
LIMNOLOGY

Morphometry refers to physical factors (shape, size, structure, etc) that determine the lake basin. Generally, lakes that are small in surface area and larger in depth exhibit higher water quality than those that are larger in surface area and shallow in depth. This is referred to as surface to volume ratio (S/V).
Maximum depth and median depth along with percentage of littoral area (part of the lake basin where enough light penetrates to allow growth of plants and algae) is important in the lake’s morphometry. If a lake has a very large watershed area compared to its surface area, it will receive larger surface runoff with substantial nutrient loads.
Depth analyses, including sediment measurement at various depths, volumes of strata and shoreline characteristics are often critical to the investigation of biological, chemical and physical properties of fresh waters. Morphometric parameters are needed to evaluate erosion, nutrient loading rates, chemical mass, heat content, thermal stability, biological productivity and effectiveness of growth, and many other structural and functional components of the ecosystem. Management techniques such as loading capacity for effluents and selective removal of undesirable components of the biota are also dependent on detailed knowledge of the morphometry and flow characteristics.
The water in lakes is balanced by the basic hydrological relationship in which change in water storage is governed by inputs from all sources minus water losses. Water income from precipitation, surface influents and groundwater sources is balanced by outflows from surface effluents, seepage to groundwater and evapotranspiration. Each of these inflows and outflows vary seasonally and geographically and is governed by the characteristics of particular lake basins, their groundwater, drainage basins and climate. The hydrological cycle, which can be altered extensively by human induced changes of surface water systems, determines the distribution of lakes in relation to the suitable catchment (watershed) area.
WATERSHED
The lowest area of the catchment where water collects to form a lake is called lake basin. The region from where water drains into the lake basin is known as catchment area or drainage basin of the lake. Water may directly drain into the lake or pass through a system of streams and rivers, and other depressions on the way to the lake. The spatial extent of the catchment and the amount of rain falling on it ultimately determine the volume of water entering the lakes.
Watershed (Figs. 1 and 2) contributes surface-runoff to the water body. Watershed on a defined drainage channel includes all the land and water areas that drain. It is marked by an elevated line that forms a division between two areas drained by separate stream or bodies of water, such as a small watershed of a few hectares that drains into a small stream forming part of a larger watershed that in turn forms a part of a still larger watershed until the combined watershed becomes a major river basin draining millions of square kilometres of land. There can be sub watersheds within a watershed like a tributary to a lake having its own watershed, which is a part of the larger total drainage area to the lake. A lake reflects the watershed size, topography, geology, land use, soil fertility, erodibility and vegetation.
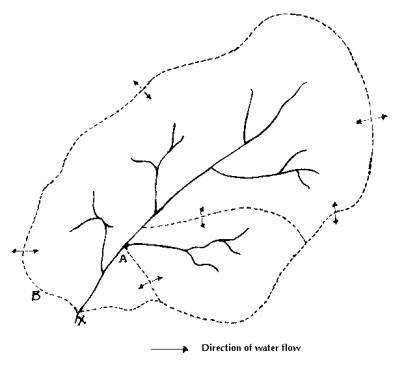
Fig 1: A Typical Watershed
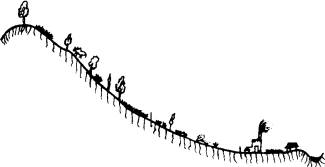
Fig 2: A Typical Watershed Transect (macro-perspective)
Water quality decreases with increasing ratio of watershed area to lake area. In a large watershed, water from precipitation will interact and leach minerals from the soil before being discharged into the lake. Lakes with small watersheds, maintained primarily by groundwater flow, are known as seepage lakes. Where as, lakes fed by inflowing streams or rivers are known as drainage lakes.
Land use has an impact on the quantity and quality of water entering into the lake. In urban areas, the high proportion of impervious surfaces prevents the seepage of rainwater into the soil increasing the rate of surface water flow to the lake. High flushing rates from urban areas can increase erosion of the watershed area providing sufficient force to carry materials (minerals, etc) along with the soil affecting the water quality.
Soil types and their susceptibility to erosion is another important component of the watershed. The type and abundance of vegetation determines the extent of erosion. Areas with native undisturbed vegetation are less prone to erosion than areas with disturbed vegetation like agricultural lands.
