
 |
| 1. Introduction |
Fresh water is already a limiting resource in many parts of the world. In the next century, it will become even more limiting due to increased population, urbanization, and climate change. This limitation is caused not just by increased demand for water, but also by pollution in freshwater ecosystems. Pollution decreases the supply of usable water and increases the cost of purifying it. Some pollutants, such as heavy metals or chlorinated organic compounds, contaminate aquatic resources and affect food supplies. This nutrient pollution, combined with human demand for water, affects biodiversity, ecosystem functioning, and the natural services of aquatic systems upon which society depends. Point sources are 'pipeline' discharges of pollutants to receiving waters, e.g. domestic sewage discharges or industrial waste effluents from factories or plants. They are relatively easy to identify and isolate. In contrast, non-point pollution results from storm runoff, which transports polluting materials diffusely and over land.
Major water pollutants include a variety of organic and inorganic chemicals such as heavy metals and industrial compounds. They can affect human health and/or interfere with industrial or agricultural water use. If the level of a pollutant in the water supply exceeds an acceptable level for a given water use (e.g., domestic or industrial water supply), the water is considered unsafe or too degraded for that use. Solutions to such pollution problems, therefore, usually focus on reduction of pollution at the source and/or treatment of the polluted water prior to use (Ahalya and Ramachandra, 2002).
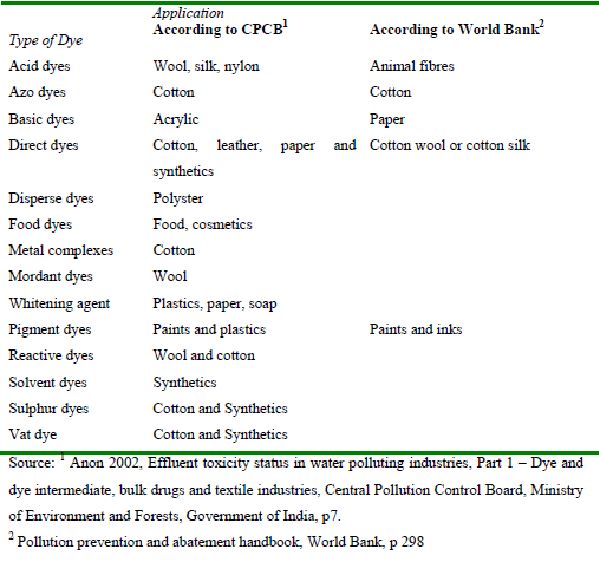
Dyes are basically chemical compounds that can attach themselves to fabrics or surfaces to impart colour. Most dyes are complex organic molecules and are resistant to weather, action of detergents, etc. Synthetic dyes are extensively used in many fields of up-to-date technology, e.g., in various branches of the textile industry (Gupta et al., 1992; Shukla and Gupta, 1992 and Sokolowska-Gajda et al., 1996), of the leather tanning industry (Tünay et al., 1999 and Kabadasil et al., 1999) in paper production (Ivanov et al., 1996), in food technology (Bhat and Mathur, 1998 and Slampova et al., 2001), in agricultural research (Cook and Linden, 1997 and Kross et al., 1996), in light-harvesting arrays (Wagner and Lindsey, 1996), in photoelectrochemical cells (Wrobel et al., 2001), and in hair colourings (Scarpi et al., 1998). Moreover, synthetic dyes have been employed for the control of the efficacy of sewage (Morgan-Sagastume et al., 1997) and wastewater treatment (Hsu and Chiang, 1997 and Orhon et al., 1999), for the determination of specific surface area of activated sludge (Sorensen and Wakeman, 1996) for ground water tracing (Field et al., 1995), etc. Dyes can be classified according to their chemical structure or according to their use. However, classifications vary from country to country though there are some fundamental categories that are common to all. According to the central pollution control board (CPCB), India there are approximately a million known dyes and dye intermediates out of which 5,000 are produced commercially. Based on their use based classification, the dyes are divided into 15 groups.
Unfortunately, the exact amount of dyes produced in the world is not known. It is estimated to be over 10,000 tonnes per year. Exact data on the quantity of dyes discharged in the environment are also not available. It is assumed that a loss of 1–2% in production and 1–10% loss in use are a fair estimate. For reactive dyes, this figure can be about 4%. Due to large-scale production and extensive application, synthetic dyes can cause considerable environmental pollution and are serious health-risk factors. The growing concern of environmental protection has influenced industrial development promoting the development of ecofriendly technologies (Desphande, 2001), reduced consumption of freshwater and lowers output of wastewater (Knittel and Schollmeyer, 1996 and Petek and Glavic, 1996), etc. However, the release of important amounts of synthetic dyes to the environment has posed challenges to environmental scientists apart from increased public concern and legislation problems.
Many industries, such as dyestuffs, textile, paper and plastics, use dyes in order to colour their products and also consume substantial volumes of water. As a result, they generate a considerable amount of coloured wastewater. It is recognized that public perception of water quality is greatly influenced by the colour. Colour is the first contaminant to be recognized in wastewater (Banat et al., 1996). The presence of very small amounts of dyes in water (less than 1 ppm for some dyes) is highly visible and undesirable (Robinson et al., 2001 and Banat et al., 1996).
