|
|
Results and Discussion
3.1 Water Quality Analysis
-
Dissolved Oxygen: Dissolved oxygen (DO) is the most essential parameter in the aquatic ecosystems as it helps in the aquatic respiration as well as detoxification of complex organic and inorganic mater through oxidation. However, the sustained disposal of organic materials after rituals can impose a very high oxygen demand leading to oxygen depletion with severe impacts on the water ecosystem. The temple debris and the left out matter from the cultural practices can impart significant amount of oils, volatile organics, nutrients and solids in the pond. The DO of the analysed water samples varied between 2.7 (site 1) to 11.3 ppm (site 3). The higher variations of DO especially lower DO values are indicative of fast oxidising chemicals/bacterial activity in the ecosystem. Lower values of DO highlight the impact of disposing organic materials (such as rice balls, flower, etc.) in the pond. The shallower region (site-3) showed the highest DO levels indicating less abuse of the place compared to other sites.
-
Total Dissolved Solids (TDS): TDS affect the water quality in many ways impacting the water usage for domestic (drinking, cleaning, bathing, etc.). Total dissolved solids originate from organic sources such as leaves, silt, plankton, organic debris and also from sewage. Other sources come from runoff from nearby areas (APHA, 2000). Surface as well as groundwater with high dissolved solids are of inferior flavour and induce an unfavourable physiological reaction to the dependent population. TDS values in the samples analyzed, ranged from 38.5 to 41 ppm across all locations. It was higher at site 2, where disposal of organic materials is higher. TDS was comparatively high at locations with higher human activities such as washing of utensils, disposing rituals organic materials (rice balls, etc.).
-
pH: pH is a numerical expression that indicates the degree to which water is acidic or alkaline, with the lower pH value tends to make water corrosive and higher pH has negative impact on skin and eyes. pH value of water samples of the pond are acidic and ranged from 5.8 – 6.1. A slightly lower pH value can be attributed to either the humic and fulvic acids (high detrital matter) or due to the volatile organic acids emanating from the decomposing and semi decomposed organic materials (of rituals). Also, aquatic macrophytes that were cut were left in the shallow region of the pond and with decomposition it is emanating low molecular weight organic acids.
- Turbidity: Turbidity indicates the amount of suspended matter that are of a colloidal dimension. All water samples showed a very low turbidity indicating no suspended micro algae. The values ranged from 1.69 (site 3) to 4.81 NTU (site 2). Slightly higher values at site 2 is due to the disposal of organic materials after ritual.
- Chlorides: Chlorides are essentially potential anionic radical and excess of chlorides leads to the formation of potentially carcinogenic and chloro-organic compounds. Chloride values in samples ranged from a mere 22.72 – 24.14 ppm. Relatively higher values were observed in sites 2 and 3. It may be due to washing (cloth and utensils) with bleaching powder or chloride detergents.
- Sodium: Sodium (Na) is one of the essential cations that stimulate various physiological processes and functioning of nervous system, excretory system and membrane transport in animals and humans. Increase of sodium ions has a negative impact on blood circulation, nervous coordination, hence affecting the hygiene and health of the nearby localities. The concentration of sodium in the pond water samples were low and ranged from 21.6 (site 2 and 3) to 22.8 ppm (at the deeper reaches, site 1). Sodium values were significantly correlated with chlorides (r= 0.99; p<0.0001) and Ca Hardness ((r= 0.99; p<0.0001).
- Potassium: Potassium (K) is an essential element for both plant and animal nutrition, and occurs in ground waters as a result of mineral dissolution, decomposing of plant materials and also from agricultural runoff. Potassium ions in the plant root systems helps in the cation exchange capacity to transfer essential cations like Ca and Mg from the soil systems into the vascular systems in the plants in replacement with the potassium ions (Mahapatra et al., 2011a). Incidence of higher potassium levels in soil system affects the solute transfer (active and passive) through the vascular conducting elements to the different parts of the plants. The potassium content in the water samples of Gokarna tank ranges between 3.2-4.8 ppm. Higher potassium values were at disturbed regions (site 2) due to the sustained disposal of organic materials (of rituals).
