Results and Discussion
|
1Energy and Wetlands Research Group, Centre for Ecological Sciences [CES],
Indian Institute of Science, Bangalore – 560012, India.
2 Centre for Sustainable Technologies [CST], Indian Institute of Science. 3Centre for Infrastructure, Sustainable Transport and Urban Planning [CiSTUP], Indian Institute of Science, Bangalore 560 012. *Corresponding author: trv@iisc.ac.in Water pollution in Varthur lake Varthur lake series receives ~590 MLD (million liters per day) of untreated and partially treated sewage daily17, which has sustained the level of nutrients (nitrogen, carbon and phosphorus) resulting in the pollution of the lake. TDS mainly consists of bicarbonates, carbonates, sulphates, chlorides, phosphates and nitrates of calcium, magnesium, sodium, potassium, iron etc. and small amount of organic matter. TDS at V1, V2 and foam were 448 mg/L, 454 mg/L and 7000 mg/L respectively. EC was 749 µS at V1; 764 µS at V2 and 17000 µS in foams. The conductivity increases due to the presence of chloride, phosphate and nitrate in wastewater entering Varthur lake. pH indicates whether water is acidic or basic, ranging from 0 -14. pH at V1, V2 and foam were 7.46, 7.35 and 6.98 respectively. DO is the amount of oxygen dissolved in water. Hypoxic/anoxic condition prevailed in Varthur Lake due to low dissolved oxygen levels, attributed to the high pollution/organic load, extensive macrophyte cover and organic matter decomposition. BOD was higher at all sites i.e., 24.39 mg/L at V1, 60.98 mg/L at V2 and 650.41 mg/L in foam. COD at V1, V2 and foam were 40 mg/L, 88 mg/L and 1140 mg/L respectively. The higher values of BOD and COD indicate increase in organic pollution due to wastewater from household and industrial waste discharges. Varthur lake behaves as an anaerobic - aerobic lagoon18. In addition, surface foams may block aeration of lakes, increase decomposition rate (BOD), and hence deplete DO levels19. Similar instances of foam/froth formation reported in lakes of Bengaluru20. Alkalinity indicates the acid-neutralizing capacity of water and it was recorded 336 mg/L at V1 and V2 whereas 12000 mg/L in foams. The hardness mainly depends on the presence of calcium and magnesium salts, linked with bicarbonates, carbonates, sulphites, sulphates etc. Total hardness at V1, V2 and foam were 206 mg/L, 224 mg/L and 13000 mg/L respectively. Calcium was 57.72 mg/L at V1, 64.13 mg/L at V2 and 3607.2 mg/L in foam whereas magnesium was 15.10 mg/L at V1, 15.58 mg/L at V2 and 974.25 mg/L in foam. Ionic content in water increases due to the water pollution. Chloride at V1, V2 and foam were 117.86 mg/L, 122.12 mg/L and 3195 mg/L respectively. Sodium at V1, V2 and foam were 169.5 mg/L, 161 mg/L and 770 mg/L respectively whereas potassium levels at V1, V2 and foam were 35 mg/L, 34 mg/L and 230 mg/L respectively. Phosphorus and nitrogen are essential nutrients required for all living organisms. Orthophosphate at V1, V2 and foam were 1.263 mg/L, 0.881 mg/L, 74.59 mg/L