Dyeing industry effluents are one of the most problematic wastewaters to be treated not only for their high chemical oxygen demand, but also for high biological oxygen demand, suspended solids, turbidity, toxic constituents but also for colour, which is the first contaminant discernible by the human eye. Dyes may affect the photosynthetic activity in aquatic life due to reduced light penetration and may also be toxic to some aquatic life due to the presence of aromatics, metals, etc. in them (Clarke and Anliker 1980; Zollinger 1987; Mishra and Tripathy 1993; Banat et al1996; Fu and Viraraghvan 2001; Robinson et al2001).
Dyes usually have a synthetic origin and complex aromatic molecular structure, which make them more stable and more difficult to biodegrade. Dyes are classified as follows: anionic – direct, acid and reactive dyes; cationic – basic dyes; non-ionic – disperse dyes (Mishra and Tripathy 1993; Fu and Viraraghvan 2001). The chromophores in anionic and non-ionic dyes are mostly azo groups or anthroquinone types. The reductive cleavage of azo linkages is responsible for the formation of toxic amines in the effluent. Anthraquinone based dyes are more resistant to degradation due to their fused aromatic structures and thus remain coloured in the wastewater. Reactive dyes are typically azo-based chromophore combined with different types of reactive groups e.g, vinyl sulphone, chlorotriazine, trichloropyrimidine, difluorochloropyrimidine. They differ from all other dyes in that they bind to textile fibers like cotton to form covalent bonds. They are used extensively in textile industries regarding favourable characteristics of bright colour, water fast, simple application techniques with low energy consumption.
Water soluble reactive and acid dyes are problematic; as they pass through the conventional treatment system unaffected, posing problems. Hence, their removal is also of great importance (Robinson et al 2001; Hu 1992; Juang et al 1997; Karcher et al1999; Sumathi and Manju 2000; Aksu and Tezer et al2000; O’Mahony et al2002; Moran et al1997).
Basic dyes have high brilliance and intensity of colours and are highly visible even in very low concentration (Clarke and Anliker, 1980; Banat et al., 1996; Fu and Viraraghavan, 2001; Mittal and Gupta, 1996; Chu and Chen, 2002; Fu and Viraraghavan, 2002). Metal complex dyes are mostly chromium based, which is carcinogenic (Clarke and Anliker, 1980; Banat et al., 1996; Mishra and Tripathy 1993; Gupta et al., 1990). Disperse dyes do not ionize in an aqueous medium and some disperse dyes have also been shown to have a tendency to bioaccumulate (Banat et al., 1996). Due to the chemical stability and low biodegradability of these, conventional biological wastewater treatment systems are inefficient in treating dye wastewater.
Dyes have generated much concern regarding its use, due to its toxic effects. It has been reported to cause carcinogenesis, mutagenesis, chromosomal fractures, teratogenecity and respiratory toxicity. McGeorge et al. (1985) reported the mutagenic activity of textile wastewater effluents, using the salmonella/microsome assay and contributed the highest percentage (67%) of mutagenic effluents. Costan et al. (1993) found that a textile effluent ranked second in toxicity, among eight industrial sectors represented, by using a series of bioassays assessing the acute, sublethal and chronic toxicity at various trophic levels.
Estimation of LC50 values of many commercial dyes at different time intervals on fish was done earlier by Clarke and Anliker 1980. Srivastava et al. (1995a) also observed changes in LC50 values of malachite green in a fresh water catfish. Gambusia affinis was used to find the LC50 value for acid red 73 and showed higher toxicity (Muthukumar et al., 2005). Over 90% of some 4000 dyes tested in an ETAD (Ecological and Toxicological Association of the Dyestuffs Manufacturing Industry) Survey had LD50 values greater than 2 X 103 mg/kg. The highest rates of toxicity were found amongst basic and diazo direct dyes (Shore, 1996).
Sub – chronic exposure (13 week) to benzidine – based dyes resulted in hepatocellular carcinomas and hepatic neoplastic nodules in rats (National Cancer Institute 1978) and carcinomas in very short duration (National Institute for Occupational Safety, 1980). Histopathological changes in the testes of textile wastewater exposed rats (sub – chronic) included a reduction in the number of germ and Leydig cells, resulting in impaired spermatogenesis (Mathur, et al. 2003).
Umbuzeiro et al. (2005) analysed the mutagenic activity of dyes in environmental samples of the Cristais River, Sao Paulo, Brazil. A low level mutagencity of textile/dye industries in the underground water of Sanganer, Jaipur (India) were also investigated (Mathur et al2005). A number of studies have demonstrated mutagenic activity in effluents from textile and dye- related industries (Mcgeorge, et al. 1985; Sanchez, et al., 1988; Wells, et al. 1994).
