4.63 |
| 17 |
Nothopegia colebrookeana |
0.325 |
1.457 |
0.462 |
2.288 |
4.21 |
| 18 |
Litsea floribunda |
0.863 |
1.610 |
1.226 |
1.307 |
4.14 |
| 19 |
Aglaia anamallayana |
0.672 |
1.380 |
0.954 |
1.634 |
3.97 |
| 20 |
Symplocos racemosa |
0.629 |
1.380 |
0.893 |
1.634 |
3.91 |
| 21 |
Syzigium hemisphericum |
1.389 |
0.613 |
1.973 |
0.980 |
3.57 |
| 22 |
Syzigium sp. |
1.671 |
0.690 |
2.374 |
0.327 |
3.39 |
| 23 |
Caryota urens |
0.483 |
0.690 |
0.686 |
1.961 |
3.34 |
| 24 |
Syzygium cumini |
1.291 |
0.613 |
1.834 |
0.654 |
3.10 |
| 25 |
Garcinia cambojia |
0.433 |
0.844 |
0.615 |
1.634 |
3.09 |
| 26 |
Ixora brachiata |
0.359 |
1.534 |
0.510 |
0.980 |
3.02 |
| 27 |
Diospyros crumenata |
0.524 |
0.920 |
0.744 |
1.307 |
2.97 |
| 28 |
Callicarpa tomentosa |
0.212 |
0.997 |
0.301 |
1.634 |
2.93 |
| 29 |
Diospyros paniculata |
0.418 |
0.690 |
0.594 |
1.634 |
2.92 |
| 30 |
Holigarna ferruginea |
0.524 |
0.844 |
0.744 |
1.307 |
2.89 |
| 31 |
Myristica dactyloides |
0.542 |
0.690 |
0.770 |
1.307 |
2.77 |
| 32 |
Mesua ferrea |
0.741 |
0.460 |
1.053 |
0.980 |
2.49 |
| 33 |
Mangifera indica |
0.236 |
0.383 |
0.335 |
1.634 |
2.35 |
| 34 |
Artocarpus hirsutus |
0.906 |
0.383 |
1.287 |
0.654 |
2.32 |
| 35 |
Beilschmiedia fagifolia |
0.456 |
0.613 |
0.647 |
0.980 |
2.24 |
| 36 |
diospyros sp |
0.162 |
0.690 |
0.230 |
1.307 |
2.23 |
| 37 |
Diospyros saldhanae |
0.067 |
0.460 |
0.095 |
1.634 |
2.19 |
| 38 |
Dimorphocalyx lawianus |
0.146 |
0.920 |
0.208 |
0.980 |
2.11 |
| 39 |
Lophopetalum wightianum |
0.725 |
0.307 |
1.029 |
0.654 |
1.99 |
| 40 |
Myristica fatua |
0.507 |
0.537 |
0.720 |
0.654 |
1.91 |
| 41 |
Cleidion javanicum |
0.121 |
0.383 |
0.172 |
1.307 |
1.86 |
| 42 |
Ficus nervosa |
0.414 |
0.230 |
0.588 |
0.980 |
1.80 |
| 43 |
Drypetes elata |
0.328 |
0.613 |
0.466 |
0.654 |
1.73 |
| 44 |
Syzigium laetum |
0.072 |
0.537 |
0.102 |
0.980 |
1.62 |
| 45 |
Canthium dicoccum |
0.172 |
0.383 |
0.244 |
0.980 |
1.61 |
| 46 |
Vepris bilocularis |
0.453 |
0.230 |
0.643 |
0.654 |
1.53 |
| 47 |
Flacourtia montana |
0.111 |
0.383 |
0.158 |
0.980 |
1.52 |
| 48 |
Polyalthia fragrans |
0.318 |
0.383 |
0.452 |
0.654 |
1.49 |
| 49 |
Ficus callosa |
0.432 |
0.153 |
0.614 |
0.654 |
1.42 |
| 50 |
Macaranga peltata |
0.086 |
0.307 |
0.123 |
0.980 |
1.41 |
| 51 |
Gymnocranthera canarica |
0.400 |
0.153 |
0.568 |
0.654 |
1.37 |
| 52 |
Ervatamia heyneana |
0.045 |
0.307 |
0.065 |
0.980 |
1.35 |
| 53 |
Glochedion javanicum |
0.077 |
0.230 |
0.109 |
0.980 |
1.32 |
| 54 |
Garcinia morella |
0.091 |
0.537 |
0.129 |
0.654 |
1.32 |
| 55 |
Canarium strictum |
0.406 |
0.230 |
0.577 |
0.327 |
1.13 |
| 56 |
Meiogyne pannosa |
0.057 |
0.383 |
0.081 |
0.654 |
1.12 |
| 57 |
Cinnamomum macrocarpum |
0.214 |
0.153 |
0.304 |
0.654 |
1.11 |
| 58 |
Mastixia arborea |
0.153 |
0.230 |
0.217 |
0.654 |
1.10 |
| 59 |
Myristica malabarica |
0.094 |
0.307 |
0.133 |
0.654 |
1.09 |
| 60 |
Pterospermum diversifolium |
0.081 |
0.230 |
0.115 |
0.654 |
1.00 |
| 61 |
Antidesma menasu |
0.079 |
0.230 |
0.112 |
0.654 |
1.00 |
| 62 |
Terminalia paniculata |
0.189 |
0.307 |
0.269 |
0.327 |
0.90 |
| 63 |
Mimusops elengi |
0.049 |
0.153 |
0.070 |
0.654 |
0.88 |
| 64 |
Diospyros oocarpa |
0.042 |
0.153 |
0.060 |
0.654 |
0.87 |
| 65 |
Walsura trifolia |
0.038 |
0.153 |
0.053 |
0.654 |
0.86 |
| 66 |
Euonymus indicus |
0.034 |
0.153 |
0.048 |
0.654 |
0.86 |
| 67 |
Hydnocarpus pentandra |
0.172 |
0.230 |
0.245 |
0.327 |
0.80 |
| 68 |
Hydnocarpus laurifolia |
0.167 |
0.230 |
0.237 |
0.327 |
0.79 |
| 69 |
Lagerstroemia microcarpa |
0.212 |
0.153 |
0.302 |
0.327 |
0.78 |
| 70 |
Mitrephora heyneana |
0.047 |
0.307 |
0.067 |
0.327 |
0.70 |
| 71 |
Phoebe cathia |
0.086 |
0.230 |
0.121 |
0.327 |
0.68 |
| 72 |
Cassine glauca |
0.189 |
0.077 |
0.268 |
0.327 |
0.67 |
| 73 |
Careya arborea |
0.038 |
0.153 |
0.055 |
0.327 |
0.53 |
| 74 |
aglaia sp |
0.037 |
0.153 |
0.052 |
0.327 |
0.53 |
| 75 |
Murraya paniculata |
0.070 |
0.077 |
0.100 |
0.327 |
0.50 |
| 76 |
Carallia brachiata |
0.047 |
0.077 |
0.067 |
0.327 |
0.47 |
| 77 |
Celtis cinnomomea |
0.042 |
0.077 |
0.060 |
0.327 |
0.46 |
| 78 |
Elaeocarpus tuberculatus |
0.039 |
0.077 |
0.055 |
0.327 |
0.46 |
| 79 |
Fahrenhetia zeylanica |
0.024 |
0.077 |
0.034 |
0.327 |
0.44 |
| 80 |
Cassine sp |
0.022 |
0.077 |
0.031 |
0.327 |
0.43 |
| 81 |
Litsea ghatica |
0.015 |
0.077 |
0.022 |
0.327 |
0.43 |
| 82 |
drypetes sp |
0.013 |
0.077 |
0.019 |
0.327 |
0.42 |
| 83 |
Tetrameles nudiflora |
0.013 |
0.077 |
0.018 |
0.327 |
0.42 |
| 84 |
Casearia sp. |
0.010 |
0.077 |
0.015 |
0.327 |
0.42 |
| 85 |
Syzygium macrophylla |
0.009 |
0.077 |
0.013 |
0.327 |
0.42 |
| 86 |
Lepisanthes deficiens |
0.008 |
0.077 |
0.012 |
0.327 |
0.42 |
| 87 |
Polyalthia sp |
0.008 |
0.077 |
0.012 |
0.327 |
0.42 |
| 88 |
Randia rugulosa |
0.007 |
0.077 |
0.010 |
0.327 |
0.41 |
BA- Basal Area, RB- Relative Basal area, RD- Relative Density, RF- Relative Frequency, IVI- Importance Value Index, Ind/ha- Individuals/ha.
