Energy and Wetlands Research Group, Centre for Ecological Sciences, Indian Institute of Science, Bangalore – 560012, India.
*Corresponding author: cestvr@ces.iisc.ac.in
|
INTRODUCTION
Diatoms (Greek = "cut in half") are the major group of unicellular, photosynthetic and eukaryotic algae. They constitute the most speciose group of organisms (worldwide distribution ~ 200,000 species, Bentley et al., 2005) and are found inhabiting a range of habitats from oceans to freshwater systems like rivers, lakes and ponds (Armbrust et al., 2004). Importance of these unique intricate cell patterned organisms, since then has increased manifold in areas of taxonomy, ecology, biomonitoring, biotechnology, etc combining microscopic observation with in situ culturing. It has taken a long time to recognize the significance of the ubiquity of the microscopic life, revealed by Robert Hooke through his compound microscope, despite of the reliance on microorganisms (Ash et al., 2002). Microscope since time immemorial has been used to understand many biological functions in prokaryotes and eukaryotes. Among all the organisms, study of diatoms was started off with microscopic observations i.e., taxonomy (Müller, 1786). Diatom taxonomy is based either on the identification of ribosomal sequences (Medlin et al., 1996) or more classically on the morphology and the shape of frustules, the extracellular silica cell walls (Karthick et al., 2010). Culturing of diatoms is followed in morphometry and phylogeny (Mann, et al., 2008) and to understand the teratological structures in diatoms (Falasco et al., 2009, Håkansson and Chepurnov, 1999) by herbicidal effects (Debenest et al., 2008), etc., which can be applied in biomonitoring practices (Debenest et al., 2009). Toxicological studies for metal contamination and bioaccumulation of trace metals is also done for biomonitoring applications (Wang and Dei, 2001; Price and Morel, 1990).The community structure (deJong and Admiraal, 1984, Debenest et al., 2009) of diatoms could be understood to unravel ecological intricacies by culturing them in an artificial media, which mimic the natural condition of diatoms.
Culturing got impetus with Cohn (1850) cultivating unicellular flagellate Haematococcus (Chlorophyceae) in situ. However, these attempts had setback due to the absence of suitable culture media or maintenance (Preisig and Andersen, 2005). Later, Famintzin (1871) cultured algae (Chloroccum infusionum (Schrank) Meneghini and Protococcus viridis Agardh) using a media with a few inorganic salts that was adopted from Knop (1865) used for vascular plants (Preisig and Andersen, 2005).
In situ culturing helps to decipher physiological and biological processes including enzymatic behavior, genetics, etc. affecting growth of an organism in an in vitro environment (except when cultured in outdoor ponds). This requires appropriate culture medium or an agar medium containing essential nutrients (macronutrients, micronutrients, vitamins) and chelator elements, etc., required for the sustained growth of cells. This is being customized considering the requirement of microorganism (Pelczar et al., 1993).
Culture media can be broadly grouped as marine or freshwater culture media based on the ecology of the diatom species. Although culturing of algae has a very long history of as old as 1871 (Famintzin 1871), researchers were intrigued with diatom culturing for various reasons. The various fields in which diatom culturing is done to unravel its mystery are illustrated in Figure 1.
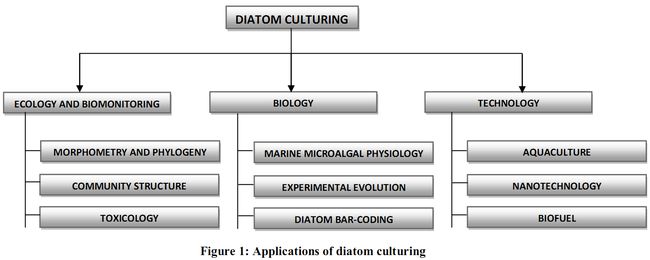
Many facets of diatom biology like sexual behavior, chloroplast and protoplast dynamics have been understood with the help of in situ culturing(Edlund and Stoermer, 1991, Mann et al., 1999, Davidovich and Bates, 1998, Chepurnov et al., 2002, Sabbe et al., 2004, Chepurnov et al., 2004). Various physiological activities (Berland et al., 1973; Lane and Morel, 2000; Reinfelder et al., 2000) and evolution related questions have also been understood by culturing (Armbrust et al., 2004 and Connolly et al., 2006). The concept of bar-coding was introduced to diatom taxonomy (Evans et al., 2007; Kaczmarska et al., 2007) on the premise that the divergence of a small DNA fragment coincides with biological separation of species. This DNA fragment becomes a DNA barcode for species which can be used to flag new species, select optimal taxa for phylogenetic studies, or to signal the geographical extent of divergences in a population (Hajibabaei et al., 2007). DNA bar-coding is used as an initial approach for diverse applications, followed by larger in-depth studies in the respective fields. Different DNA regions within the nuclear, mitochondrial and chloroplast genomes have been considered for testing as a universal DNA barcode for diatoms (Moinz and Kaczmarska, 2009). Culturing helps to isolate the specific diatom and also isolating nuclear, mitochondrial and chloroplast genomes for DNA barcode of a species (Moinz and Kaczmarska, 2009).