respectively. The high values of phosphate are mainly due to agriculture runoff, wastewater and detergents. Nitrate at V1, V2 and foam were 0.541 mg/L, 0.361 mg/L and 129.72 mg/L respectively. The major sources of nitrate are fertilizer, agricultural runoff and wastewater. Thus, foam samples collected from the lake had higher concentrations of all the physicochemical parameters compared to lake water samples (at V1 and V2). These results coincide with earlier studies as foams in lakes were enriched with organic and inorganic forms of phosphorus, carbon and nitrogen; chlorinated hydrocarbons; heavy metals and cations21. Both the natural and synthetic foams can collect and concentrate chemical contaminants22. Detergents can increase the ionic/chemical contents in water. Powder detergents add to chemical contamination than liquid detergents by increasing the concentration of TDS, chloride, sulphate, carbonate, bicarbonate and pH of wash water23. 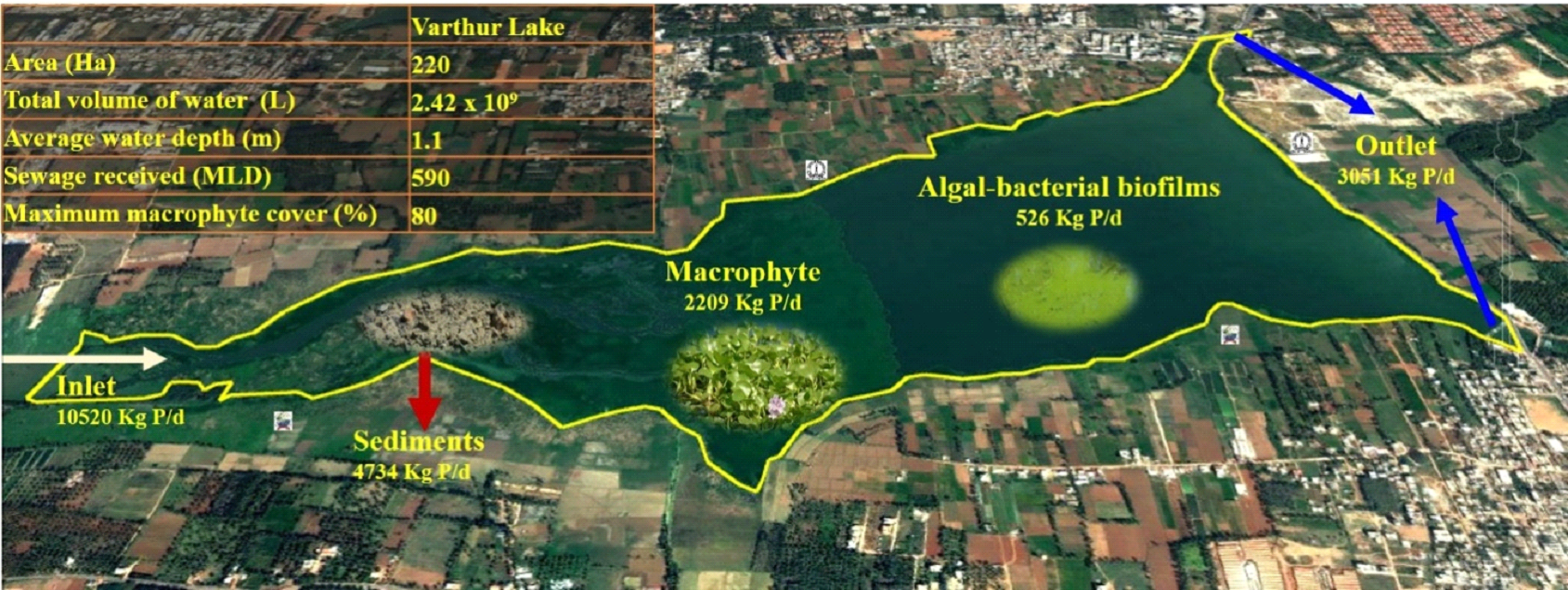 Fig. 6: Phosphorus in Varthur lake Varthur lake receives daily a considerable amount of untreated and partially treated sewage loaded with P, which gets trapped in sediments apart from assimilation by macrophyte, algal-bacterial biofilms etc. P uptake varies widely among biotic components. The foam formation is observed at outlet and is found to contain concentrated nutrients and ions (figure 6). Foam formation in lakes White coloured foam is formed by the interactions between liquid phase, gas phase and surfactants in Varthur lake. Foams have low density and large surface area, exhibiting both solid and liquid like behavior24. Stable foams generated by the process of flotation involve three components (i) air bubbles, (ii) surfactants that reduce the surface tension and (iii) hydrophobic (bacterial) cells25. Foam in Varthur lake had total phosphate (TP) of >2 g/l and orthophosphate of 0.075 g/l which is due to the sustained inflow of sewage (containing detergents) and internal P loading from the lake sediments26. TP concentrations of uncontaminated surface waters ranges between 10-50 μg P/L27. Even a concentration of phosphates (PO4-P) greater than 0.5 ppm causes foam in surface water28. Phosphates are also responsible for the formation of white foam, which acts as a barrier to entry of oxygen and light in the water29. Surface-active agents of municipal wastewater include synthetic detergents, fats, oils, greases and biosurfactants. Synthetic detergents contain phosphates to soften water, increases pH and surfactant efficiency. Detergents cause foaming, eutrophication, limits oxygen production, reduce potable water sources and threaten aquatic life. Nature of the surfactants (whether, anionic/non-ionic synthetic detergents/biosurfactants) and their adsorption to interfaces governed by electrostatic and steric repulsion determines the stability of foam30. The environmental risk associated with the use of surfactants depends on its final concentration in lake water31 since it is toxic and