Over 100,000 commercially available dyes exist and more than 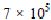 tonnes per year are produced annually (Pearce et al., 2003 and McMullan et al., 2001). Due to their good solubility, synthetic dyes are common water pollutants and they may frequently be found in trace quantities in industrial wastewater. An indication of the scale of the problem is given by the fact that two per cent of dyes that are produced are discharged directly in aqueous effluent (Pearce et al., 2003 and Robinson et al., 2001). Due to increasingly stringent restrictions on the organic content of industrial effluents, it is necessary to eliminate dyes from wastewater before it is discharged. Many of these dyes are also toxic and even carcinogenic and this poses a serious hazard to aquatic living organisms (O’Neill et al., 1999 and Vandevivere et al., 1998). However, wastewater containing dyes is very difficult to treat, since the dyes are recalcitrant organic molecules, resistant to aerobic digestion, and are stable to light, heat and oxidizing agents (Sun and Yang, 2003 and Ravi Kumar et al., 1998).
tonnes per year are produced annually (Pearce et al., 2003 and McMullan et al., 2001). Due to their good solubility, synthetic dyes are common water pollutants and they may frequently be found in trace quantities in industrial wastewater. An indication of the scale of the problem is given by the fact that two per cent of dyes that are produced are discharged directly in aqueous effluent (Pearce et al., 2003 and Robinson et al., 2001). Due to increasingly stringent restrictions on the organic content of industrial effluents, it is necessary to eliminate dyes from wastewater before it is discharged. Many of these dyes are also toxic and even carcinogenic and this poses a serious hazard to aquatic living organisms (O’Neill et al., 1999 and Vandevivere et al., 1998). However, wastewater containing dyes is very difficult to treat, since the dyes are recalcitrant organic molecules, resistant to aerobic digestion, and are stable to light, heat and oxidizing agents (Sun and Yang, 2003 and Ravi Kumar et al., 1998).
During the past three decades, several physical, chemical and biological decolorization methods have been reported; few, however, have been accepted by the paper and textile industries (Ghoreishi and Haghighi, 2003). Amongst the numerous techniques of dye removal, adsorption is the procedure of choice and gives the best results as it can be used to remove different types of coloring materials (Jain et al., 2003, Ho and McKay, 2003 and Derbyshire et al., 2001). If the adsorption system is designed correctly it will produce a high-quality treated effluent. Most commercial systems currently use activated carbon as sorbent to remove dyes in wastewater because of its excellent adsorption ability. Activated carbon adsorption has been cited by the US Environmental Protection Agency as one of the best available control technologies (Derbyshire et al., 2001). However, although activated carbon is a preferred sorbent, its widespread use is restricted due to high cost. In order to decrease the cost of treatment, attempts have been made to find inexpensive alternative adsorbents.
Recently, numerous approaches have been studied for the development of cheaper and effective adsorbents. Many non-conventional low-cost adsorbents, including natural materials, biosorbents, and waste materials from industry and agriculture, have been proposed by several workers. These materials could be used as sorbents for the removal of dyes from solution. Some of the reported sorbents include clay materials (bentonite, kaolinite), zeolites, siliceous material (silica beads, alunite, perlite), agricultural wastes (bagasse pith, maize cob, rice husk, coconut shell), industrial waste products (waste carbon slurries, metal hydroxide sludge), biosorbents (chitosan, peat, biomass) and others (starch, cyclodextrin, cotton).
In the Section below, we have reviewed the technical feasibility of various non-conventional low-cost adsorbents for dye removal from contaminated water. The Section provides a summary of recent information concerning the use of low-cost materials as sorbents. For this, an extensive list of sorbent literature has been compiled. The review (i) presents a critical analysis of these materials; (ii) describes their characteristics, advantages and limitations; and (iii) discusses various mechanisms involved. Recent reported adsorption capacities are also noted to give some idea of sorbent effectiveness. However, the reported adsorption capacities must be taken as an example of values that can be achieved under specific conditions since adsorption capacities of the sorbents presented vary, depending on the characteristics of the material, the experimental conditions, and also the extent of chemical modifications.
1.1 Technologies available for colour removalThe methods for dye wastewater treatment have been reviewed by Pokhrel and Viraraghavan, 2004, Robinson et al., 2001, Slokar and Majcen Le Marechal, 1998, Delée et al., 1998 and Banat et al., 1996 and Cooper (1993). Fungal and bacterial decolorization methods have been reviewed by Aksu, 2005, Wesenberg et al., 2003, Pearce et al., 2003, McMullan et al., 2001 and Fu and Viraraghavan, 2001a and Stolz (2001).
The reported methods for the removal of pollutants from effluents are listed in Table 2. The technologies can be divided into three categories: biological, chemical and physical (Robinson et al., 2001). All of them have advantages and drawbacks. Due to the high cost and disposal problems, many of these conventional methods for treating dye wastewater have not been widely applied at large scale in the textile and paper industries (Ghoreishi and Haghighi, 2003).