4.3.8 Important Value Index: Important Value Index is a measure of ecological success of species in an ecosystem. Based on the relative density, relative basal area and relative measures IVI can be calculated. In the current study the Hopea ponga was found to be having highest IVI value of 29.32, followed by Dipterocarpus indicus (21.77), Olea dioica (19.83), Syzygium gardneri (14.22), Holigarna grahamii (13.2), Knema attenuata (12.8), Aglaia roxburghiana (10.83), Dimocarpus longan (10.29), Calophyllum tomentosum (8.24), etc. And species having lesser IVI includes, Lagerstroemia microcarpa (0.78) Mitrephora heyneana (0.70), Careya arborea (0.53), Carallia brachiata (0.47), Elaeocarpus tuberculatus (0.46), Syzygium macrophylla (0.42), Randia rhugosa (0.41) and so on (Table 4.3.2). Based on the IVI values grid-wise typification of the forest has been attempted here on the model found in Pascal (1988). In each grid two or three top IVI species have been considered for assigning the type.
Grid 1 Litsea floribunda-Dimocarpus longan type
Grid 2 Syzigium gardneri-Hopea ponga-Knema attenuata type
Grid 4 Syzigium gardneri-Dipterocarpus indicus type
Grid 4 Dipterocarpus indicus-Hopea ponga-Dimocarpus longan type
Grid 5 Knema attenuata-Dipterocarpus indicus-Syzigium gardneri type
Grid 6 Olea dioica-Dipterocarpus indicus type
Grid 7 Dipterocarpus indicus-Holigarna grahamii-Aglaia anamallayana type
Grid 8 Hopea ponga-Olea dioica-Syzigium travancoricum type
Grid 9 Hopea ponga-Olea dioica type
One of the significant finding of this study is the high prevalence of Dipterocarpus indicus in five of the 9 grids. This hygrophilous, climax evergreen and endemic tree species of Western Ghats is more characteristic of southern forest form 8o N to 13o N. the paucity of these species and its confinement to some of its southern most kan forest patches were reported by Chandran (1997) in the Central Western Ghats (Uttara Kannada). The present study reveals that protection as was given to the kans by local communities in the pre-colonial time resulted in persistence of Dipterocarpus. Such kans are the centers of endemism which was substantially adversely affected in the other forests under anthropogenic pressure. Not only that in Kathlekan and its peripheral forest regeneration of Dipterocarpus was found to be promising. The role of kans in regeneration of secondary forests has been alluded to by Chandran and Gadgil (1993).
Table 4.3.2: Vegetational composition of savanna in Grid 5.
| Area sampled (ha) |
BA/ha (m2) |
Total Species |
Shannon Diversity |
Simpson dominance |
Pielou Evenness |
| 0.2 |
1.5916 |
5 |
1.523 |
0.235 |
0.946 |
4.3.8 Savanna vegetation composition: A transect was laid in Grid no5 exclusively for studying savanna. The species were found to be very few; just 3 species of dwarf trees, 10 species of shrubs and 12 species of herbs were recorded. Trees belong to fire tolerants like Careya arborea, Terminalia chebula, Glochidion javanicum andshrubs were Scutia myrtina, Ziziphus rugulosa, Flemingia strobilifera, Randia rugulosa, Phoenix humilis, etc. Trees and shrubs are sparsely distributed in savanna. Most of the herbs were grasses. Here Shannon index is found to be 1.523 and the species were evenly distributed with least dominance. The exposed soils of savanna are compact and dry. These extreme conditions in the elevated part of the forest .In the absence of shifting cultivation for over century the forest from valleys are found to advance towards the savanna. The pioneer species towards the exposed edge are Olea dioica, Glochidion, Carvia callosa, Strobilanthus sp, Fleminga congesta, etc. Exposed rocky area is also found along the rim of Sharavathi river gorge. These rocky areas have almost same vegetation as in savanna.
4.4 Status of endemism
Endemism, one of the main and easily identifiable components of biodiversity is the occurrence of a species with restricted range. While biodiversity is the biological Capital of the Earth, endemic flora and fauna (which includes genes, species and ecosystem) of a region or a nation are the exclusive biological capital of that region or nation (Nayar,1996). Endemic elements of region gives a picture of the biogeography centers of speciation, refugia, areas of extinction, vicariance and adaptive evolution of the flora and fauna of that region. The extent pattern of distribution of plants and animals in area is largely influenced by the biogeographic condition prevailing in that area.
Endemism encompasses taxonomic units of any rank or taxa (includes all life forms) which occur in a biological area usually isolated by geographical, ecological or temporal barriers. The degree of endemism increases with the increase in size of a homogenous biogeographical area having the same floristic history and ecological condition but the areas of endemism may be small or large (Nayar, 1996). In most of the cases the endemic flora present in a particular area like mountains, islands, peninsula etc are the remnants of the ancient flora of that area. At the global level endemic areas are of high conservation priority because if unique species are lost they can never be replaced (WCMC, 1992). The Western Ghats is included in the 35 biodiversity hotspots all over the world (Myers 2000) due to the abundance of endemic species- 57 genera and about 1600 species of flowering plants are endemic to Western Ghats. Among the endemic woody genera are Memecylon (16 spp.), Litsea (15 spp.), Symplocos (14 spp), Cinnamomum (12 spp), Syzigium (11 spp.), Actinodaphne (9 spp,), Glochidion and Grewia (9 spp each) Diospyros (8 spp), Dalbergia and jambosa (7 sp.each), Hopea and Mallotus (6 sp.each), Aglaia, Cryptocareya, Euonymus, Garcinia, Holigarna, Humboldtia and Terminalia (5 sp each) and several others. Of these 57 endemic genera, 46 are monotypic (Nair and Daniel.1986). The family Poaceae has 13 endemic genera and about 155 species (Karthikeyan, 1983). Families like Orchidaceae and Acanthaceae also have number of endemics. A good proportion of the 356 species of Impatiens is believed to be endemics to the Western Ghats (Nair and Daniels, 1986). Kathlekan is an important centre of endemism in Central Western Ghats. Of the 185 species of flowering plants noticed in the samples 60 are endemics. A good number of unidentified are also likely to be endemic.
4.4.1 Endemism among trees: Sacred groves are some of the last refugia of flora and fauna is evident from the results presented so far: 59 % of the total tree population in the 1.8 ha. were endemics. Endemism refers to the percentage of endemic individuals with in the total no. of individuals. Among the 104 tree species inventoried, 38 species (36.5 %) (number may slightly increase because of some unidentified plants) is found to be endemic to the Western Ghats. Table 4.4.1 lists the grid wise distribution of tree endemism that helps in assigning the conservation priorities of the region within the forest.
