
 |
| 3.0 Materials and methods |
In this chapter, methods for using viable non-conventional low-cost adsorbents like tur dal husk (TDH); bengal gram husk (BGH), coffee husk (CH) and tamarind husk (TH) for removal of dyes fast green (FG) , methylene blue (MB) , amaranth (AM) and rhodamine B (RB).
3.1 MaterialsTur dal (Cajanus cajan) husk (TDH) and bengal gram (Cicer arientinu) husk (BGH), was collected from a legume seed-splitting mill. The coffee husk (CH) was collected from coffee processing unit and tamarind pod shells (TH) were obtained from a de-hulling unit. The four husks were washed extensively in running tap water to remove dirt and other particulate matter. This was later subjected to colour removal through washing and boiling in distilled water repeatedly. Subsequently the husks were oven dried at 105°C for 24 hours, stored in a desiccator and used for biosorption studies in the original piece size.
3.2 Preparation of adsorbate solutionsDye solutions: Individual stock solutions of 1000 mg/L of Fast green FCF, Methylene Blue, Rhodamine B and Amaranth were prepared by dissolving dyes in water. Solutions of various concentrations were prepared by using the above stock solution.
3.3 Determination of carbon, nitrogen and sulphur in the four husksTotal carbon, nitrogen and sulphur were determined, in order to understand the dye binding mechanisms of four agricultural byproducts. Elemental analysis was carried out with a C.H.N. 1106 Carlo Erba MicroAnalysing device equipped with inductive furnace analyzer. Samples of the four husks were put in an oven at 1000°C under oxygen in order to obtain a quick and complete combustion. 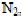 ,
, 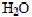 and
and 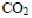 were released and conducted in a copper oven at 650°C, then passed through a 2 m column with helium vector gas, and analyzed by a catharometer detector.
were released and conducted in a copper oven at 650°C, then passed through a 2 m column with helium vector gas, and analyzed by a catharometer detector.
FT-IR spectra of the four adsorbents namely BGH, TDH, CH and TH were obtained using shimadzu, Model FTIR – 8201PC. The infrared spectral analysis was done to determine the functional groups responsible for the adsorption of dyes. As chemical bonds absorb infrared energy at specific frequencies (or wavelengths), the basic structure of compounds can be determined by the spectral locations of their IR absorptions. The plot of a compound's IR transmission vs. frequency is its "fingerprint", which when compared to reference spectra identifies the material.
3.5 Analysis of adsorbatesEstimation of dyes
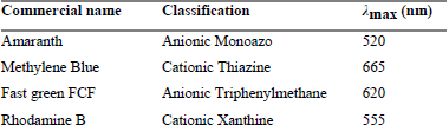
Absorbance measurements were carried out using Visible Spectrophotometer. Absorption maxima of dyes were used as the monitoring wavelengths for the estimation of dye adsorption. Absorption maxima for the dyes, Fast green, Methylene Blue, Rhodamine B and Amaranth were found to be 620, 665, 555 and 520 nm respectively.
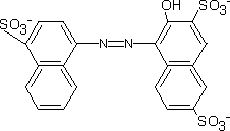 (a) Amaranth (a) Amaranth |
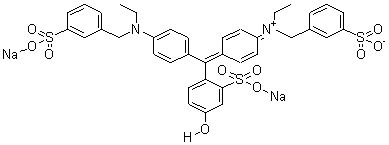 (b) Fast green
(b) Fast green |
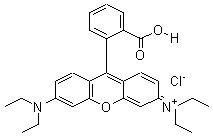
|
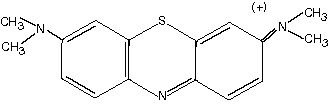 |
Batch mode adsorption studies for individual dyes were carried out to investigate the effect of different parameters such as adsorbate concentration, adsorbent dose, agitation time and pH. Solution containing adsorbate and adsorbent was taken in 250 mL capacity beakers and agitated at 150 rpm in a mechanical shaker at predetermined time intervals. The adsorbate was decanted and separated from the adsorbent using Whatman No.1 filter paper. To avoid the adsorption of adsorbate on the container walls, the containers were pretreated with the respective adsorbate for 24 hours.
3.6.1 Effect of agitation timeFor the determination of rate of dye biosorption by BGH, TDH and TH from 100 ml (at 10, 20, 50, 100 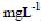 ), the supernatant was analysed for residual dye at different time intervals. The pH and the adsorbent dosage was kept constant, which varied according to the adsorbent and adsorbate under consideration.
), the supernatant was analysed for residual dye at different time intervals. The pH and the adsorbent dosage was kept constant, which varied according to the adsorbent and adsorbate under consideration.
The effect of adsorbent dosage i.e., the amount of the four husks on the adsorption of dyes was studied at different dosages ranging from 1 to 40 g/l with varied dye concentrations of 10, 20, 50 and 100 mg/L. The equilibrium time and the pH were kept constant depending on the dye under consideration.
3.6.3 pH effectTo determine the effect of pH on the adsorption of dyes solutions (100 mL) of different concentration ranges (0-100 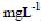 ) were adjusted to desired pH values and mixed with known weight of adsorbent and agitated at preset equilibrium time. The equilibrium time and adsorbent dosage varied with the dye and adsorbent under consideration.
) were adjusted to desired pH values and mixed with known weight of adsorbent and agitated at preset equilibrium time. The equilibrium time and adsorbent dosage varied with the dye and adsorbent under consideration.
After adsorption, the adsorbates – loaded adsorbent were separated from the solution by centrifugation and the supernatant was drained out. The adsorbent was gently washed with water to remove any unadsorbed adsorbate. Regeneration of adsorbate from the adsorbate – laden adsorbent was carried out using the desorbing media – distilled water at pH ranges 4.0 to 12.0 using dilute solutions of NaOH and HCl. Then they were agitated for the equilibrium time of respective adsorbate. The desorbed adsorbate in the solution was separated and analyzed for the residual dyes.
This Section presents the results obtained from the batch studies of biosorption of dyes by the four agricultural by products namely bengal gram husk, coffee husk, tur dal husk and tamarind husk. The four dyes include amaranth, methylene blue, fast green and rhodamine B. Preliminary results showed that coffee husk was not efficient in the biosorption of dyes. Hence the results of biosorption of dyes by coffee husk are not presented here.