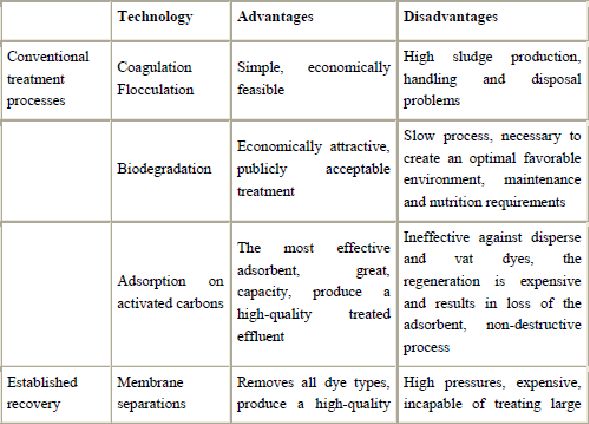
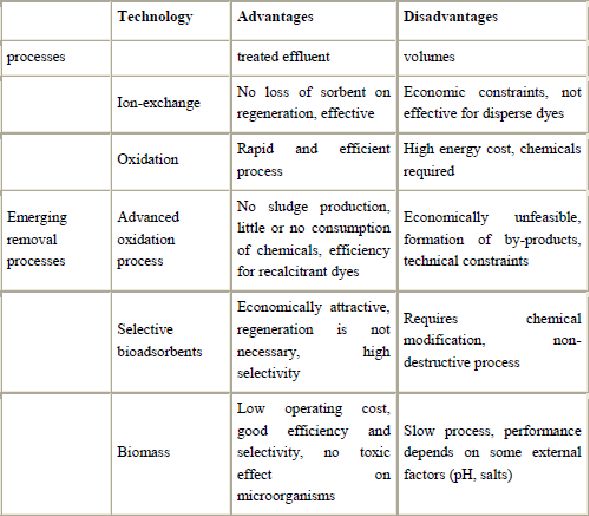
Presently, there is no single process capable of adequate treatment, mainly due to the complex nature of the effluents (Pereira et al., 2003 and Marco et al., 1997). In practice, a combination of different processes is often used to achieve the desired water quality in the most economical way. A literature survey shows that research has been and continues to be conducted in the areas of combined adsorption-biological treatments in order to improve the biodegradation of dyestuffs and minimize the sludge production.
1.1.2. Biological treatments
Biological treatment is often the most economical alternative when compared with other physical and chemical processes. Biodegradation methods such as fungal decolorization, microbial degradation, adsorption by (living or dead) microbial biomass and bioremediation systems are commonly applied to the treatment of industrial effluents because many microorganisms such as bacteria, yeasts, algae and fungi are able to accumulate and degrade different pollutants (McMullan et al., 2001, Fu and Viraraghavan, 2001a and Banat et al., 1996). However, their application is often restricted because of technical constraints. Biological treatment requires a large land area and is constrained by sensitivity toward diurnal variation as well as toxicity of some chemicals, and less flexibility in design and operation (Bhattacharyya and Sarma, 2003). Biological treatment is incapable of obtaining satisfactory colour elimination with current conventional biodegradation processes (Robinson et al., 2001). Moreover, although many organic molecules are degraded, many others are recalcitrant due to their complex chemical structure and synthetic organic origin (Ravi Kumar et al., 1998). In particular, due to their xenobiotic nature, azo dyes are not totally degraded.
1.1.3. Chemical methods
Chemical methods include coagulation or flocculation combined with flotation and filtration, precipitation–flocculation with  , electroflotation, electrokinetic coagulation, conventional oxidation methods by oxidizing agents (ozone), irradiation or electrochemical processes. These chemical techniques are often expensive, and although the dyes are removed, accumulation of concentrated sludge creates a disposal problem. There is also the possibility that a secondary pollution problem because of excessive chemical use. Recently, other emerging techniques, known as advanced oxidation processes, which are based on the generation of very powerful oxidizing agents such as hydroxyl radicals, have been applied with success for pollutant degradation. Although these methods are efficient for the treatment of waters contaminated with pollutants, they are very costly and commercially unattractive. The high electrical energy demand and the consumption of chemical reagents are common problems.
, electroflotation, electrokinetic coagulation, conventional oxidation methods by oxidizing agents (ozone), irradiation or electrochemical processes. These chemical techniques are often expensive, and although the dyes are removed, accumulation of concentrated sludge creates a disposal problem. There is also the possibility that a secondary pollution problem because of excessive chemical use. Recently, other emerging techniques, known as advanced oxidation processes, which are based on the generation of very powerful oxidizing agents such as hydroxyl radicals, have been applied with success for pollutant degradation. Although these methods are efficient for the treatment of waters contaminated with pollutants, they are very costly and commercially unattractive. The high electrical energy demand and the consumption of chemical reagents are common problems.
1.2 Physical methods
Different physical methods are also widely used, such as membrane-filtration processes (nanofiltration, reverse osmosis, electrodialysis, etc.) and adsorption techniques. The major disadvantage of the membrane processes is that they have a limited lifetime before membrane fouling occurs and the cost of periodic replacement must thus be included in any analysis of their economic viability. In accordance with the very abundant literature data, liquid-phase adsorption is one of the most popular methods for the removal of pollutants from wastewater since proper design of the adsorption process will produce a high-quality treated effluent. This process provides an attractive alternative for the treatment of contaminated waters, especially if the sorbent is inexpensive and does not require an additional pre-treatment step before its application.
