Fig. 1. Location map of Estonia within Europe.
| Previous Session | Paper 1 | Paper 2 | Next Session |
SESSION-13
PAPER-2: Nuisance alga Gonyostomum semen
: Implications for Its Gglobal Expansion
Reet Laugaste & Peeter Nõges
CONTENTS-
Abstract
Introduction
Study Site
Materials and Methods
Results
Discussion
Acknowledgements
References
| Abstract | up | previous | next | last |
The large raphidophyte Gonyostomum semen is a world-wide distributed flagellated that causes allergic reactions to people swimming in lakes. Its mass development was first recorded by Drouet & Cohen (1935) in Cedar pond, Massachusetts. Regional reviews of the distribution and ecology of G. semen are published for Sweden (Cronberg et al ., 1988) and Finland (Lepistö et al ., 1994). We analysed phytoplankton and water chemistry data collected from 269 Estonian small lakes since the 1950s. Being not found in Estonian lakes until the 1950s, this species is now common in 63 lakes with soft (HCO 3 - <25 mg l -1 ) brown water and high organic matter content (COD Cr >40 mgO l -1 ). It achieved maximum biomass (> 100 g m -3 ) in dystrophic lakes with extremely dark water (Secchi depth < 1 m; COD Cr 60-100 mg O l -1 ). During last years, the species has invaded some oligo- and semidystrophic lakes. Processes connected with lake acidification are considered the main reason for the expansion of G. semen. High phosphorus levels favour the alga. The vertical migration ability gives to the species a certain independence of the phosphorus deficiency during stratification.
| 1. Introduction | up | previous | next | last |
Gonyostomum semen (Ehrb.) Diesing is a large (length 50-100 µm) raphidophyte with two flagella. The cell membrane is thin and fragile and brakes easily on physical contacts with other bodies. Numerous trichocysts (slime bodies) located inside the cell membrane are characteristic of the species and explode on strong stimulation throwing out up to 200 µm long slime threads (Cronberg et al., 1988). On stronger physical forcing cells are totally destroyed and form together a slimy mass that covers fish nets and bodies of swimmers causing itching and allergic reactions to people swimming in lakes. Already a G. semen concentration of 100 ind. ml -1 gives a typical slimy cover on bathers' skin (Eloranta & Palomäki, 1986). With gentle stimulation only few trichocysts eject, the alga remines alive and can keep attackers at distance. The cells of G. semen break also on sampling with a plankton net and on adding formalin or Lugol solution to the samples. As mentioned by Hongve et al. (1988), bunches of hundreds of spherical chloroplasts remaining in the preserved sample are often taken for detritus or even identified as Microcystis colonies. Hard recognizability in preserved samples is probably the main reason why G. semen has been often overlooked.
G. semen was first described by Ehrenberg in 1853 under the name of Monas semen from a small pond outside Berlin. Up to now, the occurrence of G. semen has been stated from Sweden, Norway, Denmark, Finland, Austria, Slovakia, Czechia, Russia, USA, Canada, South America (Bourrelly, 1970) and Africa (Gerrath & Denny, 1980) showing a world-wide distribution of the species. Mass development of G. semen was first recorded by Drouet & Cohen (1935) in Cedar pond, Massachusetts and by Sörensen (1954, cit. Cronberg et al., 1988) in Sweden in 1948. In Finland G. semen was recorded already in 1894, but the first complaints from swimmers came in 1978 (Lepistö et al., 1994). The same authors analyse the distribution of the species in Finland during the period of 1978-1989. Starting as a small spot in Southeastern Finland, the distribution area expanded and reached almost the polar circle by the end of the period. Judging upon the distribution of G. semen by literature data, the expansion of distribution area during last decades is undisputable. Fast expansion of G. semen is expressed in 1) extension of the distribution to new areas, 2) occupying new habitats (lake types) within the distribution area, and 3) achieving mass development in several lakes.