Tab.4.4.1 Endemism among the tree layer
Grid
No. |
Total
Species |
Endemic
Species |
% of end. Species. |
Total
Individual |
Total
Endemics |
Degree of
Endemism (%) |
| 1 |
41 |
17 |
41.5 |
141 |
76 |
54.61 |
| 2 |
39 |
19 |
48.7 |
173 |
105 |
61.85 |
| 3 |
37 |
17 |
46 |
104 |
60 |
60.58 |
| 4 |
38 |
14 |
37 |
129 |
75 |
58.91 |
| 5 |
38 |
18 |
47.3 |
158 |
88 |
55.70 |
| 6 |
23 |
11 |
49 |
165 |
84 |
50.91 |
| 7 |
44 |
17 |
38.6 |
141 |
70 |
52.482 |
| 8 |
34 |
14 |
41 |
113 |
68 |
60.177 |
| 9 |
18 |
9 |
50 |
186 |
145 |
77.96 |
The percent of endemism varies between of 51% (in grid 6) to 78% (in grid 9).This study brings to the fore that the percentage of endemics tree species (not individuals) at the Kathalekan (36.5 %) compares well with Pascal’s (1988) range of 34.1 to 37.4 % for Western Ghats north of the Palghat gap. In the population of endemics within any species Hopea ponga was leading in numericity with 209 individuals in the sampled area. It was followed by Dipterocarpus indicus (109). Holigarna grahamii, Knema attenuata, Garcinia talbotii, Palaquium ellipticum, Diospyros candolleana, Actinodaphne hookeri, Litsea floribunda and Aglaia anamallayana. Trees such as Cinnamomum macrocarpum, Mastixia arborea, Myristica malabarica, Euonymus indicus, Hydnocarpus pentandra, Hydnocarpus laurifolia, Litsea ghatica and Syzigium macrophylla are rare endemics.
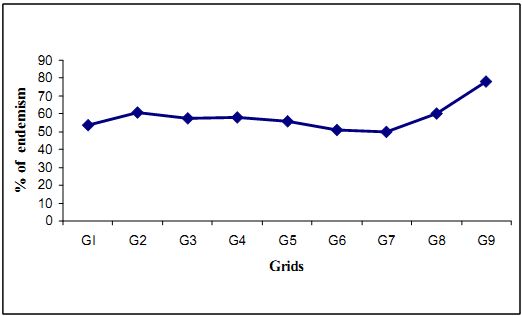
Figure 4.4.1: Grid wise percentage of endemism in Kathalekan
The distribution of tree endemism within the grid is more or less uniform except for grid 9 where the high number (100) of Hopea ponga was the reason for high percentage of endemism, though the grid had only 9 endemic species. In Fig (4.4.2) is given with number of tree species (based on the sample) and the number of endemic species. Interestingly the grid no 9 which account for largest number of endemics in the tree population has the smallest number of tree species (18).
Table 4.4.2 lists endemic flowering plant species, family wise that occurred in the grid wise sampling in Kathlekan. Lauraceae with 6 tree species and Ebenaceae, Myrtaceae with 3 species are found in the region. Considering higher number of individuals, Dipterocarpaceae (329) and Myristicaceae (98) and Anacardiaceae (78) are the leading families.
Table 4.4.2. Endemic tree species in Kathalekan
| Sl.No |
Species |
Family |
| 1 |
Holigarna arnotiana* |
Anacardiaceae |
| 2 |
Holigarna ferruginea |
Anacardiaceae |
| 3 |
Holigarna grahamii |
Anacardiaceae |
| 4 |
Meiogyne pannosa |
Annonaceae |
| 5 |
Polyalthia fragrans |
Annonaceae |
| 6 |
Segaria laurifolia* |
Annonaceae |
| 7 |
Ervatamia heyneana |
Apocynaceae |
| 8 |
Arenga wighitii |
Arecaceae |
| 9 |
Euonymus indicus |
Celastraceae |
| 10 |
Garcinia gummi gutta |
Clusiaceae |
| 11 |
Garcinia talbotii* |
Clusiaceae |
| 12 |
Mastixia arborea |
Cornaceae |
| 13 |
Dipterocarpus indicus |
Dipterocarpaceae |
| 14 |
Hopea ponga |
Dipterocarpaceae |
| 15 |
Diospyros candolleana |
Ebenaceae |
| 16 |
Diospyros paniculata |
Ebenaceae |
| 17 |
Diospyros saldhanae |
Ebenaceae |
| 18 |
Dimorphocalyx lawianus |
Euphorbiaceae |
| 19 |
Drypetes elata |
Euphorbiaceae |
| 20 |
Mallotus stenanthus* |
Euphorbiaceae |
| 21 |
Flacourtia montana |
Flacourtiaceae |
| 22 |
Hydnocarpus laurifolia |
Flacourtiaceae |
| 23 |
Hydnocarpus pentandra |
Flacourtiaceae |
| 24 |
Actinodaphne hookeri |
Lauraceae |
| 25 |
Beilschmiedia fagifolia |
Lauraceae |
| 26 |
Cinnamomum macrocarpum |
Lauraceae |
| 27 |
Litsea floribunda |
Lauraceae |
| 28 |
Litsea ghatica |
Lauraceae |
| 29 |
Litsea laevigata* |
Lauraceae |
| 30 |
Lagerstroemia microcarpa |
Lythraceae |
| 31 |
Aglaia anamallayana |
Meliaceae |
| 32 |
Artocarpus hirsutus |
Moraceae |
| 33 |
Knema attenuata |
Myristicaceae |
| 34 |
Myristica fatua |
Myristicaceae |
| 35 |
Myristica malabarica |
Myristicaceae |
| 36 |
Syzigium laetum |
Myrtaceae |
| 37 |
Syzygium macrophylla |
Myrtaceae |
| 38 |
Syzigium travancoricum |
Myrtaceae |
| 39 |
Strombosia zeylanica* |
Oleaceae |
| 40 |
Ixora brachiata |
Rubiaceae |
| 41 |
Vepris bilocularis |
Rutaceae |
| 42 |
Mitrephora heyneana |
Sapotaceae |
| 43 |
Palaquium ellipticum |
Sapotaceae |
* found in juvenile form only.
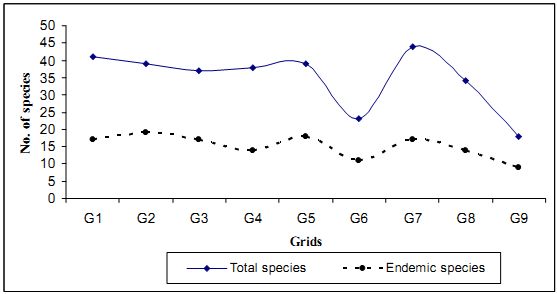
Figure 4.4.2: Grid wise total tree species and endemic species
4.4.1.1 Endemic tree species throughout the Western Ghats: Kathalekan harbours several endemic species such as Meiogyne pannosa, Knema attenuata, Myristica malabarica, Diospyros paniculata, Lagerstroemia microcarpa, Syzigium laetum, Euonymus indicus, Dimorphocalyx lawianus, Mallotus stenanthus, Holigarna arnottiana, Holigarna grahamii, Vepris bilocularis, Arenga wighitii etc. with widespread distribution in the Western Ghats..
4.4.1.2. Rare endemic species: Myristica fatua, Gymnocranthera canarica and Mastixia arborea are the rare endemic trees confined to the swamps and streams. Semecarpus kathalekanensis is an altogether new tree species discovered from the Myristica swamps of Kathalekan (Dasappa and Swaminath, 2000).
4.4.2. Endemism in the ground layer: About 56.9 % of endemism was observed in the ground layer community, which are listed in Table 4.4.3. A total of 172 flowering plant species (including juveniles of trees and lianas) were enumerated in the ground layer samples. Of the total 39 shrub species 11 were endemics and of the 11 species of herbs 3 were endemics. Saplings and seedlings of endemic trees dominated the ground layer and if adequate protection is given especially from forest encroachers that growing stock will restore a promising forest for future. Kathalekan forest could turn out to be in the near future a gene bank of endemic species, especially for Central Western Ghats. Gregarious occurrence of slender, endemic palm species Pinanga dicksonii and promising presence of juveniles of Dipterocarpus indicus are good indicators of the future of the forest as a centre of endemism.
