Results and Discussion |
The distribution of nutrients in the seed coat of tur dal is as follows: crude protein 5.6%, ether extractives 0.3%, crude fibre 31.9%, ash 3.5% and carbohydrates 58.7% (Morton, 1976; Singh et al., 1968).
The results indicated that increase in the contact time increased the metal uptake but remained constant after an equilibrium time. The uptake of Cr (VI) was rapid and the equilibrium was attained within 15 minutes of contact between the biosorbent and the metal solution. The equilibrium time remained constant for all the initial metal concentrations measured. Equilibrium time varied with the metals due to the difference in initial metal concentration and affinity of the adsorbent for the particular metal ion.
The equilibrium time for an initial Fe (III) concentration of 10 ppm and 20 ppm was 30 minutes, for 50 ppm and 100 ppm it was 60 and 240 minutes respectively. In the case of Fe (III), the percentage of heavy metal adsorbed decreased with increase in metal concentration (Fig. 1). The 100 ppm solution took longer to attain equilibrium due to the presence of proportionally high amount of Fe (III). Mameri et al. (1999), reported that the available adsorption sites on the biosorbent are the limiting factor for metal uptake. The equilibrium time required by the adsorbent to remove Cr (VI) is very less, compared to other adsorbents. This is significant as equilibrium time is one of the important considerations for economical water and wastewater applications.
The experiment was carried out with different adsorbent dosage up to the equilibrium time. It was noted that after an adsorbent dosage level of 1g/100 ml, adsorption of Cr (VI) and Fe (III) was very low or constant (Fig. 2). However, the percentage of metal removed increased with increasing initial metal concentration and increasing adsorbent dosage.
The biosorption of Cr (VI) and Fe (III) was dependent on pH. Maximum adsorption of Cr (VI) was seen at pH 2 and Fe (III) at pH 2.5. The percentage of Cr ions adsorbed at pH 2.0 decreased with increasing metal concentration. The adsorption of metal ions depends on solution pH, which influences electrostatic binding of ions to corresponding metal groups. At the optimum sorption pH 2.0, the dominant species of Cr ions in solution are
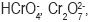

(Dakiky et al., 2002; Namasivayam and Yamuna, 1995). These chromate anions interact strongly with the negatively charged ions of the tur dal husk matrix. The experiments were carried out below pH3 for Fe (III) ions as Fe (III) precipitated as their hydroxides above pH 3. Ozer et al. (1999) and Sag and Kutsal (1996), obtained similar results.The optimum conditions for removal of heavy metals were standardized based on the results.
Langmuir isotherm was applied to the present study to estimate the adsorption capacity of tur dal husk. Langmuir isotherm is valid for monolayer adsorption onto a surface containing a finite number of identical sites (Langmuir, 1918). It is represented by the following equation:-
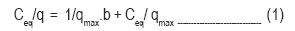
where q is milligrams of metal accumulated per gram of the biosorbent material; Ceq is the metal residual concentration in solution; qmax is the maximum amount of the metal ion per unit weight of the biosorbent to form a complete monolayer on the surface bound at high Ceq. qmax represents a practical limiting adsorption capacity when the surface is fully covered by metal ions and assists in the comparison of adsorption performance. b is the constant related to the affinity of the binding sites. The linear plots of Ceq/ q vs Ceq for Cr (VI) and Fe (III) show that adsorption follows the Langmuir adsorption model (Fig. 3i and 3ii).
The adsorption capacity (qmax) was calculated to be 96.05 and 66.63 mg/g for Cr (VI) and Fe (III) respectively. The adsorption capacity of Cr (VI) and Fe (III) by TDH with other adsorbents is compared in Table 1, which indicates that tdh has better capability than some of the biosorbents used previously.
where Ceq is the equilibrium concentration (mg/l), q is the amount adsorbed (mg/g) and Kf and n are constants incorporating all parameters affecting the adsorption process, such as adsorption capacity and intensity respectively. The linearised forms of Freundlich adsorption isotherm was used to evaluate the sorption data and is represented as:

Linear plots of ln Ce vs ln q show that the adsorption of metal ions onto the tur dal husk follows the Freundlich isotherm model (Fig. 4i and 4ii).
It also indicates that the average energy of adsorption decreases with increasing adsorption density. Values of Kf and n were calculated from the intercept and slope and are given in Table 2 along with the Langmuir constants. The values of n between 1 and 10 represent good adsorption of the adsorbate onto the adsorbent (McKay et al., 1982).
The infrared spectra of tdh before and after treatment with Cr (VI) and Fe (III) reveal the functional groups that are responsible for binding the heavy metal ions. Table 3 gives the wavenumbers with the corresponding functional groups. The results indicate that several functional groups are available on the surface of tdh for binding Fe(III) and Cr(VI).
The study reveals that tur dal husk, an agro-milling waste available in plenty at low cost is efficient in the removal of Cr (VI) and Fe (III). In batch mode studies, adsorption was dependent on contact time, pH, initial metal ion concentration and biosorbent dosage. Adsorption followed Langmuir and Freundlich isotherm models. Consisting of approximately 31.9% crude fibre composed of cellulose, hemicellulose and lignin, the TDH biomatrix indicates the presence of many OH and COOH groups in the lignocellulosic moieties. Hydrogen of these groups is capable of ion exchange with metal cations. Protein content in TDH is less than 5.6%, which is advantageous over the protein rich algal and fungal biomass projected as metal biosorbents, since proteinious materials are likely to putrefy under moist conditions. These adsorbed heavy metal ions can be easily desorbed and the biomass be incinerated for final disposal. This biosorbent is of low cost, its utility will be economical and can be viewed as a part of a feasible waste management strategy.