Another aspect of the watershed to be considered is whether the lake is a closed (no outlet) or open (with outlet) system. In the closed system, all the water draining in stays, not flowing into a channel or river, making it more sensitive than an open system. The upstream water quality has an important role in the health of the lake ecosystem. Lakes in the upper end of the watershed may have a different water quality than the downstream. Depending on the physical and biological factors of the upstream lakes, the downstream water quality may be affected either positively or negatively.
Factors influencing watershed operations:
a) Size: Both run-off volume and rate increases with increase in watershed size. However, both rate and volume per unit of watershed area decreases as the area increases. The size or area of watershed is an important parameter in determining the peak rate of run-off.
b) Shape: Long and narrow watersheds have longer times of concentration resulting in lower runoff-rates than more square watersheds of similar size, which have a number of tributaries discharging into the main channel. This type of concentration also affects the amount of water infiltrating into the soil in the watershed. The longer time it takes to leave the watershed, the greater is its seepage into the soil.
c) Land-slope: Slope has major implications on land-use. The speed and extent of run-off depend on the slope of the land. Greater the slope, greater is the velocity of flow of run-off water. If velocity is doubled, energy and consequently erosion also increases. The degree of slope sets limits on land use for annual crops, plantation and land reclamation, depending on soil depth.
d) Drainage pattern: Drainage pattern of an area depends on the course of the streams and their tributaries. Land-slope, lithology and structure influence the drainage pattern. In general, coarser the drainage texture, higher is the conductivity. Finer drainage texture results in heavier soil type. Drainage patterns act as guidelines to locate vulnerable areas requiring different kinds and degrees of soil conservation measures.
e) Soil and geology: Soil and geology of the watershed also determine the amount of water percolating into the ground. Soil character also determines the amount of silt that will be washed down into water harvesting structures.
f) Vegetative cover: The type and quality of vegetative cover of the watershed influence run-off, infiltration rates, erosion, sediment production and evapotranspiration rate. Dense vegetation reduces erosion.
g) Precipitation: Amount and nature of precipitation is an important factor determining the rate of run-off into the water body. Rainfall distributed evenly throughout the year has a different impact than a sudden sharp seasonal rainfall.
Water
budget in lakes
The water budget of lakes provides useful information about the availability of water in lakes at any time. The basic parameters of the water budget may be classified as recharge and discharge. These can be further divided as inflow from precipitation, surface runoff and groundwater and outflow from river and evaporation. Hydrogeological, hydromorphological and hydrodynamic conditions influence the water budget in the lake basin.
The water budget equation of a lake can be expressed as follows:
D S
= Is + Iu + Pl – Qs – Qu
- El
where D S = change in water storage, Is = Surface inflow, Iu = Underground inflow, Pl = Lake precipitation, Qs = Surface outflow, Qu = Underground outflow and El = Lake evaporation.
The water budget of lakes is determined by the conditions of humidification in the area where the lakes and drainage basins are located. In humid areas, precipitation is higher than evaporation from water surface, and in arid regions, evaporation from water surface is higher than precipitation. An intermediate zone may also exist where both humid and arid zones may alternate. Each zone is characterised by its own structure and dynamics. Quantitatively, water budget of lakes can be computed by using conventional techniques, the drawbacks of which can be overcome using isotopic techniques. The conventional method of computing water budget is through parameters like surface inflow, lake precipitation, lake evaporation, surface outflow, subsurface outflow and inflow and storage changes. Water budget of lakes can also be estimated using environmental isotopes and artificial isotopes.
Sedimentation
The lake bottom is covered by mud due to sedimentation of particles in the water, which comprise inorganic silt derived from surrounding lands and organic particles of dead plants and animals sinking down the water column. These act as food for the bottom dwelling animals and source chemicals to the water. In many lakes, bottom sediments are principal sites of decomposition. Sediments with high organic content contain large numbers of bacteria, which break them into smaller inorganic molecules. These bacteria require oxygen and when the sediment becomes totally devoid of oxygen, they start to adsorb it from the overlying water. If the lake is stratified, then there is no mixing of hypolimnion and hence no replenishment of oxygen to the lower layers. This leads to anoxic conditions and death of animals. If the lake is large and regularly has a deoxygenated hypolimnion, only those animals able to endure the absence of oxygen will survive. When the sediments are devoid of oxygen, some processes of decomposition cannot occur, but other chemical reactions that occur only in the absence of oxygen take over. Anaerobic bacteria can operate without oxygen and break down large quantities of organic matter in the mud to produce methane gas (made up of carbon and hydrogen CH4). The released methane is used as energy by other bacteria, which are aerobic and can live only in oxygenated conditions. Other anaerobic bacteria can use sulphates and the by-product of their activity is hydrogen sulphide. Many lakes have a layer of deoxygenated water at the bottom during summer, above which a layer of oxygenated water is present. The balance between these two layers and between the intensity of aerobic and anaerobic activities is essential to the health of the lake ecosystem.