Adsorption is a well known equilibrium separation process and an effective method for water decontamination applications (Dabrowski, 2001). Adsorption has been found to be superior to other techniques for water re-use in terms of initial cost, flexibility and simplicity of design, ease of operation and insensitivity to toxic pollutants. Adsorption also does not result in the formation of harmful substances.
1.2 Colour removal using commercial activated carbons
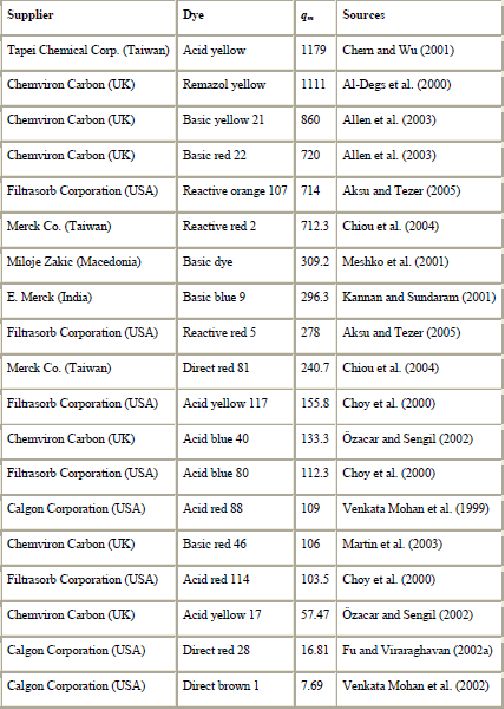
Adsorption techniques employing solid sorbents are widely used to remove certain classes of chemical pollutants from waters, especially those that are practically unaffected by conventional biological wastewater treatments. However, amongst all the sorbent materials proposed, activated carbon is the most popular for the removal of pollutants from wastewater (Babel and Kurniawan, 2003, Derbyshire et al., 2001 and Ramakrishna and Viraraghavan, 1997). In particular, the effectiveness of adsorption on commercial activated carbons (CAC) for removal of a wide variety of dyes from wastewaters has made it an ideal alternative to other expensive treatment options (Ramakrishna and Viraraghavan, 1997). CAC are the most effective adsorbents due to of their great capacity to adsorb dyes and recent reported adsorption capacities for CAC are listed in Table 3. This capacity is mainly due to their structural characteristics and their porous texture which gives them a large surface area, and their chemical nature which can be easily modified by chemical treatment in order to increase their properties. However, activated carbon presents several disadvantages (Babel and Kurniawan, 2003). It is quite expensive, the higher the quality, the greater the cost, non-selective and ineffective against disperse and vat dyes. The regeneration of saturated carbon is also expensive, not straightforward, and results in loss of the adsorbent. The use of carbons based on relatively expensive starting materials is also unjustified for most pollution control applications (Streat et al., 1995). This has led many workers to search for more economic adsorbents.
 (mg/g) for commercial activated carbons
(mg/g) for commercial activated carbons
| 1.3. Non-conventional low-cost adsorbents and removal of dyes |
 |
Due to the problems mentioned above, research interest into the production of alternative sorbents to replace the costly activated carbon has intensified in recent years. Attention has focused on various natural solid supports, which are able to remove pollutants from contaminated water at low cost. Cost is actually an important parameter for comparing the adsorbent materials. According to Bailey et al. (1999), a sorbent can be considered low-cost if it requires little processing, is abundant in nature or is a by-product or waste material from another industry. Certain waste products from industrial and agricultural operations, natural materials and biosorbents represent potentially economical alternative sorbents. Many of them have been tested and proposed for dye removal.
1.3.1. Waste materials from agriculture and industry
The by-products from the agricultural and industries could be assumed to be low-cost adsorbents since they are abundant in nature, inexpensive, require little processing and are effective materials.
a) Activated carbons from solid wastes : Commercially available activated carbons (AC) are usually derived from natural materials such as wood, coconut shell, lignite or coal, but almost any carbonaceous material may be used as precursor for the preparation of carbon adsorbents (Rozada et al., 2003, Rodriguez-Reinoso, 1997 and Pollard et al., 1992). Coal is the most commonly used precursor for AC production (Carrasco-Marin et al., 1996 and Illan Gomez et al., 1996) for its availability and cheapness. Coal is a mixture of carbonaceous materials and mineral matter, resulting from the degradation of plants. The sorption properties of each individual coal are determined by the nature of the original vegetation and the extent of the physical–chemical changes occurring after deposition (Karaca et al., 2004). Coal adsorption capacities are reported in Table 4. Coal based sorbents have been used by Karaca et al., 2004, Venkata Mohan et al., 1999 and Venkata Mohan et al., 2002 and McKay et al. (1999) with success for dye removal. However, since coal is not a pure material, it has a variety of surface properties and thus different sorption properties.