| 2. Study Site | up | previous | next | last |
Estonia is situated in the northeast of Europe, on the east coast of the Baltic Sea, north of Finland. It is the northernmost of the three Baltic states - Estonia, Latvia and Lithuania. Geologically, Estonia lies on the southern slope of the Fennoscandian shield. As Estonia is risen from the seabottom, its surface is mostly flat. The dominating bedrock is Ordovician-Silurian limestone in the northern and Devonian sandstone in the southern

Fig. 1. Location map of Estonia within Europe.
part of Estonia. The variety of landscapes is due to the retreating ice shield, which changed the appearance of the country completely after the last glaciation period some 10 thousand years ago. At present, Estonia belongs to the temperate climate region. The long-term mean air temperature in January is between -2.4 and -7.4°C and that of July between 16.3 and 17.3°C. There are about 1150 small lakes in Estonia with an aquatory of over 1 hectare. The two largest lakes Peipsi and Võrtsjärv together make up nearly 90% of the total area of lakes. There is a large variety of lake types in Estonia from calcareous to soft water lakes, from uncoloured to dark brown lakes, from alkaline to acidic lakes. The lakes are usually ice-covered from November to April, in summer the water temperature is around 20°C, on average.
We analysed phytoplankton and water chemistry data collected from 269 small lakes in Estonia since the 1950s. Sampling was performed mostly in summer (July - August), spring data were available from less than half of the lakes while whole year seasonal data were only from Lake Valguta Mustjärv. Biomasses of different species were determined as direct chamber counts multiplied by cell volumes. Partly old samples were re-examined for G. semen. Water transparency was measured using the Secchi disk. Spectrophotometric light absorbance in filtered (GF-C filters) water at 400 nm wave length against distilled water was used as an equivalent for water colour. Two methods were used to determine the concentration of organic matter: the permanganate and the bichromate methods. Both the total and mineral forms of nutrients N and P were measured using standard methods. The buffering capacity was evaluated on the basis of HCO 3 - concentration and pH. Using the biomass of G. semen as a grouping variable, all sets of parameters measured during individual visits to lakes were divided into four groups: 1 - cases where the species was absent, 2 - cases where the biomass was less than 2 g m -3 , 3 - cases when the biomass was between 2 and 10 g m -3 , and 4 - cases of mass development of G. semen characterised by a biomass of more than 10 g m -3 .
| 4. Results | up | previous | next | last |
For the first time in Estonia, G. semen was found from a small brown water lake (Orava Mustjärv) in South-eastern Estonia in 1983. However, re-examining the old materials, characteristic remains were found from a number of samples taken during earlier decades (Fig. 2). Up to now, G. semen has been found from 63 lakes. More than one half of these lakes (33) are located in Põlva and Võru counties in Southeast Estonia, and 13 in Virumaa county in North Estonia. The alga was found also in two lakes located on the Saaremaa island. The seasonally investigated L. Valguta Mustjärv is still the only lake in Tartu county where G. semen has been found.
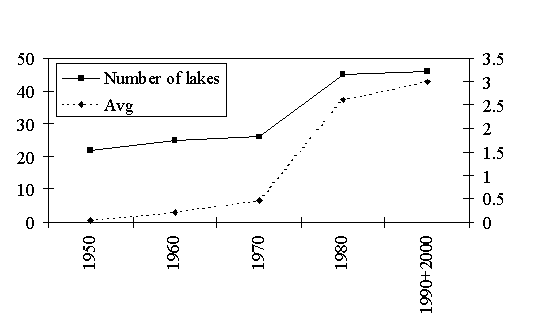
Fig. 2. Number of lakes with G. semen and the average abundance of the species on a 5-step scale by decades
Comparing cases of G. semen absence with those of its occurrence (Table 1, Fig. 3), we found that the latter cases were characterised by significantly lower water transparency, higher light absorbance in the blue region of the spectrum (browner water), lower pH, higher chemical oxygen demand, at least ten times smaller alkalinity and nitrate nitrogen content, and at least twice smaller total nitrogen content. As the standard deviation of several variables (NH 4 -N, PO 4 -P, Ptot and N/P ratio) was very high within the group of cases where G. semen was absent (this group included data from nearly all lake types), the between-group differences remained insignificant. With increasing biomass of G. semen , there occurred continuous increasing trends in water colour and chemical oxygen demand (both COD Mn and COD Cr ) and continuous decreasing trends in Secchi depth and nitrate nitrogen content. Within Gonyostomum lakes, there were significant differences in COD, total nutrients and their ratio when low biomass cases (B<2) and high biomass cases (B>10) were compared. Cases of medium (B=2…10) and high G. semen biomass (B>10) differed significantly only by total phosphorus concentration.