The endangering of lakes due to high rate of sedimentation has caused serious problems, particularly in developing countries. The loss of storage capacity of the lakes due to unwanted sedimentation may have a profound effect on the functioning and objectives of the water system. The solution to this problem requires a comprehensive study of the catchment with regard to the following aspects:
(i) Measurement of erosion effects in the catchment and identification of the sediment sources including those related to particular land use.
(ii) Identification of areas that serve as sediment storage.
(iii) Measurement of temporal changes in sediment storage.
(iv) Determination of sediment rates.
Sedimentation in lakes records the effects of climatological or man-made changes in and around the catchment. The important factors influencing this process in lakes/reservoirs are as follows:
· Nature and size of watersheds surrounding the lake basins; high relief areas contribute coarser particles whereas low-gradient areas contribute finer material.
· Size of the lake/reservoir; controls the wave action within the system and hence affects the sedimentation behaviour.
· Orientation of lake/reservoir relative to dominant wind direction.
· Thermal stratification and density of lake water; particularly important with respect to movement of fine particles.
· Climatic factors such as precipitation, evaporation, etc.
Rivers and streams provide pathways for sediment movement to lakes/reservoirs. The nature of sediment transported ranges from pebbles and coarse sands to fine sands, silts, clays (suspended/wash load), depending on a number of variables such as water discharge, bed slope, velocity, turbulence, etc. Although in most lakes, sedimentation is principally from inorganic material, in many shallow lakes (in areas of low relief, where rivers carry small loads), the indigenous phytoplankton populations may contribute significant quantities of particulate matter to the lakebed.
The most conventional technique and accurate determination of sediment load being carried to a lake by streams is to measure the flow rate and sediment concentration of the inflowing waters just upstream of the lake. The other conventional methods involve periodic bathymetric surveys of the lake. Environmental isotopes can also be used for this purpose. Increased rate of sedimentation can attenuate the light impinging on the surface of water.
LIGHT
Light plays an important role in lake ecology and determines the potential rate of photosynthesis, which supplies dissolved oxygen and food in the water. Solar radiation is the major source of heat and determines the wind patterns in the lake basin and water movement. Nearly all energy that controls the metabolism of lakes and streams is derived directly from the solar energy utilised in photosynthesis. That energy stored in photosynthetically formed organic matter is both synthesised within the lake or stream (autochthonous) or within drainage basin and brought to the lake or stream in various forms (allochthonous). Utilisation of this energy received within the water or imported from the drainage basin and factors that influence the efficiency of conversion of solar energy to potential chemical energy are fundamental to lake productivity. Light intensity varies with seasons and depth. Deeper the light penetration, higher is the rate of photosynthesis. Photosynthetic organisms include phytoplankton (algae), attached algae (periphyton) and vascular aquatic plants (macrophytes). The rate at which the penetration of light decreases with depth depends on the amount of suspended particles in water. The level of light determines the maximum depth at which algae and macrophytes can grow, which is determined to be the point where available light is reduced to 0.5% - 1% of the amount available on the surface. In a clear lake, the euphotic zone may extend down to 20 m or more but in most of the present day lakes, it is often about 3-5 m or as less as 0.5 m deep. Significant changes in lake transparency are the result of anthropogenic activities in the watershed.
| Light and Depth: When light passes down through a column of water, the amount of light available at any particular depth decreases exponentially, as most of the light is absorbed in the surface waters. The depth of light penetration depends very much on the colour and turbidity of the water. Thus, if there are 100 units of light energy at the surface, they will decrease depending on the water quality and not in a simple linear manner (like 90 at 1m, 80 at 2m and so on). |
Nutrient
cycling, distribution of dissolved gases and biota,
and behavioural adaptations
of organisms are all markedly influenced by the thermal structure and
stratification patterns. Therefore the optical properties of lakes and
reservoirs are important regulatory parameters in the physiology and behaviour
of aquatic organisms.