persistent in nature32. The decomposition products of the phytoplankton, fulvic or humic acids, lipidic, proteic or colloidal substances present in water also act as surface-active compounds, producing foams both in marine and freshwater environments33,34. Even proteinaceous and carbonaceous matter from industrial and treatment plant effluents or from natural sources (plankton, higher plants and microorganisms) acts as a surface-active agent, reducing the surface tension and create foams35. Both surfactants and cells together form stable foams, whereas only surfactant (without bacterial cell) forms unstable foams in lakes. Foam formation never occurs in the absence of surfactants36. Surfactants have a polar, hydrophilic head group and a nonpolar, hydrophobic hydrocarbon tail group37 and are of four types namely (i) anionic, (ii) cationic, (iii) amphoteric and (iv) nonionic, depending on the charge of their head group38. Surfactants form films on lakes and hinder evaporation of water and transport of gases across the aqueous interface39. Skermania piniformis, Rhodococcus sp., Microthrix parvicella and Gordonia sp. are foam-causing organisms growing on oil and hydrocarbons in wastewater40. Surfactants as well as foam are responsible for reducing oxygen levels41, which affects aquatic life. Surfactants deteriorate water quality by creating foams in water bodies42, which are harmful to fishes, vegetation and alsohuman beings. Foam transfers micro-contaminants and toxic metals into the food web, induces various chemical and physical interactions among components of foam and transports chemicals to the atmosphere through bubble breaking and wind-suspension processes43. Effect of pollution on aquatic organisms The organisms such as primary producers (phytoplankton) and consumers (zooplankton) of foodweb, which inhabit the surface of lake, will be exposed to these contaminants. Among phytoplankton, Chlorophyceae members dominated Varthur lake indicating the presence of higher amounts of dissolved carbon contents. The phytoplankton population comprised of Chlorella sp., Monoraphidium sp., Dictyosphaerium sp., Chlamydomonas sp., Micracitinium sp., Scenedesmus sp., Pandorina sp., Schroederia sp., Pediastrum sp., Golenkinia sp., Oscillatoria sp., Chroococcus sp., Spirulina sp., Anabaena sp., Planktothrix sp., Merismopedia sp., Microcystis sp., Pinnularia sp., Nitzschia sp., Navicula sp., Amphora sp., Cyclotella sp., Aulacoseira sp., Synedra sp., Euglena sp., Phacus sp., Lepocinclis sp. and Trachelomonas sp. Zooplankton in Varthur lake includes Brachionus quadridentatus, Brachionus plicatilis, Brachionus rubens, Brachionus calyciflorus, Brachionus diversicornis, Philodina sp., Brachionus angularis, Lecane luna, Platyias quadricornis, Cephalodella sp., Arcella sp., Vorticella sp., Paramecium sp., Chironomid larvae, Moina micrura, Chydorus sphaericus, Moina brachiata, Mesocyclops sp., Mesocyclops leuckarti and Microcyclops varicans. Dominant are protozoa and rotifers, which indicates