G. semen was found mainly in softwater lakes (HCO 3 - < 25 mg l -1 ) with brown water and high content of dissolved organic matter (COD Cr >40 mg O l -1 ). It achieved the highest abundance (maximum biomass > 100 g m -3 ) in dystrophic lakes with extremely dark water (Secchi depth < 1 m; COD Cr 60-100 mg O l -1 ). Most of these lakes are located in sandy areas in Põlva county. In typical bog lakes and in semidystrophic brown-water lakes the abundance was smaller. G. semen colonises typical bog pools rather seldom and with low abundance. In the second half of the1980s, the species occurred with rather high abundance in four eutrophic soft water lakes. Since the 1990s, G. semen appeared in two oligotrophic and four semidystrophic soft water lakes. As a rule, the biomass increases with increasing nutrient content and with decreasing N/P ratio.
In the seasonally studied dyseutrophic forest lake Valguta Mustjärv, G. semen occurred from may to September and reached its seasonal biomass maximum usually in July, in some years in June. It was quite rare in August and September but common in May, although, the biomass remained less than 1 g m -3 . In brown-water lakes the biomass maximum was usually found in the epilimnion, in non-coloured lakes in the metalimnion or near the bottom when the lakes were shallow.
Table 1. Significance ( p ) of the differences between average values of hydro-optical and hydrochemical variables in sets of data grouped by the biomass of Gonyostomum semen (B=0; B<2; B=2…10; and B>10). Significant differences ( p <0.05) are marked in bold
Variable |
B=0 vs. B<2 |
B<2 vs. B=2…10 |
B<2 vs. B>10 |
B=2…10 vs. B>10 |
Secchi |
0.000 |
0.382 |
0.208 |
0.579 |
A400 |
0.000 |
0.249 |
0.321 |
0.933 |
pH |
0.000 |
0.055 |
0.825 |
0.384 |
COD Mn |
0.000 |
0.102 |
0.001 |
0.232 |
COD Cr |
0.000 |
0.125 |
0.001 |
0.161 |
HCO 3 - |
0.000 |
0.878 |
0.495 |
0.459 |
NO 3 -N |
0.001 |
0.127 |
0.143 |
0.240 |
NH 4 -N |
0.461 |
0.093 |
0.297 |
0.468 |
PO 4 -P |
0.418 |
0.379 |
0.518 |
0.590 |
Ntot |
0.000 |
0.151 |
0.007 |
0.160 |
Ptot |
0.390 |
0.267 |
0.000 |
0.030 |
N/P ratio |
0.133 |
0.376 |
0.043 |
0.208 |
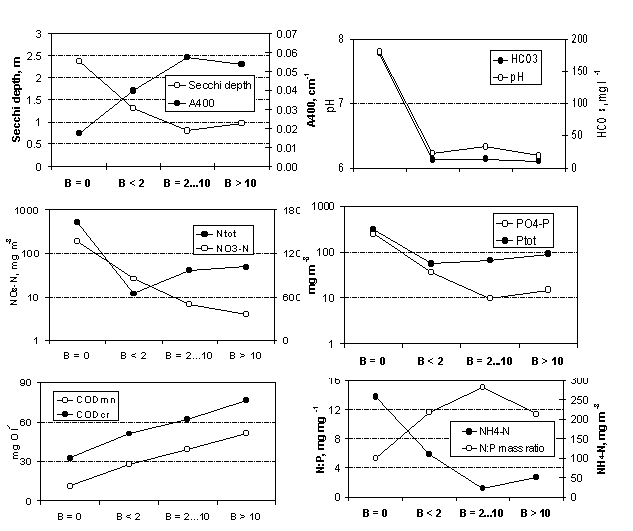
Fig. 3. Changes of hydro-optical and hydrochemical parameters of lakes grouped by the biomass of Gonyostomum semen.