Table 4.4.3: Endemic species in the ground layer
| Sl.No |
Species |
Family |
| Shrubs |
| 1 |
Blachia denudata |
Euphorbiaceae |
| 2 |
Canthium parviflorum |
Rubiaceae |
| 3 |
Croton gibsonianus |
Euphorbiaceae |
| 4 |
Dichapetalum gelonioides |
Dichapetalaceae |
| 5 |
Gymnosporia rothiana |
Celastraceae |
| 6 |
Ixora polyantha |
Rubiaceae |
| 7 |
Memecylon terminale |
Melastomaceae |
| 8 |
Pinanga dicksonii |
Arecaceae |
| 9 |
Psychotria flavida |
Rubiaceae |
| 10 |
Ixora polyantha |
Rubiaceae |
| 11 |
Strobilanthus heynianus |
Acanthaceae |
| Herbs |
| 1 |
Boesenbergia pulcherrima |
Zingiberaceae |
| 2 |
Ophiorrhiza hirsutula |
Rubiaceae |
| 3 |
Zingiber cernum |
Zingiberaceae |
| Lianas/Climbers |
| 1 |
Ancistrocladus heyneanus |
Ancistrocladaceae |
| 2 |
Calamus sp |
Arecaceae |
| 3 |
Calamus thwaitesii |
Arecaceae |
| 4 |
Cyclea peltata |
Menispermaceae |
4.5 Myristica swamps of Kathalekan
A Myristica swamp is a fresh water swamp dominated by members of the family Myristicaceae. Myristica fatua var.magnifica and Gymnacranthera of this family are exclusive to such swamps. Krishnamurthy (1960) first reported the occurrence of Myristica swamps from the Travancore region of Kerala and classified them under a newly introduced category ‘Myristica Swamp Forests’ under the sub group 4C of Champion and Seth (1968). As far as Uttara Kannada district is concerned a lone locality near Malemane in Siddapur taluk has been mentioned in the Forest Flora of the Bombay Presidency and Sind (vol, 2), by Talbot (1909) as having Myristica magnifica (presently M. fatua var.magnifica). This locality obviously could have been nothing other than Kathalekan (which is a part of Malemane revenue village). Saldanha (1984), in the Flora of Karnataka (vol, 2) reports Myristica fatua var magnifica as ‘occasional in the swampy areas of evergreen forest of Uttara Kannada. Probably alternative localities were never found when this flora was prepared.
Gadgil and Chandran (1989) observed that the Myristica swamp of Kathlekan in Malemane Ghats as the only one of its kind in the district where Myristica fatua occurred. Varghese (1992) and Varghese and Kumar (1997) and Varghese and Menon (1999) made various floristic and ecological observations of the Myristica swamps of southern Kerala. A more detailed study later by Chandran et al (1999) in Uttara Kannada district listed Myristica swamps in 51 localities. Of these 49 were in Siddapur and just two in Honnavar taluk, both towards south of the district. Most of these swamps were fractions of a hectare in area, which obviously could have been the last fragments of larger swamps that occurred before severe human impacts. Myristica Swamps of Kathlekan are also dealt with in the Cumulative Impact Assessment of the Sharavathi River Basin, prepared by Ramachandra et al. (2002-03). Chandran and Mesta (2001) characterizes Myristica swamps as the only sites of occurrence of certain members of the ancient family Myristicaceae such as Myristica fatua var magnifica and Gymnacranthera canarica. These swamps of high watershed value, with their little known biota, were described as “virtually live museum of ancient life of great interest to biologists.”
These pioneering works referred to created widespread interest in the various aspects of Myristica swamps. Dasappa and Swaminath (2000) named a new species of tree of the genus Semecarpus, discovered in the Kathalekan swamps, as Semecarpus kathalekanensis. Vasudeva et al (2001) investigated the population structure, reproductive biology and conservation of S. kathalekanensis. Ganesan (2002) documented the ‘evergreen forest swamps’ and their plant species diversity from Kalakad-Mundanthurai Tiger Reserve of southern Western Ghats. Sasidharan (2003) and Roby and Nair (2007) found out Myristica swamps as the prime habitats of the critically endangered tree Syzygium travancoricum. The faunal diversity of the Myristica swamps of southern Kerala was documented by Jose et al (2007a, b, c). A detailed work on mapping the biodiversity of the Myristica swamps in southern Kerala has been carried out by Nair et al. (1997).
4.5.1 Distribution of Myristica swamps: The swamps are associated with flat bottomed or gently slopping valleys with deep soil amidst hills of Western Ghats heavily forested with evergreen forests. Rock below the soil layer by preventing percolation downwards, probably help in developing swampy conditions. Slow seepage of water from the side hills in to the valley throughout the year, heavy annual rainfall averaging 3000mm and temperature ranging from 20-30ºC promote the occurrence of such swamps (Varghese, 1992; Chandran et. al., 1999). Hence these Swamps are highly restricted in distribution.
During the present study in Kathalekan, Myristica swamps were observed in the Grids 1, 2, 3, 4 and 8 occurring along the water-logged portions of sluggish perennial streams. The swamps occur intermittently within the Kathalekan forests wherever suitable edaphic conditions along the stream-sides. Due to the availability of water in swamps, these places are subjected to the agricultural encroachment mainly for the paddy cultivation (grid 2). This feature is clearly visible in some location where the swamp is on the edge of vanishing. One swamp is lying in the Linear Tree Increment plot of Karnataka forest Department and is relatively well protected. Another Swamp is dominated with Semecarpus kathlekanensis (grid 4).
4.5.2 Vegetational composition: Myristicaceae in the Western Ghats have altogether three genera and five species all of which are evergreen trees. Of these Gymnacranthera canarica and M. fatua var.magnifica are found only in swampy conditions. Myristica dactyloides, M. malabarica and Knema attenuata are non-swamp species. Gadgil and Chandran (1989) reported the presence of Myristica magnifica and the endemic palm Pinanga dicksonii as the most notable of Kathalekan swamps. In fact this slender palm occurs as gregarious undergrowth away from swamps as well, but only in moist and shaded soils. Myristica fatua is more restricted in distribution than Gymnacranthera canarica, present almost in every swamp. Other members of Myristicaceae such as M. dactyloides, M. malabarica and Knema attenuata are not habitually confined to swampy places. Most notable of the non-Myristicaceae tree notable in a portion of Kathalekan swamp was the newly discovered Semecarpus kathalekanensis. Of the other non-Myristicaceae may be mentioned Mastixia arborea, Syzigium hemisphericum, Syzygium spp., Lophopetalum wightianum Pandanus canarana etc. Close to the swamps were also noticed various tree species like Dipterocarpus indicus, Hopea ponga, Holigarna grahamii, Nothopegia colebrookeana, Agrostistachys indica etc. On the whole trees exclusively confined to the swamps are very few in number, and they are leading a precarious existence, always under the shadow of threat from humans. The wetness of the swamps and their immediate surroundings favour various pteridophytes like Angiopteris evecta, Blechnum orientale, Cyathtea nilgirensis (tree fern), Pteridium aquilinum, Tectaria wightii etc. Myristica swamps are unique areas at the ecosystem level than at species level as endemicity is high in these swamps though waterlogged conditions reduce species richness.
.
4.5.3 Root modifications in swamp plants: The swamp plants are well adapted morphologically, anatomically and physiologically to combat the adverse conditions of water-logged soils. Most noticeable of these modifications are in the root system:
- Stilt roots: These are woody adventitious roots from the base of the main. These roots grow obliquely to reach the soil, where they branch and give firm additional anchorage to the trees. Myristica fatua var.magnifica, M. malabarica, Elaeocarpus, Holigarna, Pandanus etc. produce such roots. In Myristica fatua, as the tree becomes older these roots become more woody, flattened and resembles buttresses.