DENSITY
Much of the radiation entering the lake, especially those with longer wavelengths, is absorbed near the surface and transformed into heat. Water differs from other compounds as it is less dense as solid than as liquid (most dense at 4°C and less dense at both higher and lower temperatures). When the surface water of the lake warms up, heat takes a long time to penetrate down through the water. During summer, temperature differences between the upper and lower layers become more distinct. Deep lakes generally become stratified into three identifiable layers known as epilimnion, metalimnion and hypolimnion.
The epilimnion is the warm upper layer, which is well mixed. Below the epilimnion is the metalimnion or thermocline region, a layer of water in which the temperature declines rapidly with depth. The hypolimnion is the bottom layer of cold water. Change in density at the metalimnion acts as a physical barrier preventing the mixing of epilimnion and hypolimnion. Thermocline is used synonymously with metalimnion, but is actually the plane or surface of maximum rate of decrease of temperature with respect to depth. Thus, thermocline is the point of maximum temperature change within the metalimnion.
There are few lakes, which are so deep and warm that they are permanently stratified, and many shallow lakes that are never stratified for more than few hours. But most lakes undergo seasonal stratification and mixing, which is a major determinant of their ecology. Thermal stratification (Fig. 3) and heat exchange depend on solar radiation and wind. In addition to climate, the size and wind exposure of a lake and water inflow are the major factors that determine the type of circulation. Lakes can be categorised according to the degree and frequency of their circulation patterns.
Circulation pattern in lakes:
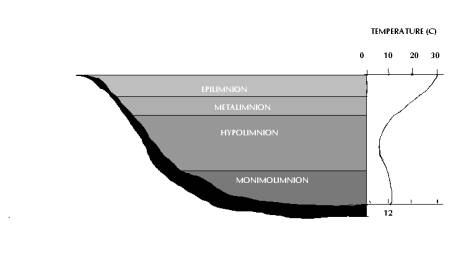
Fig 3: Thermal Stratification of a Meromictic Lake
LAKE
CHEMISTRY
The chemical and biological characteristics of the lake depend on the following:
(i) Formation
(ii) Basin size and shape
(iii) Topography and chemistry
(iv) Regional climate
(v) Biological community
(vi) Anthropogenic activity
Lakes are extremely variable in their physical, chemical and biological characteristics. Physically they vary in terms of the level of light, temperature and water currents. Chemically they vary in nutrients, major ions and contaminants, and biologically in terms of biomass, population numbers and growth.
Major ions:
The chemical composition of a lake is a function of its climate, hydrology and basin geology. Each lake has three major anions and four major cations (Table 1), which are in ionic balance. Ion balance means the sum of negative ions equals the sum of positive ions when expressed in equivalents. These ions are expressed as mg/L (ppm).
|
Anions |
Percent |
Cations |
Percent |
|
HCO3 |
73% |
Ca 2+ |
63% |
|
SO4 |
16% |
Mg2+ |
17% |
|
Cl |
10% |
Na+ |
15% |
|
|
|
K+ |
4% |
|
Other |
<1% |
Other |
< 1% |
Table 1: Ion balance for fresh water
Anthropogenic activities have profound influence on lake chemistry. Excessive landscape disturbance causes higher rates of leaching and erosion by removing vegetative cover, exposing soil and increasing water runoff velocity. Lakes with high concentrations of calcium and magnesium are called hard water lakes, while those with low concentrations of these ions are called soft water lakes.
H+ (hydrogen ion) contributes to the acidity of the lake water; while mercury (Hg) another significant pollutant, affecting the aquatic ecosystems, can bioaccumulate in the aquatic food webs, contaminating fish and causing threat to humans and wildlife. Anions and cations carry electrical charges, and the water containing them can conduct electricity and the measurement of the total quantity of charged particles is called as conductivity or TDS (total dissolved solids). TDS concentration and the relative ratios of different ions influence the type of organisms that can best survive in the lake and also affect the chemical reactions occurring in the water.
All the ions listed in the above table are vital to the health of the living organisms in the lake. For eg., calcium is essential for all the cell processes of plants and animals and serves as a structural component for the shells of invertebrate animals like molluscs. Magnesium is important for photosynthesis. Iron is a major component of the red blood pigment haemoglobin (found in all invertebrates) and also for certain cell processes. Silica is essential for the growth of diatoms as their outer cast (frustule) is entirely made up of silica.