Changes in the distribution of G. semen can be followed during the last 15 years. During this period, the frequency of occurrence as well as the biomass have undoubtedly increased. We can observe two clear tendencies: 1) the increase of G. semen biomass in dystrophic lakes around Põlva, especially in the 1990s, and 2) colonisation of new habitat types in oligotrophic and semidystrophic softwater lakes where its earlier absence is well documented.
| 5. Discussion | up | previous | next | last |
We suppose that the actual distribution of G. semen in Estonia is wider than described by us, because many of the bog lakes where the species was absent were visited for the last time in the 1960s. Also we are lacking information about a large number of big bog pools and some semidystrophic lakes.
G. semen occurs mainly in small, shallow, dyseutrophic lakes. For example, in Norway its distribution is limited by the mean lake depth of 15 m (Hongve et al, 1988). In Finland G. semen is numerous in small brown-water forest lakes. In Sweden it has been found on 70% of occasions in lakes with an area less than 50 ha and only 10% of lakes have been deeper than 5 m (Cronberg et al., 1988).
As a rule, G. semen requires pH less than 7, but there are different opinions concerning the lower limit. According to Rosén (1981), G. semen is pH-tolerant, requiring humus and occurring in lakes with a median water colour value of 60 mg Pt l -1 . Will é n et al. (1990) consider the species typical for humic (dystrophic) lakes with a median pH of 5.5, but not for very acidified (median pH 4.5) lakes. In Norwegian lakes G. semen prefers pH ranging from 5 to 7, and is absent at a pH less than 5 (Hongve et al., 1988). Rosenström & Lepistö (1996) analysing the indicator species for different types of Finnish lakes, put G. semen among species typical for dystrophic and eutrophic but not for acidic lakes (acidified non-coloured lakes with a very low pH). In Finland it occurred in the pH range of 6.2-7.5 (mean 6.8) and in the colour range of 12-149 mg Pt l -1 (mean 61). According to Cronberg et al. (1988), G. semen prefers slightly acidic or neutral environment but tolerates the pH range of 5-7.5. In two small lakes in Russia G. semen occurred at pH 4.5-6.6 (Korneva, 2000); in Japan G. semen was observed in the pH range of 4-7.5 and it disappeared at pH>8 (Kato, 1991, cit. Korneva, 2000). In Triangle Lake, a typical Gonyostomum lake in Ohio, pH is 4.9 (Havens, 1989).
Low pH is usually coupled with dark colour of water and high content of dissolved organic matter. Lepistö et al. (1994) divided their lakes into two groups on the basis of G. semen occurrence. Water colour and the contents of total P and total N were significantly higher in lakes where G. semen occurred while pH of these lakes was lower. Although the species prefers brown water, it has been seldom observed also in waters with low colour levels. Cronberg et al. (1988) point out a tendency of G. semen to invade less coloured lakes. All Gonyostomum lakes in Sweden have low buffering capacity (alkalinity < 0.1 meq l -1 ) and are therefore vulnerable to acid deposition. Low alkalinity as a main feature characterizing Gonyostomum lakes was stressed also by Korneva (2000) and LeCohu et al. (1989). Requirement of low alkalinity is probably the reason why G. semen has not spread into some semidystrophic lakes in Estonia having a nearly neutral pH but where the HCO 3 - content is about mg l -1 .
