| Back | Article |
Next |
The discharge of heavy metals into aquatic ecosystems has become a matter of concern over the last few decades. The pollutants of serious concern include lead, chromium, mercury, uranium, selenium, zinc, arsenic, cadmium, gold, silver, copper, nickel, etc. due to pollutants' carcinogenic and mutagenic nature. These toxic materials may be derived from mining operations, refining ores, sludge disposal, fly ash from incinerators, the processing of radioactive materials, metal plating, or the manufacture of electrical equipment, paints, alloys, batteries, pesticides or preservatives.
The commonly used procedures for removing metal ions from effluents include chemical precipitation, lime coagulation, ion exchange, reverse osmosis and solvent extraction (Juang and Shiau, 2000 ; Yan and Viraraghavan, 2001). These techniques apart from being economically expensive have disadvantages like incomplete metal removal, high reagent and energy requirements, and generation of toxic sludge or other waste products that require disposal. Efficient and environment friendly methods are thus needed to be developed to reduce heavy metal content
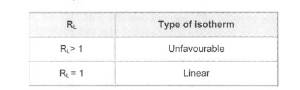
Table 1. Type of Isotherm for various RL.

In this context, considerable attention has been focused in recent years upon the field of biosorption for the removal of heavy metal ions from aqueous effluents (Volesky and Holan, 1995). The process of heavy metal removal by biological materials is known as biosorption. Biomass viability does not affect the metal uptake. Therefore any active metabolic uptake process is currently considered to be a negligible part of biosorption. Various biosorbents have been tried, which include seaweeds, moulds, yeast, bacteria, crab shells, agricultural products such as wool, rice, straw, coconut husks, peat moss, exhausted coffee (Dakiky et al. 2002), waste tea (Amir et al. 2005), walnut skin, coconut fibre (Espinola et al. 1999), cork biomass (Chubar et al. 2003), seeds of Ocimum Basilicum (Melo and dSouza, 2004), defatted rice bran, rice hulls, soybean hulls and cotton seed hulls (Marshall and Champagne, 1995 , Teixeria and Zezzi, 2004), wheat bran, hardwood ( Dalbergia sissoo ) sawdust, pea pod, cotton and mustard seed cakes, (Iqbal et al. 2002 , Saeed et al. 2002).
Figure.1. Langmuir adsorption isotherm for Cr (VI) biosorption by bgh at optimum conditions.
Chromium is a toxic metal of widespread use and exists in several oxidation states. The most stable and common forms are the trivalent Cr (III) and the hexavalent Cr (VI) species, which display quite different chemical properties. Cr (VI) considered to be the most toxic of chromium, is usually associated with oxygen as chromate (CrO42-) or dichromate (Cr2O72- ) ions. The hexavalent form of chromium is considered to be a group “A” human carcinogen because of its mutagenic and carcinogenic properties (Cieslak-Golonka, 1995). Cr (VI) is a common pollutant introduced into natural waters from a variety of industrial wastewaters including those from the textile dyeing, leather tanning, electroplating and metal finishing industries. The untreated effluent from electroplating industry contains approximately 100 mg/l Cr (VI), which is much higher than the permissible limit of 0.05 - 1 mg/l (De Filippis and Pallaghy, 1994).
Figure 2. Freundlich adsorption isotherm for Cr (VI) biosorption by bgh at optimum conditions
In the present study, Bengal gram husk (bgh) (Cicer arientinum ), which is a milling agrowaste available in plenty in a tropical country like India is used for the removal of Cr (VI) ions from aqueous solutions.