 (mg/g) for carbon materials made from solid wastes and coal-based sorbents
(mg/g) for carbon materials made from solid wastes and coal-based sorbents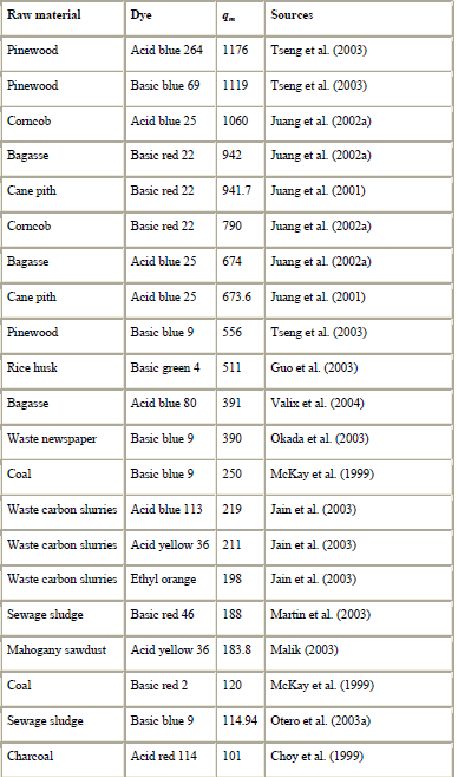
Plentiful agricultural and wood by-products may also offer an inexpensive and renewable additional source of AC. These waste materials have little or no economic value and often present a disposal problem. Therefore, there is a need to valorize these low-cost by-products. So, their conversion into AC would add economic value, help reduce the cost of waste disposal and most importantly provide a potentially inexpensive alternative to the existing commercial activated carbons.
A wide variety of carbons have been prepared from agricultural and wood wastes, such as bagasse (Valix et al., 2004, Juang et al., 2001, Juang et al., 2002a, Tsai et al., 2001 and Ahmedna et al., 2000), coir pith (Namasivayam and Kavitha, 2002 and Namasivayam et al., 2001a), banana pith (Kadirvelu et al., 2003), date pits (Banat et al., 2003), sago waste (Kadirvelu et al., 2003), silk cotton hull (Kadirvelu et al., 2003), corn cob (Juang et al., 2002a), maize cob (Kadirvelu et al., 2003), straw (Kannan and Sundaram, 2001), rice husk (Mohamed, 2004, Malik, 2003, Guo et al., 2003 and Kannan and Sundaram, 2001), rice hulls (Ahmedna et al., 2000), fruit stones (Aygün et al., 2003), nutshells (Aygün et al., 2003 and Ahmedna et al., 2000), pinewood (Tseng et al., 2003), sawdust (Malik, 2003), coconut tree sawdust (Kadirvelu et al., 2000 and Kadirvelu et al., 2003), bamboo (Wu et al., 1999) and cassava peel (Rajeshwarisivaraj et al., 2001a). There are also several reports on the production of AC from various city wastes and industrial by-products such as waste PET bottles (Nakagawa et al., 2004), waste tires (Nakagawa et al., 2004), refuse derived fuel (Nakagawa et al., 2004), wastes generated during lactic acid fermentation from garbage (Nakagawa et al., 2004), sewage sludges (Rozada et al., 2003, Otero et al., 2003a, Otero et al., 2003b and Graham et al., 2001), waste newspaper (Okada et al., 2003), waste carbon slurries (Jain et al., 2003 and Gupta et al., 2003) and blast furnace slag (Jain et al., 2003 and Gupta et al., 2003).
The excellent ability and economic promise of the activated carbons prepared from by-products have been recently presented and described. Non-conventional activated carbons exhibited high sorption properties as shown in Table 4. Juang et al. (2002a) reported that the adsorption capacities of activated carbons made from corncob had very large values of 1060–790 mg of dye per g of carbon. However, the adsorption capacities of a carbon depend on the different sources of raw materials, the history of its preparation and treatment conditions such as pyrolysis temperature and activation time. Many other factors can also affect the adsorption capacity in the same sorption conditions such as surface chemistry (heteroatom content), surface charge and pore structure. A suitable carbon should possess not only a porous texture, but also high surface area. Recently, Guo et al. (2003) showed that the adsorption does not always increase with surface area. Besides the physical structure, the adsorption capacity of a given carbon is strongly influenced by the chemical nature of the surface. The acid and base character of a carbon influences the nature of the dye isotherms. The adsorption capacity depends also on the accessibility of the pollutants to the inner surface of the adsorbent, which depends on their size. The specific sorption mechanisms by which the adsorption of dyes takes place on these adsorbents are still not clear. This is because adsorption is a complicated process depending on several interactions such as electrostatic and non-electrostatic (hydrophobic) interactions. Although much has been accomplished in terms of sorption properties and kinetics, much work is still necessary to identify the sorption mechanisms clearly.