As a rule, most of the acidic lakes are extremly oligotrophic as a result of Al precipitation of phosphorus and humic compounds in the lake (Hornström et al., 1984 cit. Morling & Will é n, 1990). The expansion of G. semen during last decades is undoubtedly related to the tendency of eutrophication that has touched even the well buffered humic lakes. According to Eloranta & Palomäki (1986) G. semen occurs in the Finnish Lake Konnevesi abundantly in the most eutrophic part of the lake receiving effluent waters from a fish farm and is common and numerous also in eutrophic brown-water lakes around Konnevesi. Manninen (1988) mentions that the biggest biomasses of G. semen can be found in lakes with a high field percentage in their watersheds. Pithart et al. (1997) showed that the conditions needed to induce a bloom of G. semen in a pond in Czechia were low water level, low content of oxygen and nitrates, high content of phosphates and the dark colour of water. Cronberg et al. (1988) found a clear linear relationship between the contents of chlorophyll a and phosphorus in Gonyostomum lakes. Lindmark (1984) described a mass development of G. semen at the depth of 4-5 m in the non-coloured, softwater Lake Lilla Galtsjön in 1980 and 1981 (biomass 45 mg l -1 , chl a 105 µg l -1 ) after liming the lake with sodium carbonate that caused a rapid phosphorus release from the sediment. Also Hongve et al. (1988) considered the increasing eutrophication one of the main reasons for the expansion of G. semen as most of the Gonyostomum lakes have received at least some extra nutrients. They showed that G. semen dominated in the total P range of 10-50 µg l -1 and at total N more than1000 µg l -1 . The preferred N/P ratio was less than 30 and the content of inorganic nitrogen forms (NO 3 -N + NH 4 -N) was always less than 10 µgN l -1 . At higher P levels bluegreens started to dominate. As favouring the development of G. semen , Brettum (1989, cit. Salonen & Rosenberg, 2000) suggests the N/P ratio of 20-50, Ptot concentration of 7-25 µg l -1 and Ntot concentration of 200-500 µg l -1 . The Gonyostomum lakes in Estonia fit well in this range of the N/P ratio, but phosphorus concentrations are generally higher.
In Estonia G. semen reaches its biomass peak usually in July or August, but in other places it has been observed around midsummer (Havens, 1989; Korneva, 2000), at the end of summer or in early autumn (Morling & Will é n, 1990; Salonen & Rosenberg, 2000; Pithart et al., 1997), in France even in October-November (LeCohu et al., 1989). Mostly the species occurs in plankton from May to September, but Korneva (2000) has found it also in March under the ice. According to Salonen et al. (2002) G. semen dominated in Lake Valkea-Kotinen in 1990-1996 from the beginning of June to the end of September, but in1991 was numerous until November.
Several investigators have studied the vertical distribution and diel migrations of G. semen and mention its avoidance of high light intensities. The vertical biomass maximum has been found at the quantum irradiadiance of 80 µE m -2 s -1 (LeCohu et al., 1989). A similar limit was pointed out by Eloranta & Räike (1995), who showed that the upward migration of G. semen stops at a light intensity of ca. 75-95 µE m -2 s -1 . In a small artificial stratified water body of dark water (oxygen disappeared at 1.5 m, 1% light penetrated to 1 m) in Texas G. semen preferred the intermediate incident light levels at the surface at 9 a.m. and migrated to deeper layers with increasing surface irradiance (Van den Avyle et al., 1982). Avoidance of higher light intensities explains probably the fact that in non-coloured lakes in Estonia G. semen colonizes deeper layers has been never found near the surface. As the whole biomass may be concentrated within a very thin layer near the bottom, it may be overlooked already in the sampling phase. F or vertical migration G. semen needs a light gradient and probably also a nutrient gradient. However, Pithart et al. (1997) observed a diel migration of G. semen in a 1.9-m deep nonstratified pool with the biomass maximum located at the depth of 10 cm at noon and near the bottom at night. A similar migration pattern was found also by Salonen et al. (2002) in Finnish dark-water lakes where the biggest biomasses of G. semen occurred in the upper 1-m layer in the day time and in the hypolimnion during night. The authors pointed out the high motility of this large flagellate that enables long distance vertical migrations to the hypolimnion during night in order to recruit its cellular nutrient supplies. This gives an important competitive advantage and can explain its high proportion in phytoplankton. Migration to the cooler hypolimnion may also help to diminish respiration losses. Salonen & Rosenberg (2000) showed that part of G. semen population remains in the anoxic hypolimnion also during the daytime, especially near the end of stratification when the nutrient concentration in these layers has dropped. It could be explained by the increased time required to reload the nutrient reserves of algae. During this time, the algal uptake of soluble reactive phosphorus could cause its decrease in the hypolimnion. Staying in the hypolimnion might be also connected with predator avoidance as G. semen is seriously threatened by grazing of Holopedium gibberum and Polyarthra vulgaris . There are different opinions with regard to the edibility of G. semen . Havens (1989) explains the domination of G. semen with its inedibility that result from its large size and ability to discharge trichocysts when contracted by a predator. He relates vertical migrations mainly with obtaining hypolimnetic nutrients. Sanders & Wickham (1993), on the contrary, consider G. semen a favourite food for Diaptomus oregonensis and Daphnia pulicaria as this alga made up 27% of the food of Daphnia in the epilimnion and 94% in the hypolimnion. Hansson (1996) also related migrations with reducing exposure to grazers. He supposed that the expansion of G. semen together with the ongoing acidification might be explained by reduced abundance of efficient grazers at lower pH. Cronberg et al.(1988) consider both the nutrient transport and the a voidance of grazing as factors stimulating the upward migration of G. semen in the morning and downward migration in the afternoon.