- Adventitious water roots: These arise from the stem above the soil usually within the flood zone. These are designated soil water roots. By trapping debris from the water it usually forms a hummock around the base of the stem.
- Pneumatophores: As in mangroves pneumatophores or breathing roots are present in Myristicaceae tree Gymnacranthera canarica. Profuse production of aerial loop like breathing roots studded with enlarged lenticels or air pores are seen protruding into the air from soft soils all around the parent tree. Pneumatophores enable the tree to combat the anaerobic conditions of the water-logged conditions. Pneumatophores of S. kathalekanensis could be seen like rude knobs that protrude into the air from the surface roots present around the tree.
- Serpentine roots: Large serpentine roots protruding high into the air are characteristic of the tree Lophopetalum wightianum.
- Rhizomes: Rhizomes are indeed below ground modifications of stems. These rhizomes have high range of tolerance against the anoxia than roots (Braendle and Crafford, 1987). The palm Pinanga dicksonii the aroid Lagenandra ovata and the zingiber Alpinia malaccensis produce rhizomes, which also function effectively in vegetative propagation.
4.5.4 Importance of Myristica swamps: Myristica swamps may be considered as one of the most threatened ecosystems of India. They are undoubtedly, priceless possessions for evolutionary biology. The Myristica swamp with its entanglement of aerial roots, and canopy of dark green, large leaves and high degree of endemism, is doubtlessly, the relic of one of the most primeval ecosystems of the Western Ghats (Chandran and Mesta, 2001). Since Myristica swamps are considered as one of the primeval tropical habitats of the earth, no wonder, in the Western Ghats they are associated with primary forest relics dominated by Dipterocarpus indicus. The swamps are treasure troves of endemic species including plants, mushrooms, mosses, pteridophytes and gymnosperms and different faunal elements. In the study area it is observed that rare or critically endangered plant spp. like Syzigium, Semecarpus kathlekanensis, Myristica fatua etc. are associated with these swamp area. These swamp forest in Siddapur form an important northern most refuge for the endangered primate lion tailed macaque.
4.5.5 Threats to the swamps: Most of the Myristica swamps are under anthropogenic pressures of varied kinds and can be easily converted into agricultural lands, mainly for growing areca garden and paddy cultivation. Some of such abandoned land is present in Kathalekan. The remaining part of the swamp is struggling to survive. Cultivation in the nearby plots also badly affect these swamps because of diverting stream and channalising water in to the field causing dryness of the streambed and its neighborhood. As a result of such water diversions in the upstream for agriculture or horticulture, most streams were found dry, during summer months of April and May. Cattle grazing in some pockets have resulted in lower regeneration. Most of the swampy areas are very near to national highway with accessible ways adds up the intensity of the impact on these unique habitats.
4.6 Regeneration Status of Trees
The trees are the principal components in forming the ecological framework of the forest. The microclimatic conditions persist in a forest ecosystem depend upon the abundance and size of woody plants. The nature of forest communities largely depends on the ecological characteristics of sites, species diversity and regeneration status of species. Micro environmental factors vary with seasonal changes, which affect the growth stage i.e. seedling, sapling and young trees of the plant communities that maintain the population structure of any forest. Hence, it becomes an important issue to understand the tree diversity, population structure and regeneration status of forest communities. The satisfactory natural regeneration behavior of the forests largely depends on population structure characterized by the production and germination of seed, establishment of seedlings and saplings in the forest (Rao 1988). The evaluation of the regeneration status of the woody trees is being a key element in the ecological assessment of a forest community. The sustainability and future of the ecosystem depending mainly on regeneration of trees
Regeneration of plant species in a vegetation type is an important indicator of how stable it will be in the long run (Connel, 1978). Species are occurring in various life stages from seedlings to a mature individual and forming different strata. It is distributed in various girth classes. Complete absence of seedlings and saplings of tree species in a forest indicates poor regeneration, while presence of sufficient number of young individuals in a given species population indicates successful regeneration (Saxena and Singh 1984). Tripathi and Khan (1990) stated that microsite characteristics of forest floor and micro environmental conditions under the forest canopy also influence the regeneration of trees by seeds.
Kumbhongmayum, (2003) study in four sacred groves in Manipur indicate that the occurrence of large number of species as new colonizers in the groves and have managed to reach there due to invasion of ‘new’ species through seed dispersal from other areas. Invasion of new species to the groves may be regarded as a possible factor to the co-existence of the tree species. The overall population structure of selected woody species reveals that seedling populations dominate tree populations and the fluctuation in population density in various seasons is related to the prevailing environmental factors. Germination of freshly dispersed seeds is high for most of the species during the monsoon season. Therefore, recruitment of all the species increases in the rainy season attaining peak during June. Species diversity and population structure will be stable if all the species are represented in all stages. A stable community is known as climax community. If only adult trees are there without regeneration the community is in the process of succession.
In nine transects, each representing a grid, a total of 2000 m2 area were sampled for enumerating trees (≥ 30 cm gbh).Therefore in nine grids together total of 1.8 ha was studied for trees. The girth class distribution in a given tree species is reflectance of its regeneration status. Figure 4.6.1 depicts collectively the girth status of all the trees from nine transects.
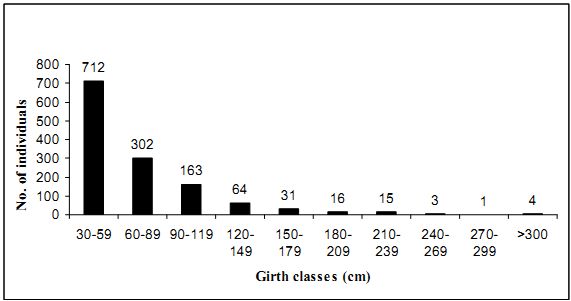
Figure 4.6.1 Girth classes of trees in Kathalekan.
The distribution pattern of trees in different girth classes produce a ‘L’ shaped curve According to Pascal (1988) the ‘L’ shaped distribution indicates the stable equilibrium of the forest, i.e. mortality being compensated by regeneration. So it can be clearly stated that Kathlekan is a well established climax forest. Moreover it harbors many well known climax evergreens of Western Ghats in all girth class. Grid wise girth classes of trees is given in Table 4.6.1. The abundance of lower girth classes especially in 30-59 cm range indicates good regeneration status. The rapid decline in higher girth classes could be attributed to the intensity of industrial logging that plagued the forest in 1940-1985 periods. Only one individual was noted in the 270-299 cm range. There were 4 individuals recorded in exceeding 300 cm. The higher girth of 407 cm was of Syzygium gardneri.