deterioration of water quality with nutrient enrichments (eutrophic conditions). Earlier, Varthur lake supported species like Catla catla, Labeo rohita, Cirrhinus mrigala, Clarias gariepinus, Oreochromis mossambica, Clarias batrachus, Heteropneustes fosslis, Mystus dittatus and Minor carps44. The sustained inflow of untreated or partially treated wastewater has contributed to nutrient enrichments leading to the profuse growth of macrophytes, which has hindered the solar energy penetration in most part of the lake and affected the producers. This has led to the decline of native species of fish and the frequent mortality of Clarias gariepinus were reported. However, the fish culture involving exotic species of fish has led to the dominance of exotic invasive species. Phosphate increases primary productivity but several studies showed the adverse effects of detergent on aquatic life45-47. Even low concentrations (0.003 mg/L) of detergent effluent induce various toxicological effects and histological abnormalities in Clarias gariepinus, which depends on exposure time and toxicant concentration48,49. Wastewater from automobile service stations (depending on wastewater concentration and exposure period) are toxic to freshwater fish, Clarias gariepinus50. Life Cycle Assessment (LCA) of detergents indicates that during production and consumption stages, affects severely ecosystems. Thus, recovery of detergents would help in reducing the environmental impacts of laundering industry51. Phosphate loadings and accumulation in urban lakes Phosphorus (P) plays a crucial role in the productivity of aquatic ecosystems. Various abiotic and biotic processes control P dynamics in sediments containing varied quantities of organic, inorganic and microbial P52. Phosphorus input to water bodies occurs in rural areas through agricultural run-off from adjacent fields with an increased use of phosphate-containing fertilizers and manure, while in urban localities through anthropogenic activities involving excessive use of laundry detergents and discharges from industries. The threshold values for anionic, cationic and non-ionic detergents that are detrimental for aquatic life are 3-12, 20 and 3-38 mg/l respectively53. Increase in phosphorus concentrations promote algal blooms and aquatic plant (macrophyte/weed) growth that adversely affects the biodiversity, water quality, fish population as well as the recreational value of lakes. During algal bloom, luxury P uptake by algae will occur and thus P accumulates as inositol polyphosphate. If concentration of orthophosphate in lake water reduces, about 90 percent of polyphosphates within the algal cell are released enzymatically back into lake water within 24 hours. Similar P release (60% of OP) occurs under anaerobic conditions within 3 hours54. Suspended particulate matter (SPM) includes all suspended particles, both inorganic and organic in freshwater ecosystems55. Generally, litter in water decomposes rapidly than standing dead