

 |
Adsorption of methylene blue and amaranth on to tamarind pod shells |
 |
N. Ahalya, M.N. Chandraprabha, R.D. Kanamadi, T.V. Ramachandra*
| Citation: N Ahalya, MN Chandraprabha, RD Kanamadi, T.V. Ramachandra. Adsorption of methylene blue and amaranth on to tamarind pod shells. J Biochem Tech (2012) 3(5): S189-S192ISSN: 0974-2328 |
Results and Discussion Characterisation of tamarind husk The carbon, hydrogen and nitrogen content of the husk showed very low percentage of nitrogen (0.94%) in comparison to the carbon quantities (46.01%). This indicates that few nitrogen containing compounds are involved in the adsorption of dyes. A relatively larger percentage of hydrogen (6.14%) in comparison to nitrogen compounds indicates that carbon-hydrogen groups might be available for adsorption of metals and dyes. The relatively low percentage of nitrogen shows that very less percentage of protein might be present in the husks. This is advantageous over protein rich adsorbents since proteinious materials are likely to putrefy under moist conditions The FT-IR spectra of the tamarind husk in the range of 400-4000 cm -1 were taken in order to obtain information on the nature of functional groups on the husk and dye interaction. It exhibits absorption bands at 3430, 2924, 1654 and 1030 cm-1, which indicate the presence of –OH, -COOH, C=O and C-O groups respectively. The effect of initial pH Initial pH is one of the most important environmental factors influencing not only site dissociation, but also the solution chemistry of the dyes: hydrolysis, complexation by organic and/or inorganic ligands, redox reactions, precipitation are strongly influenced by pH and, on the other side, strongly influence the speciation and the adsorption availability of the dyes. The effect of initial pH on the biosorption of methylene blue and amaranth is presented in Figure 1 a and b. Methylene blue, a cationic dye showed maximum adsorption in the pH range 6-10. Therefore, the best pH range for adsorption of MB was from 6.0 to 10.0. This result could be explained considering the electrostatic interaction between the surface of the biosorbent, negatively charged, mainly due to COO− species, since the pKa values of carboxylic acids range from 3.8 to 5.0 (Roberts and Caserio, 1977), with the cationic dye MB. At lower pH a possible protonation of COO− occurs, precluding the electrostatic attraction with the MB dye, decreasing the adsorbate uptaken. Thus, at pH values ranging from 6.0 to 10.0, the carboxylic groups are available to adsorb the positively charged dye, increasing the removal of MB from aqueous solution. .The maximum removal of the anionic dye amaranth occurred at pH 2 for the different dye concentrations 10, 20 and 50 mg/L. The percent removal decreased with increase in initial pH. Similar results for methylene blue (Kumar et al. 2005; Bhattacharyya and Sharma 2005; Kavitha and Namasivayam 2005; Rahman and Saad 2003) and amaranth (Mittal et al. 2005; Gong et al. 2005) were reported.