Table 4.6.1: Grid wise Girth classes of trees
| Grids |
Girth classes (cm) |
Total |
| 30-59 |
60-89 |
90-119 |
120-149 |
150-179 |
180-209 |
210-239 |
240-269 |
270-299 |
>300 |
| G1 |
72 |
40 |
18 |
5 |
3 |
0 |
1 |
0 |
0 |
0 |
139 |
| G2 |
99 |
39 |
26 |
4 |
1 |
1 |
3 |
1 |
0 |
0 |
174 |
| G3 |
44 |
23 |
17 |
7 |
2 |
1 |
1 |
0 |
1 |
3 |
99 |
| G4 |
68 |
29 |
15 |
7 |
5 |
4 |
1 |
0 |
0 |
0 |
129 |
| G5 |
93 |
38 |
16 |
8 |
3 |
1 |
3 |
0 |
0 |
1 |
163 |
| G6 |
97 |
22 |
18 |
10 |
6 |
6 |
2 |
0 |
0 |
1 |
162 |
| G7 |
82 |
30 |
14 |
5 |
3 |
1 |
0 |
0 |
0 |
0 |
135 |
| G8 |
56 |
22 |
14 |
6 |
6 |
2 |
4 |
2 |
0 |
0 |
112 |
| G9 |
100 |
45 |
24 |
13 |
4 |
0 |
0 |
0 |
0 |
0 |
186 |
All grids have good number of younger trees. In general, regeneration of a. species is affected by anthropogenic factors (Khan and Tripathi 1989; Sukumar et al. 1994; Barik et al. 1996) and natural phenomena (Welden et al., 1991). As mentioned earlier due to selection felling in the past, the forest suffered lot of damage. Pomery et al., (2003) who studied Kathalekan, state that the forest suffered maximum damage during 1977-84 periods with the heavy extraction of large trees. The density was reduced to 70 % of the original. By 1993, there has been some pronounced recovery. Cattle grazing was noticed during present study, which affects regeneration especially of sensitive species and fodder value species. Table 4.6.2 provides an overall summary of species-wise girth classes. It is found that regeneration status of most of the species in the forest was good. Emergent climax species like Calophyllum tomentosum, Dipterocarpus indicus, and Syzygium gardneri found to be having profuse regeneration. Aglaia roxburghiana, , Dimocarpus longan, Garcinia cambojia, Garcinia talbotii, Holigarna ferruginea, Holigarna grahamii, Hopea ponga, Knema attenuata, Litsea floribunda, Lophopetalum wightianum, Mesua ferrea, Myristica fatua, Olea dioica, Palaquium ellipticum, Persea macrantha, Syzigium cumini, Syzigium hemisphericum, and Syzigium sp.are also having good regeneration. However, there are many rare species in the area having fewer individuals.
4.6.1 Seedlings and saplings: The forest is noted for good representative of climax species in their seedling and sapling stages. The saplings of ≥1 m height (< 30 cm girth) were counted in a total area of 250 sq.m in each transect. The climax tree species like Dipterocarpus indicus, Syzigium gardneri, Calophyllum tomentosum, etc. are well represented in ground layer. In the ground layer, tree saplings were found to overwhelm shrubs and other juvenile plants. In fact 60% of the individuals in shrub layer and 63 % of the individuals in shrub layer and 63 % of individuals in herb layer quadrats were the progeny of trees. From this observation, it seems that status of regeneration is in a progressive state. The more similar the lower strata in a vegetation type is to the tallest stratum, the greater the chance the assemblage maintains itself in the same state or nearly so far a greater period of time (Daniels, 1989). Some tree species like Aporosa lindleyana, Litsea laevigata, Strombosia ceylanica, Sagaeria laurifolia and Mallotus sp are found to be present only in ground vegetation, they have no representatives in top layer. So it is assumed that those species may be new arrivals in to the forest recently.
Table 4.6.2 Girth classes of trees
| Species |
Family |
| 30-59 |
60-89 |
90-119 |
120-149 |
150-179 |
180-209 |
210-239 |
240-269 |
270-299 |
>300 |
| Actinodaphne hookeri |
Lauraceae |
9 |
7 |
1 |
1 |
|
|
|
|
|
|
| aglaia sp |
Meliaceae |
2 |
|
|
|
|
|
|
|
|
|
| Aglaia anamallayana |
Meliaceae |
10 |
3 |
5 |
|
|
|
|
|
|
|
| Aglaia roxburghiana |
Meliaceae |
28 |
11 |
6 |
5 |
|
1 |
1 |
|
|
|
| Antidesma menasu |
Euphorbiaceae |
2 |
1 |
|
|
|
|
|
|
|
|
| Artocarpus hirsutus |
Moraceae |
1 |
1 |
1 |
1 |
|
|
|
|
1 |
|
| Beilschmiedia fagifolia |
Lauraceae |
1 |
5 |
1 |
1 |
|
|
|
|
|
|
| Casearia sp. |
Verbenaceae |
1 |
|
|
|
|
|
|
|
|
|
| Callicarpa tomentosa |
Clusiaceae |
16 |
|
|
|
|
|
|
|
|
|
| Calophyllum tomentosum |
Burseraceae |
6 |
3 |
3 |
2 |
2 |
2 |
2 |
|
|
1 |
| Canthium dicoccum |
Rubiaceae |
2 |
2 |
1 |
|
|
|
|
|
|
|
| Canarium strictum |
Rhizophoraceae |
3 |
|
|
|
|
|
|
|
|
|
| Careya arborea |
Lecythidaceae |
5 |
1 |
1 |
|
|
|
|
|
|
|
| Carallia integrifolia |
Arecaceae |
|
1 |
|
|
|
|
|
|
|
|
| Caryota urens |
Flacourtiaceae |
1 |
6 |
2 |
|
|
|
|
|
|
|
| Cassine glauca |
Celastraceae |
|
|
|
|
1 |
|
|
|
|
|
| Cassine sp |
Celastraceae |
1 |
|
|
|
|
|
|
|
|
|
| Celtis cinnomomea |
Ulmaceae |
|
1 |
|
|
|
|
|
|
|
|
| Cinnamomum macrocarpum |
Lauraceae |
|
|
1 |
1 |
|
|
|
|
|
|
| Cleidion javanicum |
Euphorbiaceae |
5 |
|
|
|
|
|
|
|
|
|
| Dimorphocalyx lawianus |
Sapindaceae |
12 |
|
|
|
|
|
|
|
|
|
| Dimocarpus longan |
Euphorbiaceae |
16 |
11 |
8 |
4 |
3 |
|
1 |
|
|
|
| Diospyros candolleana |
Ebenaceae |
24 |
3 |
|
1 |
|
|
|
|
|
|
| Diospyros crumenata |
Ebenaceae |
3 |
6 |
3 |
|
|
|
|
|
|
|
| Diospyros oocarpa |
Ebenaceae |
1 |
1 |
|
|
|
|
|
|
|
|
| Diospyros paniculata |
Dioscoriaceae |
4 |
3 |
1 |
1 |
|
|
|
|
|
|
| Diospyros saldhanae |
Ebenaceae |
6 |
|
|
|
|
|
|
|
|
|
| diospyros sp |
Ebenaceae |
7 |
2 |
|
|
|
|
|
|
|
|
| Dipterocarpus indicus |
Dipterocarpaceae |
71 |
26 |
7 |
7 |
6 |
5 |
1 |
|
|
1 |
| Drypetes elata |
Euphorbiaceae |
4 |
2 |
2 |
|
|
|
|
|
|
|
| drypetes sp |
Euphorbiaceae |
1 |
|
|
|
|
|
|
|
|
|
| Elaeocarpus tuberculatus |
Elaeocarpaceae |
|
1 |
|
|
|
|
|
|
|
|
| Ervatamia heyneana |
Apocynaceae |
4 |
|
|
|
|
|
|
|
|
|
| Euonymus indicus |
Celastraceae |
2 |
|
|
|
|
|
|
|
|
|
| Fahrenhetia zeylanica |
Euphorbiaceae |
1 |
|
|
|
|
|