b) Agricultural solid wastes : Raw agricultural solid wastes and waste materials from forest industries such as sawdust and bark have been used as adsorbents. These materials are available in large quantities and may have potential as sorbents due to their physico-chemical characteristics and low-cost. Sawdust is an abundant by-product of the wood industry that is either used as cooking fuel or as packing material. Sawdust is easily available in the countryside at zero or negligible price (Garg et al., 2004a). It contains various organic compounds (lignin, cellulose and hemicellulose) with polyphenolic groups that might be useful for binding dyes through different mechanisms. The role of sawdust materials in the removal of pollutants from aqueous solutions has been reviewed recently (Shukla et al., 2002). Some valuable guidelines can be drawn from the review. Sawdust has proven to be a promising effective material for the removal of dyes from wastewaters (Özacar and Sengil, 2005, Garg et al., 2004a, Garg et al., 2004b, Baouab et al., 2001 and Ho and McKay, 1998a). Sawdust adsorption capacities are reported in Table 5. The sorption mechanisms can be explained by the presence of several interactions, such as complexation, ion-exchange due to a surface ionisation, and hydrogen bonds. One problem with sawdust materials is that the sorption results are strongly pH-dependent (Garg et al., 2003, Garg et al., 2004b, Khattri and Singh, 2000 and Ho and McKay, 1998a). There is a neutral pH beyond which the sawdust will be either positively or negatively charged. Ho and McKay (1998a) showed that the sorption capacity of basic dye is much higher than that of acid dye because of the ionic charges on the dyes and the ionic character of sawdust. Khattri and Singh (2000) also noted that the adsorption capacity of Neem sawdust was highly concentration dependent. Chemical pretreatment of sawdust has been shown to improve the sorption capacity and to enhance the efficiency of sawdust adsorption (Garg et al., 2003, Garg et al., 2004a, Garg et al., 2004b and Batzias and Sidiras, 2004).
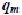 (mg/g) for waste materials from agriculture and industry
(mg/g) for waste materials from agriculture and industry
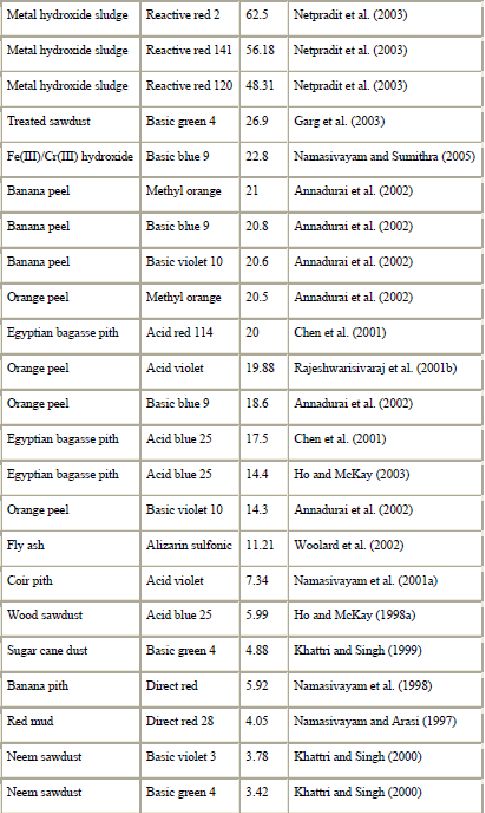
Another waste product from the timber industry is bark, a polyphenol-rich material. Bark is an abundant forest residue which has been found to be effective in removing dyes from water solutions. Because of its low cost and high availability, bark is very attractive as an adsorbent. Like sawdust, the cost of forest wastes is only associated with the transport cost from the storage place to the site where they will be utilized (Palma et al., 2003). Bark is an effective adsorbent because of its high tannin content (Bailey et al., 1999 and Morais et al., 1999). The polyhydroxy polyphenol groups of tannin are thought to be the active species in the adsorption process. Morais et al. (1999) studied adsorption of Remazol BB onto eucalyptus bark from Eucalyptus globulus. The adsorption capacity at pH = 2.5 and 18 °C was found to be 90 mg of dye/g of dry bark. Parallel sorption tests, under similar conditions, carried out with a commercial activated carbon and with bark, showed for the latter an adsorption capacity about half that of the former. The authors concluded that there are promising perspectives for the utilization of eucalyptus bark as sorbent on an industrial scale. However, there are still several important aspects such as the sorption mechanism to clarify.
Tree fern, an agricultural by-product, has been recently investigated to remove pollutants from aqueous solutions (Ho et al., 2005 and Ho, 2003). Tree fern is a complex material containing lignin and cellulose as major constituents. Maximum adsorption capacity of tree fern for basic red 13 was 408 mg/g (Ho et al., 2005). The capacity increased as the sorbent particle size decreased. The sorption mechanism involves chemical bonding and ion-exchange.