Diatoms, in particular, were regarded as useful neutral lipid sources, as liquid fuel precursors, as foods for marine culture of zooplankters (Ahlgren et al., 1990), larval and postlarval shrimp (Chu, 1989), copepods (Bourdier and Amblard, 1989), juvenile oysters (Tsitsa-Tzardis et al., 1993) and as micromachines in nanotechnology (Drum and Gordon, 2003). Many diatoms (Chaetoceros muelleri Schütt, McGinnis et al., 1997; Thalassiosira pseudonana Hasle & Hemidal, Pheodactylum tricornutum Bohlin., Yu et al., 2009; Melosira varians Agardh., Stephanodiscus binderanus (Kütz.) Krieger, Cyclotella meneghiniana Kütz., Sicko-Goad and Andresen, 1991) have been screened through culturing to assess its relevance as prospective biofuel feedstock. Gordon et al., 2005 suggest the need for standardizing and scaling up of diatom in situ culturing to track and prevent diatom malformations associated with culturing. Silica being the component of diatom cell wall, understanding its silicification process through genetic transformation experiments, is essential in the field of diatom nanotechnology.
In the preceding sections, we explain the evolution of the successive marine diatom media, since Miquel (1892-93)’s work. As a result, this deals with primitive to a modernized isolation techniques as it forms a defining step for any species-specific experiments. We then focus on the significance of recipe compositions from 19th to 21st century.
|
|
Citation : Supriya. G. and Ramachandra. T.V., 2011. Chronicle of Marine Diatom Culturing Techniques., Indian Journal of Fundamental and Applied Life Sciences, Vol. 1 (3) July-September, pp. 282-294.
|
Dr. T.V. Ramachandra
Energy & Wetlands Research Group,
Centre for Ecological Sciences, Indian Institute of Science, Bangalore – 560 012, INDIA.
E-mail : cestvr@ces.iisc.ac.in
Tel: 91-080-22933099/23600985,
Fax: 91-080-23601428/23600085
Web: http://ces.iisc.ac.in/energy
G. SupriyaEnergy and Wetlands Research Group, Centre for Ecological Sciences, Indian Institute of Science, Bangalore – 560 012, India
E-mail:
supriya@ces.iisc.ac.in
Citation: Supriya. G. and Ramachandra. T.V., 2011. Chronicle of Marine Diatom Culturing Techniques., Indian Journal of Fundamental and Applied Life Sciences, Vol. 1 (3) July-September, pp. 282-294.
Bentley K, Cox EJ and Bentley PJ (2005). Nature’s batik: a computer evolution model of diatom valve morphogenesis. Journal of nanoscience and nanotechnology, 5 25-34.
Armbrust EV, Berges JA, Bowler C, Green BR, Martinez D, Putnam N, Zhou S, Allen AE, Apt KE, Bechner M, Brzezinski MA, Chaal BK, Chiovitti A, Davis AK, Demarest MS, Detter JC, Glavina T, Goodstein D, Hadi MZ, Hellsten U, Hilldebrand M, Jenkins BD, Jurka J, Kapitonov VV, Kröger N, Lau WWY, Lane TW, Larimer FW, Lippmeier JC, Lucas S, Medina M, Monstant A, Orbornik M, Parker MS, Palenik B, Pazour GJ, Richardson PM, Rynearson TA, Saito MA, Schwartz DC, Thamatakoln K, Valentin K, Vardi A, Wilkerson FP and Rokshar D (2004). The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science, 306 79–86.
Ash C, Hanson B and Norman C. (2002). Earth, air, fire, and water. Science, 296 1055.
Müller OF (1786). Diatomaceen (Vibrio paxillifer, V. bipunctatus, V. tripunctatus, Gonium pulvinatum). Animalcula infusoria fluviatilia et marina quae detexit, systematice, descripsit et ad vivum delineare curavit O.F. Muller. Havniae.
Medlin LK, Kooistra WHCF, Gersonde R and Wellbrock U (1996). Evolution of the diatoms (Bacillariophyta). II. Nuclear-encoded small-subunit rRNA sequences comparisons confirm paraphyletic origin for centric diatoms. Molecular and Biological Evolution, 13 67-75.