material56. Both macrophyte and periphyton plays an important role in P retention and nutrient turnover. During photosynthesis and at high pH, periphytons induce P precipitation with calcium and increases P adsorption at oxic conditions near sediment surface57. Among periphytons, P uptake rate was higher in epiphyton compared to metaphyton and epipelon58. The P content in lakes vary widely (figure 7 and table 1). The organic phosphorus compounds include inositol phosphates, monoesters, diesters and phosphonates59. In India, total inputs of P through wastewater (from detergents) is between 41,000 to 145,555 tonnes/annum. These detergent phosphates in the form of STPP (sodium tri-polyphosphates) and phosphate from human waste (feces and urine) reaches surface water bodies and with the sustained inflow of untreated wastewater promotes eutrophication and froth formation. During pre-monsoon, high velocity wind coupled with the high intensity rainfall leads to the churning of the lake with upwelling of sediments and release of trapped phosphates, which contributes to the large-scale frothing. Figure 8 illustrates pollution in urban lakes with the sustained inflow of phosphorus enriched municipal wastewater and its consequent effects. Various sources of P like untreated domestic and industrial sewage, detergents and fertilizers from agricultural fields reach urban lakes through run-off and leaching. These phosphates will stimulate the growth of phytoplankton and aquatic plants (macrophyte). Phytoplankton forms the base of the food chain, provides food, and transfers energy to higher trophic levels (zooplanktons, fishes, birds and humans). This leads to eutrophication with the extensive and dense growth of macrophytes creating anoxic condition in the underlying layers with malodor generation, which chokes fishes eventually leading to their death, reducing the overall biodiversity and productivity of lake ecosystems. Table 1: Phosphorus content in urban lakes
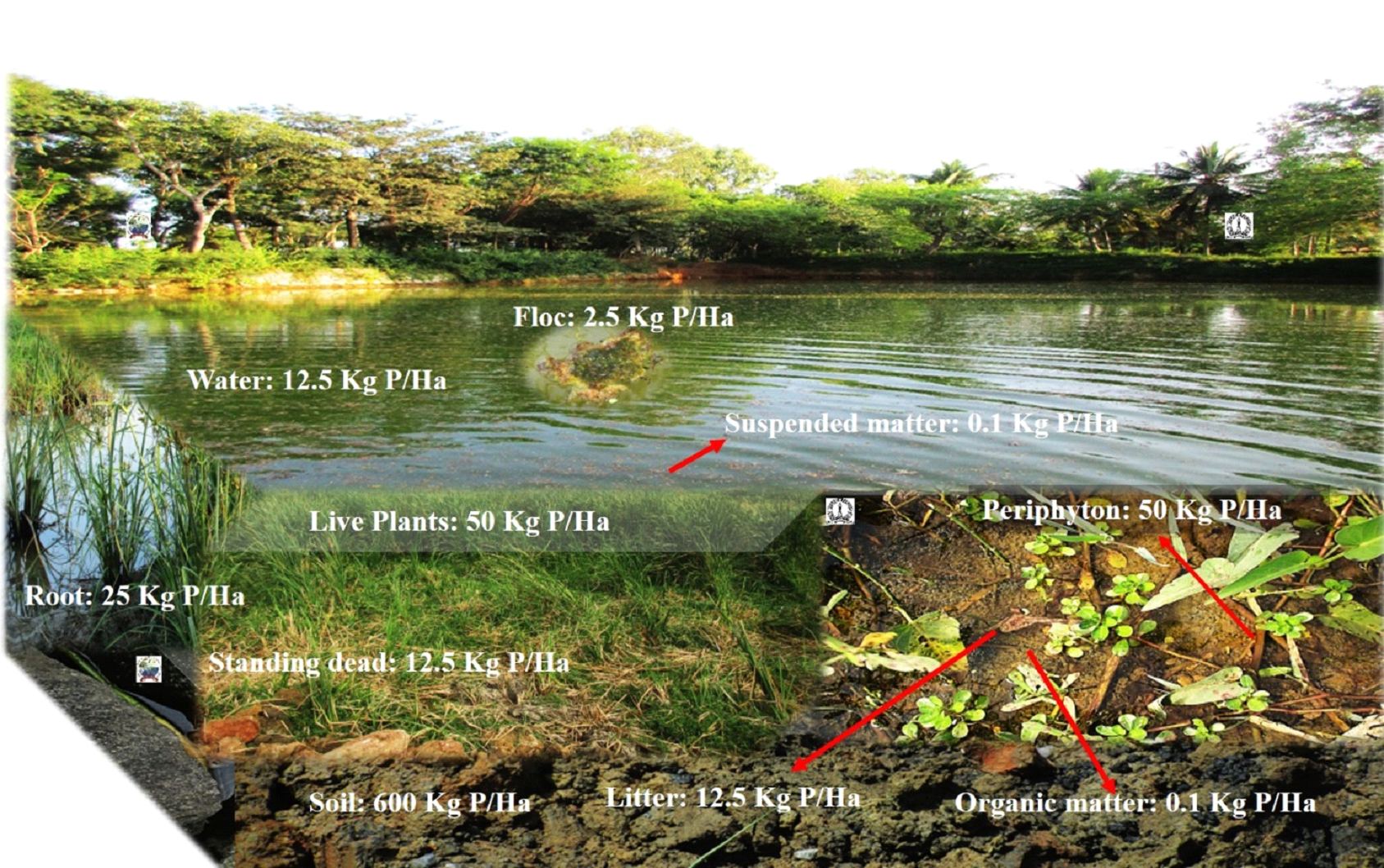 Fig. 7: Phosphorus dynamics in sewage fed urban lakes 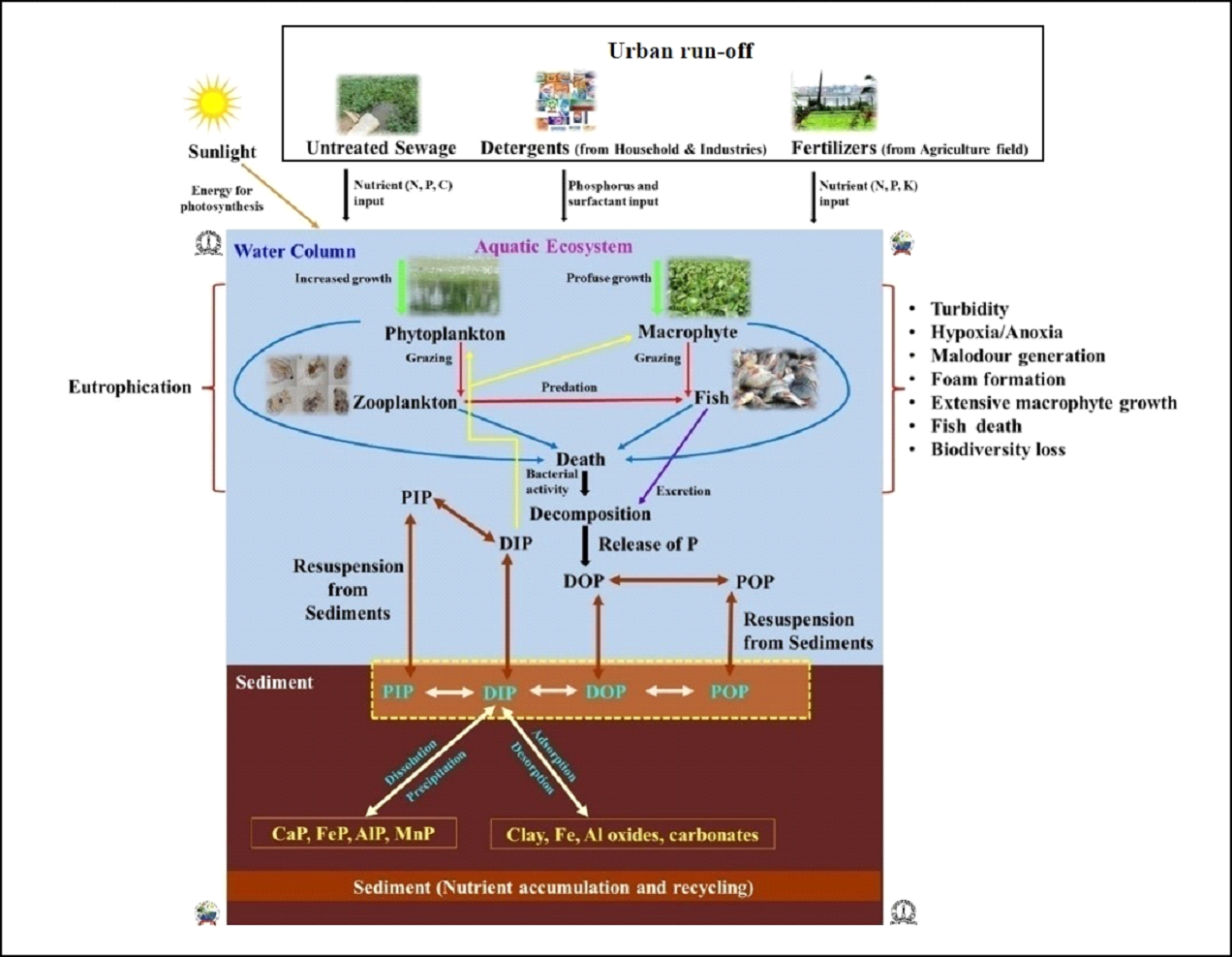 Fig.8: Sources of phosphorus and its adverse effects on lakes P found as particulate organic P (POP), dissolved organic P (DOP), particulate inorganic P (PIP) and dissolved inorganic P (DIP). Phytoplankton can readily utilize dissolved inorganic phosphates (DIP) which, later incorporated into cells in the form of organic molecules. Upon excretion, death and decomposition of aquatic inhabitants, DOP releases. In the sediment part, heterotrophs mediate transformations from POP to DOP to DIP to PIP. The DIP and PIP again released back to water column. Conversion of POP to DOP facilitated by phytoplankton and prokaryotes. These conversions are mainly dependent on pH, alkalinity, temperature, redox potential, mineral concentration and oxic/anoxic conditions (figure 8). DIP levels increases to 50-100 μg P/L due to agricultural runoff and to above 1000 μg P/L from municipal sewage69. As the pollution