LeCohu et al. (1989) found no relationship between vertical migration and nutrients. During homothermal periods G. semen was evenly distributed within the water column. During thermal stratification in summer, surface-avoidance by cells was observed, especially between noon and 4 p.m. and the biomass was strongly fluctuating. Maximum population doubling time ranged from 1.5-3.2 days.
Besides strong light avoidance, the incline of G. semen towards dark water lakes has been explained by its ability of heterotrophic carbon uptake (Havens, 1989). During a G. semen bloom located within a 1-m layer (between 0.5 and 1.5 m) in Triangle Lake (Ohio), light stimulated 3 H-glucose uptake. Basing on autoradiographic method, Buchanan (1982) supposed that it was not necessarily the direct uptake by algae but could be performed by intracellular bacteria. Korneva (2000) relates the occurrence of G. semen with high trophic state and high abundance of bacteria and supposes that the alga might be bacterivorous. In Triangle Lake Jiang et al. (1993) studied to what extent G. semen uses DOC and to what extent bacteria. The lake is rich in both of these potential food items. Experiments with labelled DOC and bacteria showed that G. semen is mainly autotrophic getting only a minor part of assimilated carbon from DOC but having no bactivorous activity.
Discussing the observed fast expansion of G. semen , Cronberg et al. (1988) conclude that the reason for that cannot be the changes in pH or water colour per se , although it might be related to acidification of lakes. Eloranta & Räike (1995) consider the expansion partly seeming, caused by more intensive investigation of small lakes but also being caused by different actions in forestry and peat processing that have increased water colour and turbidity, and also by agricultural eutrophication, fish farming and wood processing industry. Hongve et al. (1988) supposes that the expansion of G. semen can be also explained by recent implanation of species, or eventually a new genotype.
| 6. Acknowledgements | up | previous | next | last |
The investigation was supported by Estonian Science Foundation grants No. 3689
| References | up | previous | next | last |
Bourelly, P. (1970): Les algues d'eau douce. III. N. Boubee et Cie. Paris, 512 pp.
Buchanan, E.L. ( 1982): Planktonic photoheterotrophic and heterotrophic uptake of glucose in two acid lakes and one eutrophic lake in northeastern Ohio. – A dissertation submitted to the Kent State University Graduate College.
Cronberg, G., Lindmark, G. & Björk, S. (1988): Mass development of flagellate Gonyostomum semen (Raphidophyta) in Swedish forest lakes - an effect of acidification? Hydrobiologia 161: 217-236.
Drouet, F. & A. Cohen (1935): The morphology of Gonyostomum semen from Woods Hole, Massachusetts. Biol. Bull. 68: 422-439.
Eloranta, P. & Palomäki, A. (1986): Phytoplankton in Lake Konnevesi with special reference to eutrophication of the lake by fish farming. – Aqua Fennica 16, 1:37-45.
Eloranta, P. & Räike, A. (1995): light as a factor affecting the vertical distribution of Gonyostomum semen (Ehr.) Diesing (Raphidophyceae) in lakes. – Aqua Fennica 25: 15-22.