Dissolved
oxygen:
Most aquatic organisms require oxygen for their metabolic activities. The supply of oxygen in water comes from the exchange with the atmosphere or from photosynthesis in flora and cyanobacteria (blue green algae). Photosynthesis produces organic matter and releases oxygen, whereas aerobic respiration consumes organic matter and uses oxygen. Oxygen production (photosynthesis) predominates in the light whereas oxygen consumption takes place in the dark. Based on these processes lakes can be divided into two zones namely lighted trophogenic zone (where organic matter is synthesised and oxygen generated) and tropholytic zone (leads to decomposition of organic matter and lowering of oxygen level).
The trophogenic zone often corresponds to the epilimnion. The organic matter produced during photosynthesis sinks to the bottom but the oxygen remains in the surface layers (epilimnion). Hence, there is a sharp delineation between oxygen production and consumption. The entire water mass is oxygenated only during periods of circulation, which occur when the surface waters are disturbed by wind. During stagnation periods (absence of wind), only the epilimnion is oxygenated. The oxygen required for the decomposition of organic matter that sinks to the bottom must therefore come from the supply obtained during periods of circulation. Thus the oxygen levels in the hypolimnion are constantly depleted.
The critical determinants of the oxygen balance in a lake are morphometry and productivity. Among the lakes having same productivity, those with greater depth will decompose organic matter with little changes in the dissolved oxygen while shallow lakes lose oxygen completely. The partially decomposed organic matter settles as sediments in the bottom. The layer of water that is in contact with the sediments tends to lose oxygen as decomposition process continues in the sediment. Lower dissolved oxygen in the water overlying the sediments can aggravate water-quality deterioration because lowered DO levels (below 1 mg/L) initiate chemical reactions at the sediment-water interface, releasing phosphorous from the sediments into the water. When the lake mixes due to wind during summer, phosphorous and ammonium that is built up in the sediment triggers algal growth.
The vertical change in oxygen concentration is dependent on the pattern of circulation. In holomictic lakes, water is saturated with oxygen from the top to the bottom. Such oxygen profiles are called as orthrograde curves. In productive lakes where oxygen levels drop to zero in the hypolimnion, the oxygen curve is called as clinograde curve. Aquatic life in lakes has evolved many adaptations for the range of oxygen conditions in the lake. Many organisms have life cycles adapted to the predictable changes in the oxygen conditions such as obligate anaerobic microorganisms, which can live only in regions devoid of oxygen.
Determination of DO concentrations is a fundamental part of water quality assessment since dissolved oxygen is involved in or influences all the chemical and biological reactions in water. Concentrations below 5 mg/L may adversely affect the functioning and survival of biological communities and below 2 mg/L may lead to fish mortality. The measurement of DO can be used to indicate the degree of pollution by organic matter, destruction of organic matter and level of self purification of the water.
pH:
The pH is a measure of the acid-base balance of a solution and is defined as the negative of the logarithm to the base 10 of the hydrogen ion concentration. The pH scale runs from 0 to 14 (i.e., very acidic to very alkaline) with pH 7 representing a neutral solution. At a given temperature pH indicates the acidic or basic character of the solution and is controlled by chemical and biochemical processes in the solution. Acid-base balance of a waterbody can be affected by inflow of industrial effluents, domestic sewage and atmospheric deposition of acid-forming substances. Diurnal variations in pH can take place due to photosynthesis and respiration cycles of algae in eutrophic waters. Acidity of water is controlled by strong mineral acids, weak acids such as carbonic, humic and fulvic acids, and hydrolysing salts of metals such as iron and aluminium.
Vertical difference in biological activity in the lake may lead to vertical changes in pH. The three main processes that affect lake pH are photosynthesis, respiration and nitrogen assimilation. The effect of photosynthesis and respiration depends on carbonate-bicarbonate-carbon dioxide equilibrium. The simplified formulae for photosynthesis using carbon dioxide or bicarbonate are]
6CO2 + 6 H2O ® C6H12O6 + 6O2
6 HCO3 + 6 H ® C6H12O6 + 6O2
From the above equations it is obvious that no protons are used when carbon-di-oxide is taken up during photosynthesis, whereas one proton per carbon atom is used when bicarbonate ions are used for the same. The reverse happens during respiration. When pH is less than 6.3 and only carbon dioxide is present, photosynthesis and respiration have no effect on the pH. At higher pH values, when other forms of inorganic carbon are available, photosynthesis and respiration alter the uptake and release of protons. This affects the alkalinity or acid-neutralising capacity of the water.