Karthick B, Taylor JC, Mahesh MK and Ramachandra TV (2010). Protocols for collection, preservation and Enumeration of diatom from Aquatic habitats for water quality monitoring in India. The IUP Journal of Soil and Water Sciences, 3(1) 25-60.
Mann DG, Thomas SJ and Evans KM (2008). Revision of the diatom genius: Sellaphora: a first account of the larger species in the British Isles. Fottea, 8(1) 15-78.
Falasco E, Bona F, Badino G, Hoffmann L and Ector L (2009). Diatom teratological forms and environmental alterations: a review. Hydrobiologia, 623 1–35.
Håkansson H and Chepurnov V (1999). A study of variation in valve morphology of the diatom Cyclotella meneghiniana in monoclonal cultures: effect of auxospore formation and different salinity conditions. Diatom Research, 14:251–272.
Debenest T, Silvestre J, Coste M, Delmas F and Pinelli E (2008). Herbicide effects on freshwater benthic diatoms: Induction of nucleus alterations and silica cell wall abnormalities. Aquatic Toxicology, 88(1) 88-94.
Debenest T, Silvestre J, Coste M and Pinelli E (2009). Effects of pesticides on freshwater diatoms. Review of Environmental Contamination and Toxicology, 203 87- 103.
Wang WX and Dei RCH (2001). Metal uptake in a coastal diatom influenced by major nutrients (N, P, and Si). Water Research, 35(1) 315-321.
Price NM and Morel FMM (1990). Cadmium and cobalt substitution for zinc in a marine diatom. Nature, 344 658–60.
deJong L and Admiraal W (1984). Competition between three estuarine benthic diatom species in mixed cultures. Marine Ecology-Progress Series, 18 269 - 275.
Debenest T, Silvestre J, Coste M and Pinelli E (2009). Effects of pesticides on freshwater diatoms. Review of Environmental Contamination and Toxicology, 203 87- 103.
Cohn F (1850). Nachträge zur naturgeschichte des Protococcus pluvialis Kützing (Haematococccus pluvialis Flotow). Nova Acta Leop Carol, 22(2) 605-764.
Preisig HR and Andersen RA (2005). Historical review of Algal culturing techniques, Chapter 1. in: Algal culturing techniques edited by Andersen RA, Elsevier, pp 1-12.
Famintzin A (1871). Die anorganischen salze als ausgezeichnetes hilfsmittel zum studium der entwicklung neiderer chlorophyllhaltiger organismen. Bulletin of the Academy of Sciences, St. Petersburg, 17 31-70.
Knop W (1865). Quantitative untersuchung über die ernährungsprocess der pflanzen, landwirtsch. Ver-Sta., 7 93–107.
Preisig HR and Andersen RA (2005). Historical review of Algal culturing techniques, Chapter 1. in: Algal culturing techniques edited by Andersen RA, Elsevier, pp 1-12.
Pelczar JM, Chan LEA and Krieg NR (1993). Microbiology, Concept and Application, McGraw-Hill Inc. p 847.
Famintzin A (1871). Die anorganischen salze als ausgezeichnetes hilfsmittel zum studium der entwicklung neiderer chlorophyllhaltiger organismen. Bulletin of the Academy of Sciences, St. Petersburg, 17 31-70.
Edlund MB and Stoermer EF (1991). Sexual reproduction in Stephanodiscus niagarae (Bacillariophyta). Journal of Phycology, 27 780-793.
Mann DG, Chepurnov VA and Droop SJM. Sexuality, incompatibility, size variation and preferential polyandry in natural populations and clones of Sellaphora pupula (Bacillariophyta). Journal of Phycology, 35 152-170.
Davidovich NA and Bates SS (1998). Sexual reproduction in the pennate diatoms Pseudo-nitzschia multiseries and P. pseudodelicatissima (Bacillariophyceae). Journal of Phycology, 34 126–137.
Chepurnov VA, Mann DG, Vyverman W, Sabbe K and Danielidis DB (2002). Sexual reproduction, mating system, and protoplast dynamics of Seminavis (Bacillariophyceae). Journal of. Phycology, 38 1004-1019.
Sabbe K, Hodgson DA, Verleyen E, Taton A, Wilmotte A, Vanhoutte K and Vyverman W (2004). Salinity, depth and the structure and composition of microbial mats in continental Antarctic lakes. Freshwater Biology, 49 296–319.
Chepurnov VA, Mann DG, Sabbe K and Vyverman W (2004). Experimental studies on sexual reproduction in diatoms. International Review of Cytology, 237 91-154.
