|
Materials and Methods
Bangalore is the principal administrative, cultural, commercial,
industrial and knowledge capital of the state of Karnataka.
Greater Bangalore spatial extent was expanded to 741 km²
includes the city, neighboring municipal councils, and
outgrowths, in December 2006. The field
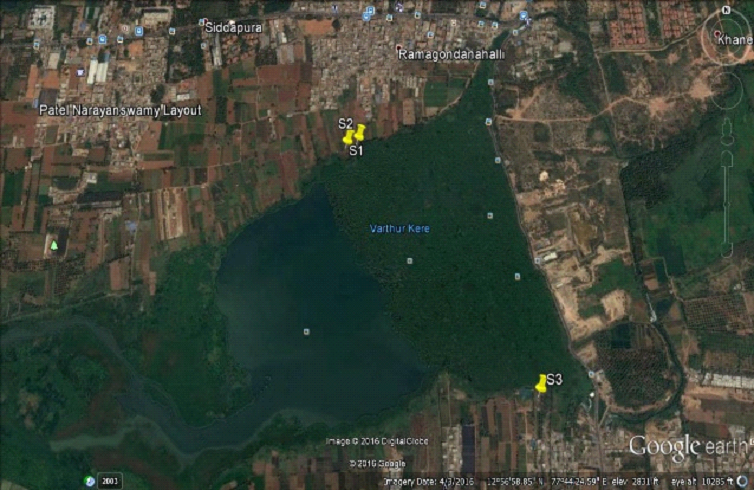
sampling was done in the vicinity of Varthur Lake.
Varthur Lake: Varthur Lake is situated in the
eastern periphery of Bangalore City and a part of the
internationally famous Whitefield Township. Varthur is a Hobli
and part of Bruhat Bangalore Mahanagara Palike (BBMP). The lake
ecosystem is an integral part of Bangalore, although unplanned
urbanization and industrialization have led to the contamination
of these water bodies. Varthur Lake, with a spatial extent of
216 ha, is the second largest lake in Bangalore city. Recently,
siltation and encroachment, the spatial extent of the lake has
been reduced to 165.75 ha and also one of the most polluted
lakes in Bangalore due to (i) the sustained inflow of untreated
and partially treated domestic sewage, (ii) discharge of
untreated industrial effluents (through stormwater drains and
through trucks (who discharge untreated effluents), (iii)
dumping of solid waste in the lake, drains and in the buffer
zone. The wetland water accounts to irrigate 625 ha of
agricultural fields in the command area for growing crops like
rice, ragi millet, coconut, flowers, and various fruits and
vegetables. Earlier reports have indicated the possible uptake
of trace elements by plants grown in the command area of the
lake (Ramachandra et al., 2011; Jumbe and Nandini,
2009). Field samples of vegetables grown using the lake water in
the command area of Varthur Lake (Varthur) were collected to
assess the level of heavy metal uptake (figures 1 - 3).
Heavy metal analysis: The collected vegetable
samples were analyzed using ICP-OES (Inductively Coupled
Plasma-Optical Emission Spectroscopy) technique to assess the
level of heavy metal in acid digested samples.
Digestion procedure: For the water sample, the
digestion was carried out with an acid combination of nitric and
sulfuric acids at a ratio of 10:1. The plant samples were
digested using nitric, sulfuric, and perchloric acids
combinations of 5:1:1. Nitric and sulfuric acid combination 5:1
was used for the digestion of soil samples. A temperature range
of 60-70˚C was maintained during digestion of samples and
additional volumes of acids were added till the sample becomes
transparent. The digested samples were transferred into a 50 ml
standard and made up to the mark using distilled water. The
samples were then transferred into small plastic (tarsons)
bottles, capped and labelled (APHA, 1999).
Preparation of standards: A stock solution of
1000 ppm was prepared for the analysis of heavy metals such as
Cd, Cu, Pb, Cr, and Ni. The chemicals used for standard
preparation are; Cadmium sulphate (Cd), Potassium dichromate
(Cr), Copper sulfate (Cu), Lead nitrate (Pb), and Nickel sulfate
(Ni). Standards were prepared in a range of 1, 10, and 20 ppm
from the stock solution in 2% HNO₃. The prepared standards were
then fed into ICP-OES to obtain a calibration curve.