increases with the sustained loading of P, leads to accumulation in sediments. Algae prefers non-apatite inorganic P for their growth and metabolism70. Dissolved organic phosphorus (DOP) can play an important role in biological and biogeochemical processes71 and occurs commonly in agricultural, municipal and animal wastewaters72. Domestic and industrial wastewater contains increased level of P due to usage of detergents, soaps and cleaning materials73, which may increase pollution load in lakes74,75. This will lead to algae bloom and massive growth of aquatic macrophytes76 that threatens the freshwater resources and recreation77. Oxygen depletion due to increased decomposition of different aquatic macrophytes, weeds and phytoplankton leads to fish kill and loss of biodiversity in eutrophic lakes78,79. Approximately, 20% of phosphate rock is mined for making detergents, animal feeds and industries80. Thus, it is better to use green and eco-friendly detergents to avoid adverse effects on lakes81. 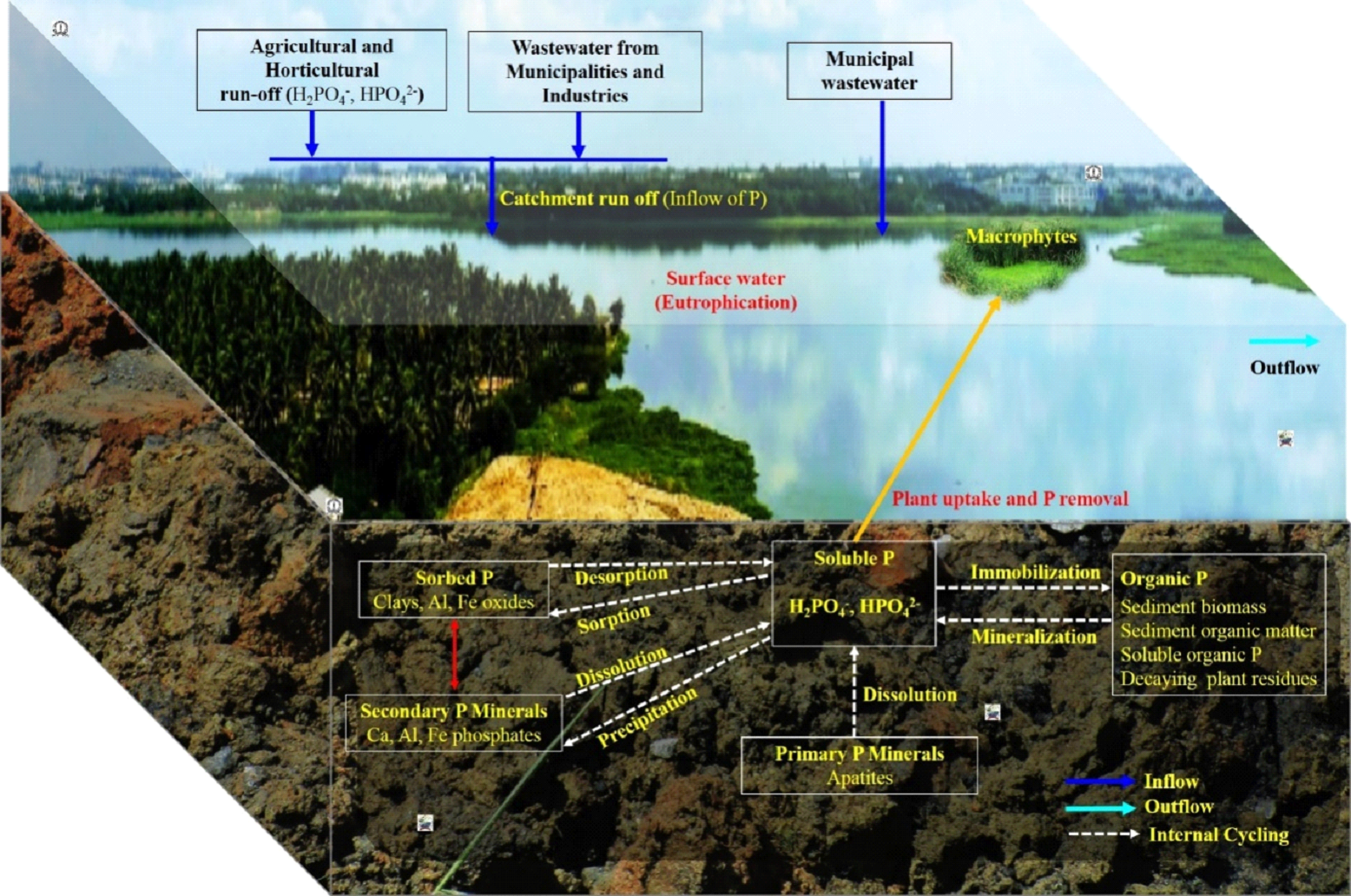 Fig. 9: Phosphorus cycling in lakes The dynamics of phosphorus in sediments of lakes are shown in figure 9. The external loading of nutrients from agricultural, horticultural and urban sources (wastewater) contributes to eutrophication. Inorganic orthophosphate (HPO42− or H2PO4−) is the most bioavailable and mobile form of P exchanged between the lake sediments and water column. The bacterial community, algae, biofilms and aquatic macrophytes contributes to organic forms of P. Organic P is not available directly to aquatic organisms and thus need to convert to inorganic orthophosphate. P transformations in sediments involves a series of processes (sorption/desorption; dissolution/precipitation; immmobilisation/mineralisation) depending upon factors like temperature, pH, redox reactions and the available concentrations of iron, calcium and aluminum. Inorganic P can be either adsorbed (chemically bound) to suspended/settled sediments or desorbed (release of adsorbed P). During adsorption, phosphorus is bound to the sediment surface i.e., on clay surfaces or iron and aluminum oxides and hydroxides in sediments. While by desorption, the adsorbed phosphorus is released into the water. Orthophosphate remain adsorbed under aerobic conditions with high redox potential whereas desorbed under anaerobic conditions with low redox potential54. P gets precipitated with Ca (calcium), Fe (iron), Al (aluminium) and Mn (manganese) complexes (illustrated with equations next). Thus, ferric oxyhydroxides, calcium phosphate (apatite), aluminium oxyhydroxides, carbonates and clay are carriers of phosphates in lake sediments. These metal phosphates can release P back into water upon dissolution. Organic phosphorus is converted into inorganic phosphorus with the help of microorganisms through mineralization process. Whereas, inorganic phosphorus is converted back to organic forms and are absorbed by the microbial cells through immobilization. The internal