- Alkalinity: Alkalinity is a measure of the buffering capacity of water contributed by the dynamic equilibrium between carbonic acid, bicarbonates and carbonates in the water. Sometimes excess of hydroxyl ions, phosphate, and organic acids in water causes alkalinity. High alkalinity imparts bitter taste. The acceptable limit of alkalinity is 200 ppm. The alkalinity of the samples ranges from 64 – 76 ppm. A comparatively higher value was observed in site 2 owing to the disposal of organic materials (rice balls, flowers, etc.) after performing rituals.
- Total hardness: Hardness is the measure of dissolved minerals that decides the utility of water for domestic purposes. Hardness is mainly due to the presence of carbonates and bicarbonates. It is also caused by variety of dissolved polyvalent metallic ions predominantly calcium and magnesium cation although, other cations like barium, iron, manganese, strontium and zinc also contribute. In the present study, the total hardness ranged between 22 to 24 ppm. It was relatively high at site 2. Hardness is significantly correlated with the EC values (r= 0.9; p<0.05).
- Calcium hardness: Calcium (Ca) is one of the major macro nutrients which are needed for the growth, development and reproduction of both plants and animals. The presence of Ca in water is mainly due to its passage through deposits of limestone, dolomite, gypsum and other gypsiferous materials (APHA, 2000). It contributes to the total hardness of the water. Ca hardness concentration in the samples analysed varied from 3.74 to 4.81 ppm.
- Magnesium hardness: Magnesium (Mg) in one of the most essential macro nutrients that helps as a co-factor in the enzyme systems and in the central metal ions that constitutes the chlorophyll molecule essential for plant photosynthesis. According to WHO guidelines the maximum admissible limit is 50 ppm. In this study the concentration of magnesium hardness ranged from 4.18-4.66 ppm. Mg hardness was significantly correlated with turbidity (r= -0.9; p<0.05).
- Nutrients (nitrates and phosphates): Nutrients essentially comprise of various forms of N and P which readily dissolve in solutions that are uptaken in the form of inorganic mineral ions by plant root systems through microbes. Accumulation of N (as nitrates) and P (as inorganic P) in aquatic ecosystems leads to higher net productivity causes with significant water quality problems. Sustained input of organic materials (of rituals consisting of large quantity of rice balls, banana, flower, etc.) beyond the supportive and assimilative capacities of the pond has resulted in nutrient enrichment and profuse growth of invasive floating plant species. Together with phosphorus, nitrates in excess amounts in streams and other surface waters can accelerate aquatic plant growth causing rapid oxygen depletion or eutrophication in the water. Nitrates at high concentrations (10 mg/l or higher) in surface and groundwater used for human consumption are particularly toxic to young children affecting the oxygen carrying capacity of blood cells ( RBC) causing cyanosis (methemoglobinemia). In the present study, nitrate values ranged from 0.15 to 0.23 ppm. Nitrate values were significantly correlated with TDS (r= -0.9; p<0.05). The phosphate values ranged between 0.2 to 0.4 ppm. Ponds with phosphate values >0.02 ppm have been reported as eutrophic in nature.
- BOD and COD: BOD and COD are important parameters that indicate contamination with organic wastes. Biochemical oxygen demand (BOD) is the amount of oxygen required by bacteria while stabilizing decomposable organic matter under aerobic. These parameters help to assess the extent of pollution of surface and ground water where contamination occurred due to the organic inputs (of rituals) to the pond. Figure 3.1 indicates the type of activities around the pond.

Chemical oxygen demand (COD) determines the oxygen required for chemical oxidation of most organic matter and oxidisable inorganic substances with the help of strong chemical oxidant. In conjunction with the BOD, the COD test is helpful in indicating toxic conditions and the presence of biologically resistant organic substances (Sawyer and McCarty 1978). In this study the BOD values ranged from 4.07-8.13 ppm. BOD values were negatively correlated with EC values (r= -0.9; p<0.05). The COD values ranged from 12 to 24 ppm. The COD were relatively high at site 2. The ORP values indicate highly oxidising conditions in the temple pond. The physicochemical analysis of the select sampling sites is elucidated in Table 3.1.