Nutrients:
Nutrients are the basic requirements of plants for their growth along with water and sunlight. Aquatic plants and algae respond to even small changes in the amount of nutrients present in the water. Hence it is necessary to estimate the concentrations of nutrients in the lake water and inflows to the lake. The identification of the watershed areas and land use activities that contribute to these nutrients in the lake water is essential. The two most important nutrients contributing to anthropogenic or cultural eutrophication are nitrogen and phosphorous. Both these nutrients are present in sufficient concentrations in fresh waters to maintain a healthy ecosystem, but anthropogenic activities may alter their concentrations contributing to algal blooms.
Phosphorous and nitrogen enter the lake not only as inorganic ions but also as inorganic polymers, organic compounds, living micro organisms and detritus. Only a few of these forms are readily available for plant and algal growth. A nutrient-poor lake may have only about 1mg/l of phosphorous or 50 mg/L of nitrogen, while the most fertile lake may have up to a milligram of phosphorous or 20-30 mg/L of nitrogen.
Nitrogen
compounds:
Plants and microorganisms convert inorganic nitrogen to organic nitrogen. The inorganic compounds include nitrite, nitrate, ammonium ions and molecular nitrogen. These undergo biological and non-biological transformations in the environment. The major non-biological transformations include sorption (absorption and adsorption), voltalisation and sedimentation. Biological transformations include: a) assimilation of inorganic ions (ammonia and nitrate) by plants and microorganisms to form organic nitrogen (amino acids); b) reduction of nitrogen gas to organic nitrogen and ammonia by microorganisms; c) oxidation of ammonia to nitrite and nitrate (nitrification); d) conversion of organic compounds to ammonia during the decomposition of organic matter; e) bacterial reduction of nitrate to nitrous oxide and molecular nitrogen under anoxic conditions (denitrification).
In lakes where the concentration of nitrogen compounds is extremely low, plants can take up additional inorganic nitrogen immediately. The discharge of sewage into the water body causes large growth of algae (if discharged directly into the lake or river). Raw sewage normally contains complex organic compounds of nitrogen that is decomposed by bacteria before they are used by plants. Eventually bacteria use up all oxygen present in the water, which leads to the death of aquatic plants and animals. Along with sewage, sometimes, fertilisers are washed off from the surrounding agricultural land, which adds to eutrophication (the process of enrichment of lakes due to addition of nutrients).
Ammonia:
Ammonia occurs naturally in water due to the breakdown of nitrogenous organic and inorganic matter in soil and water, excretion by biota, reduction of nitrogen gas in water by micro-organisms and gas-exchange in the atmosphere. At certain pH levels, high concentrations of ammonia are toxic to aquatic life and are detrimental to the ecological balance of water bodies. In aqueous solution, un-ionised ammonia exists in equilibrium with ammonium ion. Total ammonia is the sum of these two forms, also forming complexes with several metal ions, and may be adsorbed into colloidal particles, and suspended and settled sediments. The concentrations of un-ionised ammonia depend on pH, temperature and total ammonia concentration. Unpolluted waters may contain small amounts of ammonia and ammonia compounds (less than 0.1mg/L as nitrogen), but may reach 2-3 mg/L N. Higher concentrations are an indication of organic pollution due to sewage, industrial waste and fertiliser run-off. Natural seasonal fluctuations also occur as a result of death and decay of aquatic organisms, particularly phytoplankton and bacteria in nutritionally rich waters.
Nitrate and nitrite:
Nitrate ions are commonly found in natural waters. It may be reduced to nitrite by denitrification process (usually under anaerobic conditions). The nitrite ions rapidly oxidise to nitrate, which is an essential constituent of aquatic plants, although seasonal fluctuations can occur due to plant growth and decay. The natural concentration of 0.3 mg/L of nitrate may be enhanced by fertilisers in the runoff, and industrial and municipal wastewaters (to 5 mg/L). In lakes, concentration of nitrate in excess of 0.2 mg/L of nitrate nitrogen stimulates algal growth leading to eutrophication. Nitrite concentrations in freshwaters are usually low (0.001 mg/L). High nitrite concentrations in water are indicative of poor microbiological quality of water. Determination of nitrite and nitrate in surface waters gives a general indication of the nutrient status and level of organic pollution. As the determination of nitrate is difficult due to interferences from other substances in the water, the precise choice of method may vary according to the expected concentration of nitrogen as nitrate.