P loading from sediments to surface water increases the concentrations of P in water column even though there is a reduction in external loads (figure 9). Organic matter is a heterogeneous mix of decayed plant tissues and animal tissues; microbes (fungi and bacteria); humic substances; carbohydrates; lipids and amino acids. Organic matter competes with P for binding sites and inhibits the Fe and Al oxides crystallization, thus, reducing the P sorption capacity82. Ionic strength affects phosphate sorption at the sediment-water interface83. Phosphate uptake and solubility in lake sediments is controlled by either redox potential/pH/both84. At high temperatures during summer, the pH at sediment-water interface increases whereas the redox potential decreases. Variations in redox potential also occur due to changes in DO (dissolved oxygen) and bacterial metabolism. Under anoxic conditions at the sediment-water interface, P release to overlying water is 7 to 10 times higher than under aerobic conditions54. Lastly, biological P uptake by benthic organisms also cause P removal from the lakes. Phosphorus recovery from wastewater The global phosphate demand can be met efficiently through recovering P from wastewater. Estimates indicate that phosphorus recovered from human wastes (urine and feces) could account for 22% of the total global phosphorus demand85 as an individual excretes about 550 L urine/year, which is equivalent to 0.4 kg of phosphorus (P), 4 kg of nitrogen (N) and 0.9 kg of potassium (K) per year86. Urine is rich in nutrients and has high hygienic quality for utilizing as a fertilizer87. Urine contains ions like Na, K, NH4, Ca, C1, PO4, SO4 and HCO388. Many studies have reported recovery of phosphorus from municipal wastewater, sewage sludge and sewage sludge ash89-91. Biomass ash derived from olive, sludge, meat and bone meal (MBM) and poultry litter has high P content (~5.4% by weight) useful for agricultural land92. P recovery from sewage sludge ash (SSA) is most promising than liquid phase and sewage sludge as it ensures high recycling rate, eliminates organic micropollutants, heavy metal decontamination, reduces gaseous emissions and energy demand93,94. Recovery of P from sludge is achievable by anaerobic digestion, wet chemical extraction (comprising either acid or alkaline dissolution) and incineration of sludge at high temperatures95. The overall P recycling efficiency from wastes was 51% in France, wherein the efficiency was 74.6% for food processing waste, 43.1% for household wastewater and 47.4% for municipal waste96. Recovering P from human discharge (a maximum of 3.7 Mt P) added to wastewater can satisfy major fraction of the global agricultural/fertilizer demand97. Phosphorus control and alternatives In order to reduce the nutrient level in lakes, treated water from sewage treatment plants (STPs) should be allowed to pass through an integrated wetland model that consists of constructed wetlands and shallow algae pond as in Jakkur