ICP-OES: Emission spectroscopy using ICP was
developed in the mid-1960’s as a rapid, sensitive, and
convenient method for the determination of metals in water and
wastewater samples. Dissolved metals are determined in filtered
and acidified samples. Total metals are determined after
appropriate digestion. Care must be taken to ensure that
potential interferences are dealt with, especially when
dissolved solids exceed 1500 mg/L.
Principle: ICP source consists of a flowing
stream of argon gas ionized by an applied radiofrequency field
typically oscillating at 27.1 MHz. This field is inductively
coupled to the ionized gas by a water-cooled coil surrounding a
quartz ‘‘torch’’ that supports and confines the plasma. A sample
aerosol is generated in an appropriate nebulizer and spray
chamber and is carried into the plasma through an injector tube
located within the torch. The sample aerosol is injected
directly into the ICP, subjecting the constituent atoms to
temperatures of about 6000 to 8000°K. Significant reduction in
chemical interferences is achieved with the almost complete
dissociation of molecules. The light emitted from the ICP is
focused onto the entrance slit of either a monochromator or a
polychromator that affects dispersion. A precisely aligned exit
slit used to isolate a portion of the emission spectrum for
intensity measurement using a photomultiplier tube. The
monochromator uses a single exit slit/photomultiplier and may
use a computer-controlled scanning mechanism to examine emission
wavelengths sequentially. The polychromator uses multiple fixed
exit slits and corresponding photomultiplier tubes; it
simultaneously monitors all configured wavelengths using a
computer-controlled readout system (APHA, 1999).
Procedure: Initially, the instrument was
calibrated using blank. After calibration with blanks, standards
of three different concentrations were fed alternating with
blank to obtain a calibration curve. The samples were fed into
the instrument in a similar way to standards. At the end of the
analysis, blank was again fed into the instrument. The results
were obtained via a computerized readout system.
Calculations: Dilution correction factor = Final
weight or vol. / Initial weight or vol.
Interference correction factor, K = Apparent conc. of element /
Actual conc. of an interfering element.
Figure 1: Varthur lake and sampling sites
|
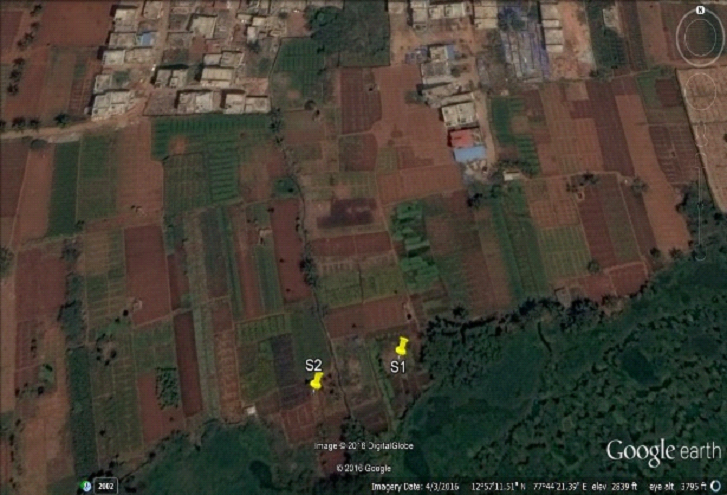
Figure 2: Sampling sites 1 and 2 (N: 12.95247, E:
077.73963 and N:12.95277, E: 077.73876) |
|
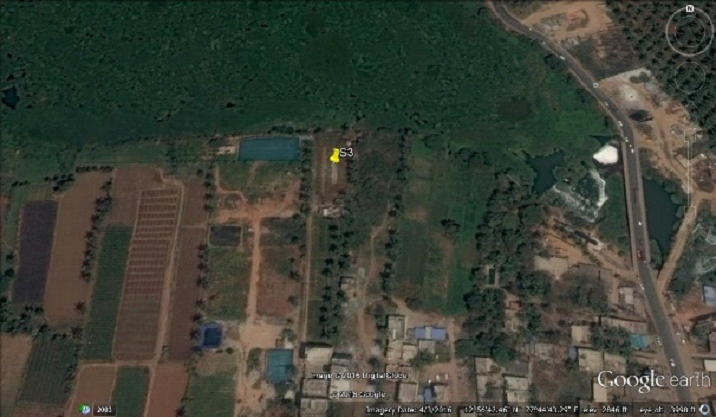
Figure 3: Sampling site 3 (N: 12. 94473, E:
077.74554)
|
|