Organic nitrogen:
Organic nitrogen consists of protein substances and the product of their transformations. It is subject to seasonal fluctuations of the biological community, formed in water by phytoplankton and bacteria, and recycled within the food chain.
Phosphorous:
Phosphorous is an essential nutrient for living organisms and exists in water bodies as dissolved and particulate matter. In natural waters, it occurs mostly as dissolved orthophosphates and polyphosphates and organically bound phosphates. Changes between these forms occur continuously due to the degradation and synthesis of organically bound forms and oxidised inorganic forms. Phosphorous is rarely found in high concentrations in freshwaters and ranges from 0.005 to 0.020 mg/L. High concentrations of phosphates can indicate the presence of pollution and are responsible for eutrophic conditions. Carbon and nitrogen are abundant in natural waters and additionally available from gases in the atmosphere. In majority of lakes, availability of phosphorous is the limiting factor, which controls the rate at which plants grow and therefore the productivity of the whole plant community. Phosphorous is much more readily lost from an ecosystem than nitrogen and carbon as it reacts with mud and chemicals in water in ways that make it unavailable for plants. Plants can absorb phosphorous only as dissolved inorganic phosphorous, which is rapidly taken up by algae and macrophytes.
Bacteria in the sediments at the bottom of the lake break down organic content of dead plants and animals and phosphate is released into the water in the spaces between the sediment particles. This process is rapid in sediments devoid of oxygen. In a lake with oxygenated water, even a thin layer of sediment having oxygen will act as a barrier and prevent the release of phosphate from the sediments below (which is deoxygenated). If water becomes deoxygenated (as in summer), phosphate is slowly released from the sediments. In a shallow lake, however, the phosphate may stay locked in the deep layers, but due to the lack of depth the wind mixes the water and releases the phosphate from the sediment. Aquatic plants and algae absorb the released phosphate in the water and their population’s increase. This enhances the death and decomposition of more phosphate containing materials in the water, which in turn reduces the oxygen levels and speeds up the release of more phosphate. This is a cyclical process and explains why shallow lakes are productive.
|
Photosynthesis
and respiration All green plants make their own food by the process known as photosynthesis. They trap energy from sunlight with the aid of chlorophyll and with carbon dioxide and water form sugar. The sugars are stored and used as a source of chemical energy and combine with other molecules to form proteins, oils, fats and carbohydrates. The chemical energy stored as carbohydrates are used for respiration. All plants and animals must respire in order to stay alive, so unlike photosynthesis, it continues even in the dark. During respiration, the oxygen is taken in and combined with cell sugars to release energy. Water and carbon dioxide is released as waste products. |
Carbon:
Carbon atoms link to form complex organic materials, as carbon is the structural basis of all living material on earth. During photosynthesis, energy is used to link carbon compounds forming sugars, starch and cellulose. Plants take up carbon dioxide and fix it in plant material. The amount of carbon dioxide fixed in the plant material is measured as the amount of carbon fixed per volume of the water. The amount of carbon fixed in a given time is an important measure of the productivity of the lake. Plants obtain carbon from the atmosphere in the form of carbon dioxide and animals from plant material. Aquatic plants use carbon dioxide dissolved in the water but the amount depends on the acidity of the water. Above pH 5.0, some of the carbon dioxide molecules form bicarbonate ions and by pH 9 all the molecules are incorporated into bicarbonate plus some carbonate ions. Above pH 9.5 carbonate ions dominate and there is a lack of free carbon dioxide. Majority of living organisms also release carbon dioxide when they respire and this includes many of the bacteria involved in decomposition. These bacteria also release carbon from the bodies of dead plants and animals back into the water for use by living plants.
Organic
matter:
Organic matter in freshwater arises from living material (directly from photosynthesis or indirectly from terrestrial organic matter) and as a constituent of many waste materials and effluents. The total organic matter can be a useful indication of pollution. In surface waters, concentration of total organic carbon is less than 10 mg/L
Chemical Oxygen Demand (COD) is a measure of the oxygen equivalent of the organic matter in a water sample that is susceptible to oxidation by strong chemical oxidant such as dichromate. The concentration of COD in surface water ranges from 20mg/L oxygen or less in unpolluted waters to greater than 200 mg/L (in waters receiving industrial effluents).