|
|
|
|
| Ficus callosa |
Moraceae |
|
|
|
|
|
|
|
|
|
|
| Ficus nervosa |
Moraceae |
1 |
1 |
|
|
|
|
1 |
|
|
|
| Flacourtia montana |
Flacourtiaceae |
4 |
1 |
|
|
|
|
|
|
|
|
| Garcinia cambojia |
Clusiaceae |
7 |
2 |
1 |
1 |
|
|
|
|
|
|
| Garcinia morella |
Clusiaceae |
7 |
|
|
|
|
|
|
|
|
|
| Garcinia talbotii |
Clusiaceae |
5 |
9 |
8 |
3 |
1 |
|
|
|
|
|
| Glochedion javanicum |
Euphorbiaceae |
1 |
2 |
|
|
|
|
|
|
|
|
| Gymnocranthera canarica |
Myristicaceae |
|
1 |
|
|
|
|
1 |
|
|
|
| Holigarna ferruginea |
Anacardiaceae |
4 |
4 |
2 |
1 |
|
|
|
|
|
|
| Holigarna grahamii |
Anacardiaceae |
27 |
19 |
14 |
5 |
|
1 |
1 |
|
|
|
| Hopea ponga |
Dipterocarpaceae |
128 |
58 |
19 |
4 |
3 |
|
|
|
|
|
| Hydnocarpus laurifolia |
Flacourtiaceae |
1 |
|
2 |
|
|
|
|
|
|
|
| Hydnocarpus pentandra |
Flacourtiaceae |
2 |
|
|
1 |
|
|
|
|
|
|
| Ixora brachiata |
Rubiaceae |
17 |
3 |
|
|
|
|
|
|
|
|
| Knema attenuata |
Myristicaceae |
64 |
14 |
7 |
2 |
|
|
|
|
|
|
| Lagerstroemia microcarpa |
Lythraceae |
|
|
1 |
1 |
|
|
|
|
|
|
| Lepisanthes deficiens |
Sapindaceae |
1 |
|
|
|
|
|
|
|
|
|
| Litsea floribunda |
Lauraceae |
14 |
8 |
|
1 |
1 |
|
|
|
|
|
| Litsea ghatica |
Lauraceae |
1 |
|
|
|
|
|
|
|
|
|
| Litsea sp. |
Lauraceae |
12 |
5 |
4 |
|
|
|
|
|
|
|
| Lophopetalum wightianum |
Celastraceae |
1 |
|
3 |
|
1 |
|
1 |
|
|
|
| Macaranga peltata |
Euphorbiaceae |
3 |
1 |
|
|
|
|
|
|
|
|
| Mangifera indica |
Anacardiaceae |
2 |
2 |
2 |
|
|
|
|
|
|
|
| Mastixia arborea |
Cornaceae |
1 |
1 |
1 |
|
|
|
|
|
|
|
| Meiogyne pannosa |
Annonaceae |
5 |
|
|
|
|
|
|
|
|
|
| Mesua ferrea |
Clusiaceae |
1 |
2 |
1 |
|
1 |
|
1 |
|
|
|
| Mimusops elengi |
Sapotaceae |
1 |
1 |
|
|
|
|
|
|
|
|
| Mitrephora heyneana |
Annonaceae |
4 |
|
|
|
|
|
|
|
|
|
| Murraya paniculata |
Rutaceae |
|
|
1 |
|
|
|
|
|
|
|
| Myristica dactyloides |
Myristicaceae |
2 |
3 |
4 |
|
|
|
|
|
|
|
| Myristica fatua |
Myristicaceae |
3 |
2 |
3 |
1 |
|
|
|
|
|
|
| Myristica malabarica |
Myristicaceae |
3 |
1 |
|
|
|
|
|
|
|
|
| Nothopegia colebrookeana |
Anacardiaceae |
16 |
3 |
|
|
|
|
|
|
|
|
| Olea dioica |
Oleaceae |
38 |
25 |
14 |
11 |
6 |
3 |
1 |
|
|
|
| Palaquium ellipticum |
Sapotaceae |
12 |
4 |
7 |
1 |
1 |
1 |
|
|
|
|
| Persia macrantha |
Lauraceae |
2 |
4 |
4 |
1 |
1 |
|
|
|
|
|
| Phoebe cathia |
Lauraceae |
2 |
1 |
|
|
|
|
|
|
|
|
| Polyalthia fragrans |
Annonaceae |
|
1 |
|
|
|
|
|
|
|
|
| Polyalthia sp |
Annonaceae |
1 |
|
|
|
|
|
|
|
|
|
| Pterospermum diversifolium |
Sterculiaceae |
3 |
1 |
|
|
|
|
|
|
|
|
| Randia rhugosa |
Rubiaceae |
1 |
|
|
|
|
|
|
|
|
|
| Symplocos racemosa |
Symplocaceae |
10 |
4 |
4 |
|
|
|
|
|
|
|
| Syzygium cumini |
Myrtaceae |
|
1 |
4 |
|
1 |
1 |
1 |
|
|
|
| Syzygium gardneri |
Myrtaceae |
18 |
7 |
10 |
4 |
1 |
1 |
|
1 |
|
3 |
| Syzigium hemisphericum |
Myrtaceae |
|
3 |
1 |
1 |
1 |
|
1 |
1 |
|
|
| Syzigium laetum |
Myrtaceae |
7 |
|
|
|
|
|
|
|
|
|
| Syzygium macrophylla |
Myrtaceae |
1 |
|
|
|
|
|
|
|
|
|
| Syzigium sp. |
Myrtaceae |
23 |
6 |
1 |
2 |
|
|
|
|
|
|
| Syzigium travancoricum |
Myrtaceae |
3 |
2 |
|
|
1 |
|
2 |
1 |
|
|
| Terminalia paniculata |
Combretaceae |
1 |
2 |
1 |
|
|
|
|
|
|
|
| Tetrameles nudiflora |
Datiscaceae |
1 |
|
|
|
|
|
|
|
|
|
| Vepris bilocularis |
Rutaceae |
|
1 |
1 |
|
|
1 |
|
|
|
|
| Walsura trifolia |
Meliaceae |
2 |
|
|
|
|
|
|
|
|
|
4.6.2 Regeneration status of some selected species: Dipterocarpus indicus is considered to be the characteristic of the climax forests of the southern Western Ghats. Extraction of the valued timber of this species for railways and industrial purposes, beginning with the 1940’s from Kathlekan did not cause its elimination and regeneration (Chandran,1993). Altogether 120 trees in almost all girth classes were recorded from the sampled area (Figure 4.6.2). It was well represented in ground layer also (160 individuals from shrub layer and 11 seedling in herb layer samples). This clearly indicates that species otherwise rare in the northern latitude of 14º has safe future in Kathalekan. This sustainable regeneration is noticed in kan forest only.
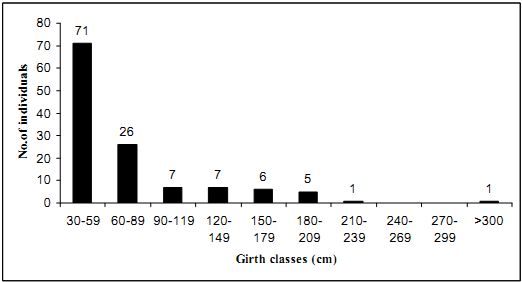
Figure 4.6.2: Girth classes of Dipterocarpus indicus
Calophyllum tomentosum was another prime species that was logged from time immemorial for ship mast and later for plywood or construction purpose. Although the species represented in all girth classes the population is low. There are 21 individual encountered in the survey (Figure 4.6.3). In ground layer it was represented by 6 individuals in shrub layer and 5 in herb layer. Syzigium gardneri is a very large evergreen tree with huge buttress. It occurs in most of the evergreen forest of this district. It is well established because it is represented in every strata in various girth classes. In Kathalekan, 46 trees were counted 25 juveniles in the shrub layer and 8 in the herb layer (Figure 4.6.4). The largest tree in the forest was also Syzigium gardneri with a girth of 405 cm.
Figure 4.6.3: Girth classes of Calophyllum tomentosum
Figure 4.6.4: Girth classes of Syzygium gardneri
4.6.3 Tree regeneration - future scenario: From the regeneration study, it is observed that the regeneration is in progressive way. Since the occurrence of more juvenile recruitment in the ground layer indicates that the disturbance on the forest is at minimum level otherwise it will never survive. The ‘L’ shaped distribution of girth classes among most of the species indicating their climax nature. The flourishing regeneration of some critically endangered species is also hopeful. The rapid recovery from the past annoyance found in Kathlekan is also a good sign of future and indicates the potentiality of the forest.