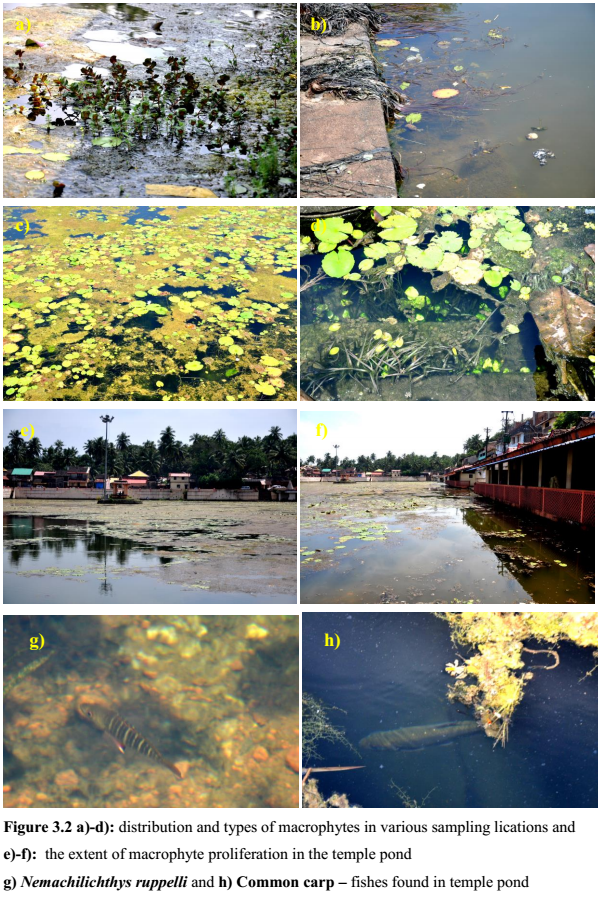
During the site visit it was observed that ~70 % of the temple pond surface was covered by macrophytes and to minor extent filamentous algae. The extent and spread of macrophytes was higher in the shallower regions of the pond. Eleven macrophytes species (Table3.2) were recorded during the field investigation.
Table 3.2: Macrophytes of Gokarna temple pond
Macrophytes observed in the pond are i) submerged floating leaf type e.g. water lily etc., and ii) submerged e.g. Ceratophyllum, Blyxa, Valisneria. Macrophytes specimen were collected through opportunistic sampling from accessible parts of the pond. Most of the samples were collected either by hand or with the help of a wooden pole. Macrophytes near the shallow regions were trapped between filamentous cyanophycean members as Oscillatoria and Pseudoanabaena.
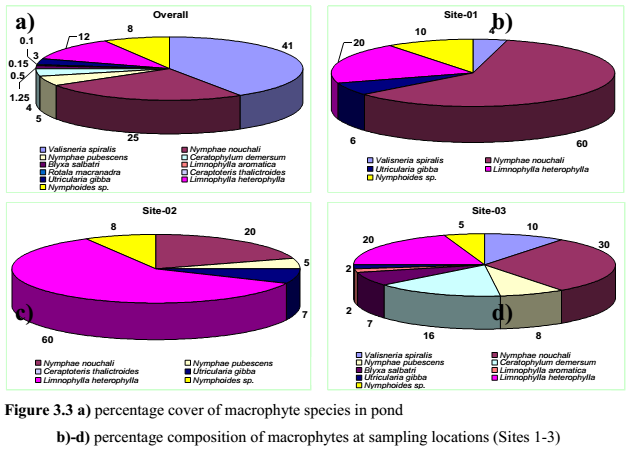
The proliferation of macrophytes in the ponds highlights of nutrient enrichment and internal recirculation of nutrients especially P from the dead and decaying plant matter, organic inputs of rituals and nutrient laden sediments. Deposition and decay of organic matter has contributed to the foul odour. Higher macrophyte density also indicates of increased primary productivity due to nutrient enrichment both from autochthonous and allochthonous sources, which eventually leads to conversion of ponds into swamps and marshes. No emergent macrophytes were recorded during the site inspection. Macrophytes recorded at sampling locations areSite 1, corresponding to deeper part of the pond comprised of Nymphae and Limnophylla species.Site 2, mainly comprised of Lymnophylla, Nymphae and Nymphoides species andSite 3 with the higher macrophyte diversity (Figure 3.2c and d). Water lily is the dominant macrophytes in the deeper reaches where as plants like Valisneria and Ceratophyllum along with that Nymphae, Limnophylla and Blyxa species were dominant in the shallower reaches.