Figure 1a & b: Effect of pH on the methylene blue and amaranth adsorption respectively by tamarind pod shells (¨ 10 mg/L ■ 20 mg/L ▲ 50 mg/L) Effect of adsorbent dosage The biosorption of methylene blue and amaranth was studied at various biosorbent concentrations ranging from 0.5 to 5 mg/L. The percentage removal of the dye increased with increase in the sorbent dosage (Fig 2 a and b). The results revealed that the colour removal was increased up to adsorbent dosage of 1 g l−1 and 3 g l−1 for methylene blue and amaranth respectively and then it remained 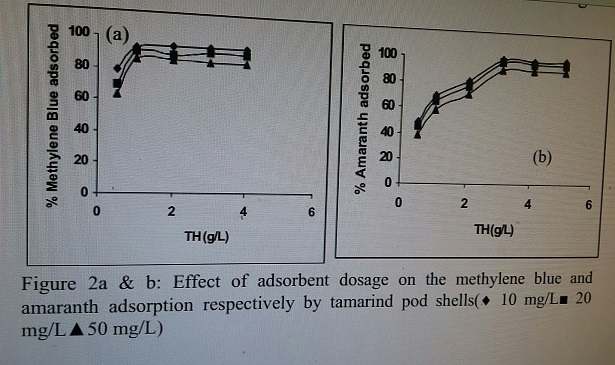
Figure 2a & b: Effect of adsorbent dosage on the methylene blue andamaranth adsorption respectively by tamarind pod shells( 10 mg/L■ 20mg/L▲ 50 mg/L) Effect of contact time
The uptake of methylene blue and amaranth by tamarind husk increased with the increase in contact time and it remained constant after an equilibrium time as shown in Figure 3 a and b. 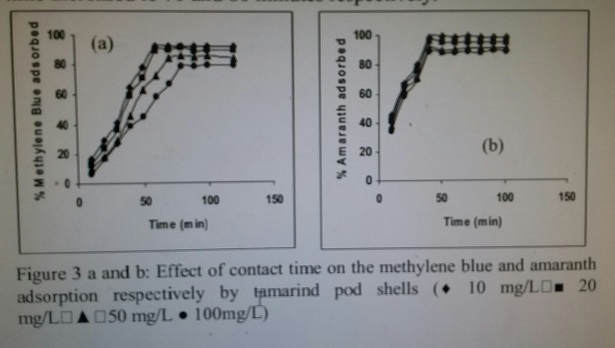
Figure 3 a and b: Effect of contact time on the methylene blue and amaranthadsorption respectively by tamarind pod shells ( 10 mg/L ■ 20mg/L ▲ 50 mg/L ● 100mg/L) Kinetic Studies sotherm Modelling> In order to optimize the design of a sorption system to remove dyes
from aqueous solutions, it is important to establish the most
appropriate correlation for the equilibrium curves. The isotherms
data were analyzed using two of the most commonly used
equilibrium models, Langmuir (Langmuir 1918) and Freundlich
(Freundlich 1906) isotherm models (Fig). The mathematical
expressions are given by Equations 2 and 3, respectively, as follows:
max he best-fit equilibrium model was determined based on the linear
squared regression correlation coefficient R2. From Table 1, it was
observed that the equilibrium sorption data were very well
represented by Langmuir isotherms followed by the Freundlich
model with high correlation coefficients. Hence, the best fit of
equilibrium data in Langmuir isotherm expressions confirm the monolayer coverage process of methylene blue and amaranth by
tamarind husk. Furthermore, the value of Freundlich exponent n in
the range of 1–10, indicates a favourable adsorption (Ho and McKay
1998). Also, high adsorption capacity indicates the strong
electrostatic force of attraction between dye molecules and
biosorbent binding-sites (Kaewsarn and Yu 2001). The comparison
of results of this work with the others found in the literature showed
that tamarind husk has a significantly high adsorption capacity for
binding the dyes (Table 2) Desorption studies Both incineration and land disposal represent possible options for
final disposition of spent adsorbent material. However, both
methods directly or indirectly pollute the environment. If
regeneration of dyes from the spent adsorbent were possible then it
would not only protect the environment but also help recycle the
adsorbate and adsorbent and hence contribute to the economy of
wastewater treatment.
Desorption experiments were carried out at different pH values.
Desorption of the dyes with water was not significant. If the
adsorption is by physical bonding then the loosely bound solute can
be easily desorbed with distilled water in most of the cases (Agarwal
et al., 2006). Hence, physical adsorption is ruled out. Among the
dyes, the percentage of amaranth desorbed was the highest with
increase in pH. About 46.32% of the dye was desorbed from
tamarind husk. The percentage of methylene blue desorbed did not
exceed 2.45%. (Figure 4)
Mechanism of adsorption of dyes Based on the results obtained, the mechanism of adsorption of methylene blue and amaranth is discussed below. Adsorption of methylene blue: The mechanism of methylene blue adsorption by tamarind pod shells is depicted below.
|