lake, Bengaluru98. Dredging of lakes will remove nutrient rich bottom sediments and reduces the internal phosphorus loading99. Phosphorus control measures include removal of phosphorus from municipal and industrial wastewater, ban of phosphorus in laundry detergents and other cleaning agents, control of agricultural and urban runoff100. Pollution prevention measures should be adopted by reducing phosphate use, reusing or removing phosphorus from wastewater through improved wastewater treatment plants which aids in the phosphorus recovery (by precipitating struvite) process. In addition, the use of locally recovered phosphorus can provide farmers with fertilizer as well as food security101. The replacement of sodium tripolyphosphate (STPP) as builder in detergents with Zeolite A helps to prevent eutrophication of surface waters. Since phosphate rocks are depleting, use of STPP need to be minimized as STPP is produced mainly from phosphate rock, sulphuric acid and sodium hydroxide/soda ash102. The internal loading of phosphate can be reduced by adopting different restoration methods such as dredging of lake sediments. P recycling (through urban wastewater treatment and food waste recycling) and use reduction (phosphorus substitution in beverages with alternatives, substitution of P in laundry detergents with zeolites, food wastage reduction, application of phosphorus solubilizing biofertilizing micro-organisms etc.) is expected to significantly improve the longevity of P resource103. Restrictions on phosphate use in detergents manufacturing and ban on detergents containing phosphorous would lower the phosphate load. Entry of P rich detergents to aquatic environments needs to be restricted to avoid eutrophication104. The BIS (Bureau of Indian Standards)105 has laid down the standards for eco-labelling of detergents (known as Ecomark) in India. The standards recommend product shall not contain phosphates and need to replace phosphates with alternatives/substitutes that are environment-friendly and biodegradable surfactants used for manufacturing of laundry detergent powders, and packaging material of the product must be recyclable, reusable or biodegradable. However, none of the detergent brands in the Indian market has opted for the Eco-mark or demonstrated environment-friendliness of a product or enlisted critical ingredients in terms of quantity (active ingredients, builders, soda ash, fillers and enzymes). In India, the expensive and popular brands of detergents still have high phosphates and STPP (sodium tripolyphosphate) compared to the cheaper detergents. This emphasizes the need for stringent environmental norms to mitigate wasteful use of phosphates in manufacturing detergents and ban on phosphate-based detergents to save fragile water bodies from eutrophication.
Citation : Ramachandra T V, Asulabha K S and Sincy, V. 2021. Phosphate Loading and Foam Formation in Urban Lakes, G P Globalize Research Journal of Chemistry
5(1)33 – 52
|
||||||||||||||||||||||||||||||||||