4.7 Rare, Endangered and Threatened Species (IUCN)
Species can be classified according to their vulnerability to extinction. On the global scale, IUCN provided rigorous definitions known as the ‘IUCN Red List Criteria’ which attempts to classify species according to the likelihood of extinction within a given period. A list of species from Kathalekan which are under the Red listed species based on the Red Data Book on Indian Plants and Report of Conservation Assessment and Management (CAMP) are indicated in Table 4.7.1.
Table 4.7.1: RET (IUCN) species of Kathlekan
| Species |
Family |
Status (IUCN) |
Density (/ha) |
IVI |
| Holigarna grahamii |
Anacardiaceae |
RA |
37.22 |
13.12 |
| Semecarpus kathlekanensis* |
Anacardiaceae |
CR |
|
NE* |
| Sageraea laurifolia |
Annonaceae |
NT |
|
J* |
| Canarium strictum |
Burseraceae |
VU |
1.67 |
1.13 |
| Garcinia gummi-gutta |
Clusiaceae |
LR-NT |
- |
J |
| Garcinia talbotii |
Clusiaceae |
VU |
14.44 |
6.44 |
| Hopea ponga |
Dipterocarpaceae |
EN |
116 |
29.18 |
| Dipterocarpus indicus |
Dipterocarpaceae |
EN |
66.67 |
21.66 |
| Diospyros candolleana |
Ebenaceae |
VU |
15 |
5.45 |
| Diospyros paniculata |
Ebenaceae |
VU |
5 |
2.89 |
| Hydnocarpus pentandra |
Flacourtiaceae |
VU |
1.67 |
0.79 |
| Cinnamomum macrocarpum |
Lauraceae |
VU |
1.11 |
1.10 |
| Persea macrantha |
Lauraceae |
VU |
6.67 |
4.59 |
| Artocarpus hirsuta |
Moraceae |
VU |
2.78 |
2.31 |
| Knema attenuata |
Myristicaceae |
LR-nt |
48.33 |
12.71 |
| Myristica dactyloides |
Myristicaceae |
VU –R |
5 |
2.74 |
| Myristica fatua var. magnifica |
Myristicaceae |
EN |
3.89 |
1.9 |
| Vepris bilocularis |
Rutaceae |
RA |
1.67 |
1.51 |
RA-Rare, CR-Critically endangered, EN-Endangered Nt-Near threatened, Vu-Vulnerable, VU-R-Vulnerable (regional), LR-nt-Lower Risk Near Threatened. (CAMP Workshop, 1997) (NE*-Not Enumerated, J*- Juveniles only)
It is found that 18 out of 111 tree species that occurred in the sampled areas come under the IUCN threat categories. Semecarpus kathlekanensis (though not found in the sampled transects), does occur in a part of the Myristica swamp. As the tree has been newly discovered (Dasappa and Swaminath, 2000), it is yet to gain entry into the Red List. Ecologists working in Kathalekan, however, consider it as “Critically Endangered” (Vasudeva et al., 2001), on account of its extreme rarity and occurrence in the threatened swamps of Kathalekan. Of the total of 18 RET species identified from Kathlekan, 14 species are found to be endemic to the Western Ghats. In fact, bulk of the RET species are found in southern Western Ghats. The occurrence of 18 RET plant species together in Kathalekan is a pointer towards the conservation value of the forest. The 18 RET species belong to 11 families and 15 genera. Prolific growth of endangered species like Hopea ponga and Dipterocarpus indicus enhances the conservation importance of Kathalekan. The endangered Myristica fatua has home in the Myristica swamps of Kathalekan. The swamps as such may be considered northernmost stronghold for the species in the Western Ghats. Among all species Hopea ponga has an estimated high density of 116 individuals/ha and high IVI (29.18) followed by Dipterocarpus indicus (67 individual/ha). Kathlekan is found to be the northernmost limit for distribution of D. indicus in the Western Ghats, sparing one more such locality at Karikan in the adjoining Honnavar taluk. Nine of the species are included in the “Vulnerable” category and two species i.e., Holigarna grahamii and Vepris bilocularis come under the “Rare” category. Lower risk species include Garcinia gummi-gutta and Knema attenuata and Myristica dactyloides, a regionally vulnerable species is also included in the list. As detailed studies on the herbs and epiphytes and climbers could not be undertaken due to the short term nature of the present we are not in a position to comment on RET herbs and climbers. However no RET shrubs occurred in the transect samples.
4.7.1 Regeneration status of RET species: Taking into account the girth classes of most RET species that occurred in the samples it was found that most of the RET individuals occur in lower girth classes. Trees below 30 cm in girth were not considered for this estimate. Of the 335 individuals (trees only) of RET species, only 3 individuals had girth of more than 240 cm. The ‘L’ shaped or inverted “J’ shaped curve, as in Figure 4.7.1 indicates equilibrium condition i.e., mortality is compensated by recruitment. The species like Sageraea laurifolia, Garcinia gummi gutta, were found only in juvenile forms and there were no larger trees identified for these species except outside the transects.
Figure 4.7.1 Girth classes of RET species in Kathalekan
Dipterocarpus indicus was found to be more frequently distributed in nearly all girth classes, indicating that Kathalekan is good refugia for it. Specieslike Knema attenuata, Artocarpus hirsuta, Garcinia talbotii, Hopea ponga, etc also occurred in a wide range of girth classes. Though Semecarpus kathlekanensis did not occur in the transects, it was found that underneath the small population of adult parental trees, there were many saplings and seedlings. The efforts to encroach its habitat by arecanut farmers became a major threat to the existence of the species. However, due to the timely intervention from the Forest Department of Karnataka such a catastrophe was prevented.
Table 4.7.2: Girth classes of RET species
| Species |
Girth classes (cm) |
| Tot. Ind |
30-59 |
60-89 |
90-119 |
120-149 |
150-179 |
180-209 |
210-239 |
240-269 |
270-299 |
>300 |
| Holigarna grahamii |
67 |
27 |
19 |
14 |
5 |
|
1 |
1 |
|
|
|
| Semicarpus kathlekanensis |
NE |
|
|
|
|
|
|
|
|
|
|
| Sageraea laurifolia |
J |
|
|
|
|
|
|
|
|
|
|
| Canarium strictum |
3 |
3 |
|
|
|
|
|
|
|
|
|
| Garcinia gummi-gutta |
J |
|
|
|
|
|
|
|
|
|
|
| Garcinia talbotii |
26 |
5 |
9 |
8 |
3 |
1 |
|
|
|
|
|
| Hopea ponga |
209 |
128 |
58 |
19 |
4 |
3 |
|
|
|
|
|
| Dipterocarpus indicus |
120 |
71 |
26 |
7 |
7 |
6 |
5 |
1 |
|
|
1 |
| Diospyros candolleana |
27 |
24 |
3 |
|
1 |
|
|
|
|
|
|
| Diospyros paniculata |
9 |
4 |
3 |
1 |
1 |
|
|
|
|
|
|
| Hydnocarpus pentandra |
3 |
1 |
|
2 |
|
|
|
|
|
|
|
| Cinnamomum macrocarpum |
2 |
|
|
1 |
1 |
|
|
|
|
|
|
| Persea macrantha |
12 |
2 |
4 |
4 |
1 |
1 |
|
|
|
|
|
| Artocarpus hirsutus |
5 |
1 |
1 |
1 |
1 |
|
|
|
|
1 |
|
| Knema attenuata |
87 |
64 |
14 |
7 |
2 |
|
|
|
|
|
|
| Myristica fatua |
7 |
3 |
2 |
3 |
1 |
|
|
|
|
|
|
| Myristica dactyloides |
9 |
2 |
3 |
4 |
|
|
|
|
|
|
|
| Vepris bilocularis |
3 |
|
1 |
1 |
|
|
1 |
|
|
|
|
| Total |
589 |
335 |
143 |
72 |
27 |
11 |
7 |
2 |
0 |
1 |
1 |
NE: Not enumerated because of occurrence outside the transects, J- Juvenile form only.