Berland BR, Bonin DJ, Maestrini SY and Pointer JP (1973). Étude de la fertilité des eaux marines au moyen de tests biologiques effectuées avec des cultures d’algues. International Review of Hydrobiology, 58 473–500.
Lane TW and Morel FMM (2000). A biological function for cadmium in marine diatoms. Proceedings of the National Academy of Sciences, USA, 97 4627–4631.
Reinfelder JR, Kraepiel AML and Morel FMM (2000). Unicellular C4 photosynthesis in a marine diatom. Nature, 407 996–999.
Armbrust EV, Berges JA, Bowler C, Green BR, Martinez D, Putnam N, Zhou S, Allen AE, Apt KE, Bechner M, Brzezinski MA, Chaal BK, Chiovitti A, Davis AK, Demarest MS, Detter JC, Glavina T, Goodstein D, Hadi MZ, Hellsten U, Hilldebrand M, Jenkins BD, Jurka J, Kapitonov VV, Kröger N, Lau WWY, Lane TW, Larimer FW, Lippmeier JC, Lucas S, Medina M, Monstant A, Orbornik M, Parker MS, Palenik B, Pazour GJ, Richardson PM, Rynearson TA, Saito MA, Schwartz DC, Thamatakoln K, Valentin K, Vardi A, Wilkerson FP and Rokshar D (2004). The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science, 306 79–86.
Connelly TL, Tilburg CM and Yager PL (2006). Evidence for psychrophiles outnumbering psychrotolerant marine bacteria in the springtime coastal Arctic. Limnology and Oceanography, 51 1205–1210.
Evans KM, Wortley AH and Mann DG (2007). An assessment of potential diatom “Barcode” genes (cox1, rbcL, 18S and ITS rDNA) and their effectiveness in determining relationships in Sellaphora (Bacillariophyta). Protist, 158 349-364.
Kaczmarska I, Reid C and Moniz M (2007). Diatom taxonomy: morphology, molecules and barcodes, In: Proceedings of the 1st Central-European Diatom Meeting edited by Kusber WH and Jahn R, Botanic Garden and Botanical Museum Berlin-Dahlem, FU-Berlin. pp 69-72.
Hajibabaei M, Singer GAC, Hebert PDN and Hickey DA (2007). DNA barcoding: how it complements taxonomy, molecular phylogenetics and population genetics. Trends in Genetics, 23 167–172.
Moniz MBJ and Kaczmarska I (2009). Barcoding diatoms: Is there a good marker? Molecular Ecology Resources, 9 65-74.
Moniz MBJ and Kaczmarska I (2009). Barcoding diatoms: Is there a good marker? Molecular Ecology Resources, 9 65-74.
Ahlgren G, Goedkoop W, Markensten H, Sonesten L and Boberg, M (1997). Seasonal variations in food quality for pelagic and benthic invertebrates in Lake Erken - the role of fatty acids. Freshwater Biology, 38 555 - 570.
Chu KH (1989). Chaetoceros gracilis as the exclusive feed for the larvae and postlarvae of the shrimp Metapenaeus ensis. Aquaculture, 83 281–287.
Bourdier G and Amblard C (1989). Lipids in Acanthodiaptomus denticornis during starvation and fed on three different algae. Journal of Plankton Research, 11 1201– 1212.
Tsitsa-Tzardis E, Patterson GW, Wikfors GH, Gladu PK and Harrison D (1993). Sterols of Chaetoceros and Skeletonema. Lipids, 28 465–467.
Drum RW and Gordon R (2003). Star Trek replicators and diatom nanotechnology. Trends in Biotechnology, 21 325–328.
McGinnis KM, Dempster TA and Sommerfeld MR (1997). Characterization of the growth and lipid content of the diatom Chaetoceros muelleri. Journal of Applied Phycology, 9 19–24.
Yu ET, Zendejas FJ, Lane PD, Gaucher S, Simmons BA and Lane TW (2009). Triacylglycerol accumulation and profiling in the model diatoms Thalassiosira pseudonana and Phaeodactylum tricornutum (Bacillariophyceae) during starvation, Journal of Applied Phycology, 21 669-681.
Sicko-Goad L and Andresen NA (1991). Effect of growth and light/dark cycles on diatom lipid content and composition. Journal of Phycology, 27 710-8.
Gordon R, Sterrenburg F and Sandhage K (2005). A special issue on diatom nanotechnology. Journal of Nanoscience and Nanotechnology, 5(1) 1-178.
Miquel P (1892-1893). De la culture artificielle des diatomees. Le Diatomiste, 93–99, 121–128, 149–156, 165–172. In: Reviews of foreign literature edited by C.H.K., T.M., E.G.B., 1893. Bulletin Torrey Botanical Club, 20 259–260.