Biochemical Oxygen Demand (BOD) is an approximate measure of the biochemically degradable organic matter present in the water sample. It is defined as the amount of oxygen required for aerobic microorganisms in the sample to oxidise the organic matter to a stable inorganic form. Unpolluted water typically has BOD value of 2mg/L, but those receiving effluent may have more than 10mg/L.
Humic and Fulvic acids: Organic matter from flora and fauna makes a major contribution to the natural quality of surface water, the composition of which is extremely diverse. Natural organic matter is not toxic but exerts major influence on biochemical and hydrochemical processes in the water body. Humus is formed by biochemical and chemical decomposition of vegetative residues and microorganism activity. It enters directly from the soil or is a result of biochemical transformations within the lake. Humus is divided into humic and fulvic acids. These concentrations are highly dependant on the physical-geographical conditions and range from 10 to 100 mg of carbon/litre.
Major ions: These include calcium, magnesium, sodium, potassium, chlorides, sulphates, bicarbonates, etc.
Calcium is present in all surface waters as Ca2+ and is usually dissolved from rocks rich in calcium minerals. The cation is abundant in surface water and along with magnesium is responsible for the hardness in water. It is an important constituent in all organisms and is incorporated into the shells of many invertebrates and bones of vertebrates. Calcium concentration in natural water is <15 mg/L.
Magnesium also contributes to hardness in water. It occurs in many of the organometallic compounds and in organic matter since it is an important element for living organisms. Natural concentrations of magnesium vary from 1 to > 100 mg/L.
The presence of carbonates and bicarbonates influences the hardness and alkalinity of water. Inorganic CO2 arises from atmosphere and biological respiration. The relative amounts of carbonates, bicarbonates and carbonic acid are related to pH. As a result of weathering of rocks combined with the pH range of surface waters (6-8.2), bicarbonate is found as the dominant anion in most surface waters. Carbonate is uncommon in natural surface waters because they rarely exceed pH 9.0.
Most chlorine occurs as chloride in solution. In a clean surface water, chloride concentrations are less than 10 mg/L and sometimes less than 2 mg/L. Higher concentrations can occur due to sewage, industrial effluents and agricultural and road run-off.
Sulphate is naturally present in surface water as SO4. It is the stable, oxidised form of sulphur that is readily available in water. Industrial discharges can also add significant amounts of sulphate to surface waters. Sulphate can be used as an oxygen source by bacteria that convert it into hydrogen sulphide (H2S) under anaerobic conditions. Sulphate concentrations in natural waters are usually between 2mg/L to 80 mg/L and exceed 1000 mg/L near industrial discharge.
Sodium is found in the ionic form and in plant and animal matter. All natural waters contain some sodium, as its salts are highly soluble in water. Sewage and industrial effluents increase the presence of sodium, commonly measured where water is used for drinking or agriculture, particularly irrigation. The surface waters, including those receiving wastewater have concentrations well below 50 mg/L.
Potassium is found usually in the ionic form, its concentration is low in natural water (less than 10 mg/L). Potassium salts are highly soluble. It is readily incorporated into mineral structures and accumulated by aquatic biota, as it is an essential nutrient.
Sulphide formation in surface waters is principally through anaerobic, bacterial decay of organic substances in the sediment. Traces of sulphide ion occur in unpolluted bottom sediments due to the decay of vegetation but higher concentration often indicates the presence of sewage or industrial effluents. Due to its high concentration, toxicity and strong odour make the water unsuitable for drinking and other uses.
Fluoride originates from weathering of fluoride containing minerals and enters surface waters from run-off and groundwater. Its concentrations vary from 0.05 to 100 mg/L.
Metals: The metals in freshwater ecosystems include aluminium, cadmium, chromium, copper, iron, mercury, zinc, nickel, manganese and lead. The ability of a water body to support aquatic life as well as its suitability to other uses depends on the concentration of metals. Some metals are important for physiological functions of living tissue and regulate many biochemical processes. The same metals, discharged at increased concentrations in sewage and industrial effluents, can have severe toxicological effects on humans and aquatic ecosystems. Due to lack of proper elimination measures, metals move from one trophic level to the other bioaccumulating, often with detrimental effects. The toxicity of metals depends on the degree of oxidation of a given metal ion together with the forms in which it occurs. Metals in natural waters can exist in truly dissolved, colloidal and suspended forms. The concentrations of different metals in water vary over a wide range (0.1 – 0.001 mg/L) and can rise to higher concentrations due to anthropogenic activities.