Gerrath, J.F. & P. Denny (1980): Freshwater algae of Sierra Leone III. Cyanophyta, Chrysophyta, Xanthophyta, Chloromonadophyta, Cryptophyta, Dinophyta. - Nova Hedwigia 33: 933-947.
Hansson, L.-A. (1996): Behavioural response in plants: adjustment in algal recruitment induced by herbivores. Proc. R. Soc. London 263: 1241-1244.
Havens, K.E. (1989): Seasonal succession in the plankton of a naturally acidic, highly humic lake in Northeastern Ohio, USA. – Journal of Plankton Research 11, 6: 1321-1327.
Hongve, D., Løvstad, Ø. & Bjørndalen, K. (1988): Gonyostomum semen – a new nuisance to bathers in Norwegian lakes. – Verh. Internat. Verein. Limnol. 23:430-434.
Jiang, Ping & Heath, R.T. (1993): Carbon sources of the alga Gonyostomum semen in an acid bog lake. – Ohio J. Sci. 93, 2: 45.
Korneva, L.G. (2000): Ekologicheskie aspekty massovogo razvitiya Gonyostomum semen (Ehr.) Dies. (Raphidophyta). - Al'gologiya 10, 3:265-277. Ecological aspects of mass development of Gonyostomum semen (Ehr.) Dies. (Raphidophyta), in Russian.
LeCohu, R., Guitard, J., Comoy, N. & Brabet, J. (1989): Gonyostomum semen (Raphidophyc é es), nuisance potentielle des grands r é servois français? L'exemple du lac de Pareloup. – Arch. Hydrobiol. 117, 2: 225-236.
Lepistö, L., Antikainen, S. & Kivinen, J. (1994): The occurrence of Gonyostomum semen (Ehr.) Diesing in Finnish lakes. - Hydrobiologia 273: 1-8.
Lindmark, G. (1984): Acidified lakes: Ecosystem response following sediment treatment with sodium carbonate. – Verh. Inter. Verein. Limnol. 22: 772-779.
Manninen, P. (1988): Gonyostomum semen (Ehr.) Dies. kannan tiheys ja elinolosuhteet humuspitoisissa lammissa. vesi- ja Ympäristöhallinnon Julkaisuja 14: 55-56.
Morling, G. & Will é n, T. (1990): Acidification and Phytoplankton Development in Some West-Swedish Lakes 1996-1983. – Limnologica (Berlin) 20, 2: 291-306.
Pithart, T., Pechar, L. & Mattsson, G. (1997): Summer blooms of raphidophyte Gonyostomum semen and its diurnal vertical migration in a floodplain pool. – Algol. Studies 85: 119-133.
Ros é n, G. (1981): Tusen sjöar - växtplanktons miljökrav. Statens Naturv å rdsverk. pp. 119.
Rosenström, U. & Lepistö, L. (1996): Phytoplankton indicator species of different types of boreal lakes. – Algol. Studies 82: 131-140.
Salonen, K. & Rosenberg, M. (2000): Advantages from diel vertical migration can explain the dominance of Gonyostomum semen (Raphidophyceae) in a small, steeply stratified humic lake. – J. Plankton Research 22, 10: 1841-1853.
Salonen, K., Holopainen, A.-L. & Keskitalo, J. (2002): regular high contribution of Gonyostomum semen to phytoplankton biomass in a small humic lake. – Verh. Internat. Verein. Limnol. 28, 488-491.
Sanders, R.W. & Wickham, S.A. (1993): Planktonic protozoa and metazoa: predation, food quality and population control. – Marine Microbial Food Webs 7, 2: 197- 223.
Van den Avyle, M.J., Allard, D.W., Dreier, D.M. & Clark, W.J. (1982): - Texas J. Science 34, 1: 69-78.
Will é n, E., Hajdu, S. & Pejler, Y. (1990): Summer phytoplankton in 73 nutrient-poor Swedish lakes. – Limnologica 20, 3: 217-227.
| Address: | up | previous |
Institute of Zoology and Botany,
Estonian Agricultural University,
Võrtsjärv Limnological Station,
61101 Rannu, Tartumaa, Estonia.