Decomposed plant debris (uprooted and dead Valisneria species) and organic inputs (of ritual) have contributed to foul odour as well as lowering of DO that might eventually harm the entire aquatic biota. The large floating leafs of lily provides aesthetic looks to the pond but at the same time their over growth acts as an obstacle in light penetration, that is detrimental to the submerged algae or macrophyte community and this ultimately results in reduction of the overall dissolved oxygen creating partial anoxia in deeper zones.
During the field investigations, it was observed that Valisneria spiralis domination were mainly confined to the littoral zone (1.5–2.0 m), which had higher transparency and also observed at higher depth (>3 m) locations. This indicates its potential in stabilising the suspended particles and making the water clear. Such type of tropical pond systems with submerged plants like Valisneria spiralis have the potential to maintain clarity of the water through out the year. These submerged species restricted suspended algal growth, even though the nutrients levels and the organic loads were slightly high. Higher phosphates (0.2 – 0.4 mg/l; i.e. > 0.02 mg/l) value indicates an eutrophic status of the lake. Figure 3.2 depicts the extent and spread of macrophytes communities in the temple pond. Figure 3.3 elucidates sampling location wise, the percentage composition/distribution of macrophytes in the lake. Macrophyte specimen collected are depicted in figure 3.4.

|
T.V. Ramachandra
Centre for Sustainable Technologies, Centre for infrastructure, Sustainable Transportation and Urban Planning (CiSTUP), Energy & Wetlands Research Group, Centre for Ecological Sciences, Indian Institute of Science, Bangalore – 560 012, INDIA.
E-mail : cestvr@ces.iisc.ac.in
Tel: 91-080-22933099/23600985,
Fax: 91-080-23601428/23600085
Web: http://ces.iisc.ac.in/energy
Durga Madhab MahapatraEnergy & Wetlands Research Group, Centre for Ecological Sciences, Indian Institute of Science, Bangalore – 560 012, INDIA.
E-mail: durgamadhab@ces.iisc.ac.in
Subash Chandran M.DEnergy & Wetlands Research Group, Centre for Ecological Sciences, Indian Institute of Science, Bangalore – 560 012, INDIA.
E-mail: mds@ces.iisc.ac.in
Sincy V.
Energy & Wetlands Research Group, Centre for Ecological Sciences, Indian Institute of Science, Bangalore – 560 012, INDIA.
E-mail: sincy@ces.iisc.ac.in
Asulabha K. S.
Energy & Wetlands Research Group, Centre for Ecological Sciences, Indian Institute of Science, Bangalore – 560 012, INDIA.
E-mail: asulabha@ces.iisc.ac.in
G.R. Rao
Energy & Wetlands Research Group, Centre for Ecological Sciences, Indian Institute of Science, Bangalore – 560 012, INDIA.
E-mail: grrao@ces.iisc.ac.in
Vishnu D. Mukri
Energy & Wetlands Research Group, Indian Institute of Science, Bangalore – 560 012, INDIA.
Akhil C. A.
Energy & Wetlands Research Group, Centre for Ecological Sciences, Indian Institute of Science, Bangalore – 560 012, INDIA.
E-mail: akhilajayaksil123@gmail.com
Citation: Ramachandra T V, Durga Madhab Mahapatra, Subash Chandran M D, Sincy V, Asulabha K S, Rao, G R, Vishnu D. Mukri, Akhil C A., 2015.Rejuvenation and Sustainable Management of Gokarna Temple Pond - Kotiteertha, ENVIS Technical Report 99, CES, Indian Institute of Science, Bangalore 560012.
| Contact Address : |
| |
Dr. T.V. Ramachandra
Energy & Wetlands Research Group,
Centre for Ecological Sciences,
New Biological Sciences Building, 3rd Floor, E-Wing, Lab: TE15
Indian Institute of Science, Bangalore – 560 012, INDIA.
Tel : 91-80-22933099 / 22933503(Ext:107) / 23600985
Fax : 91-80-23601428 / 23600085 / 23600683 [CES-TVR]
E-mail : cestvr@ces.iisc.ac.in, energy@ces.iisc.ac.in,
Web : http://wgbis.ces.iisc.ac.in/energy |
|