Other agricultural solid wastes from cheap and readily available resources such as date piths (Banat et al., 2003), pith (Ho and McKay, 1999a, Ho and McKay, 2003, Chen et al., 2001, Namasivayam et al., 1993, Namasivayam et al., 1998, Namasivayam et al., 2001b, Namasivayam and Kadirvelu, 1994 and McKay et al., 1987), corncob (Robinson et al., 2002a), barley husk (Robinson et al., 2002a), wheat straw (Robinson et al., 2002b, Robinson et al., 2002c and Nigam et al., 2000), wood chips (Kun-She et al., 2000, Low et al., 2000 and Nigam et al., 2000) and orange peel (Rajeshwarisivaraj et al., 2001b and Namasivayam et al., 1996) have also been successfully employed for the removal of dyes from aqueous solution (Table 5).
c). Industrial by-products : Because of their low cost and local availability, industrial solid wastes such as metal hydroxide sludge, fly ash and red mud are classified as low-cost materials and can be used as adsorbents for dye removal (Namasivayam and Sumithra, 2005, Netpradit et al., 2003, Netpradit et al., 2004a, Netpradit et al., 2004b, Acemioglu, 2004, Janos et al., 2003, Mohan et al., 2002, Gupta et al., 2000, Ho and McKay, 1999b, Namasivayam and Arasi, 1997, Namasivayam et al., 1994 and Namasivayam and Chandrasekaran, 1991).
Recently, Netpradit et al., 2003, Netpradit et al., 2004a and Netpradit et al., 2004b studied the capacity and mechanisms of metal hydroxide sludge in removing azo reactive dyes. The sludge is a dried waste from the electroplating industry, which is produced by precipitation of metal ions in wastewater with calcium hydroxide. It contains insoluble metal hydroxides and other salts. The authors demonstrated that metal hydroxide sludge was an effective positively charged adsorbent with a high maximum adsorption capacity (48–62 mg dye/g material) for azo reactive (anionic) dyes. The charge of the dyes is an important factor for the adsorption due to the ion-exchange mechanism.
Another industrial by-product shown to adsorb dyes is fly ash (Wang et al., 2005, Acemioglu, 2004, Mohan et al., 2002, Gupta et al., 1990, Gupta et al., 2000, Ramakrishna and Viraraghavan, 1997 and Khare et al., 1987). Fly ash is a waste material originating in great amounts in combustion processes. Although it may contain some hazardous substances, such as heavy metals, it is widely utilized in industry in many countries (Janos et al., 2003). However, bagasse fly ash generated in the sugar industry does not contain large amounts of toxic metals and has been widely used for adsorption of dyes (Mohan et al., 2002 and Gupta et al., 2000). Fly ash has a surface area of 15.6 m2/g (Wang et al., 2005). Its properties are extremely variable and depend strongly on its origin (Wang et al., 2005, Janos et al., 2003 and Ho and McKay, 1999b).
Another abundant industrial by-product is red mud (Wang et al., 2005, Namasivayam and Arasi, 1997 and Namasivayam and Chandrasekaran, 1991). Waste red mud is a bauxite processing residue discarded in alumina production. Namasivayam and Arasi (1997) proposed red mud as adsorbent for the removal of congo red. The maximum capacity was 4.05 mg/g. Wang et al. (2005) showed that physical and chemical treatment can significantly change the adsorption capacity.
1.3.2. Natural materials
a) Clays : Natural clay minerals are well known and familiar to mankind from the earliest days of civilization. Because of their low cost, abundance in most continents of the world, high sorption properties and potential for ion-exchange, clay materials are strong candidates as adsorbents. Clay materials possess a layered structure and are considered as host materials. They are classified by the differences in their layered structures. There are several classes of clays such as smectites (montmorillonite, saponite), mica (illite), kaolinite, serpentine, pylophyllite (talc), vermiculite and sepiolite (Shichi and Takagi, 2000). The adsorption capabilities result from a net negative charge on the structure of minerals. This negative charge gives clay the capability to adsorb positively charged species. Their sorption properties also come from their high surface area and high porosity (Alkan et al., 2004). Montmorillonite clay has the largest surface area and the highest cation exchange capacity. Its current market price (about US$ 0.04–0.12/kg) is considered to be 20 times cheaper than that of activated carbon (Babel and Kurniawan, 2003).
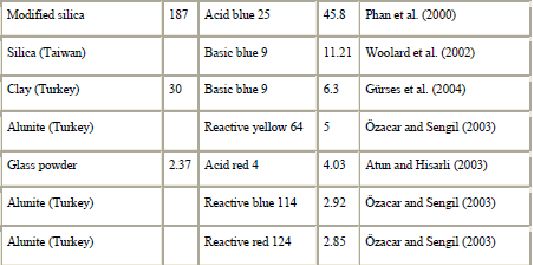
In recent years, there has been an increasing interest in utilizing clay minerals such as bentonite, kaolinite, diatomite and Fuller’s earth for their capacity to adsorb not only inorganic but also organic molecules. In particular, interactions between dyes and clay particles have been extensively studied (Alkan et al., 2005, Alkan et al., 2004, Gürses et al., 2004, Wang et al., 2004, Al-Bastaki and Banat, 2004, Özcan et al., 2004, Ozdemir et al., 2004, Al-Ghouti et al., 2003, Atun et al., 2003, Espantaleon et al., 2003, Orthman et al., 2003, Lazaridis et al., 2003, Shawabkeh and Tutunji, 2003, Neumann et al., 2002, Ghosh and Bhattacharyya, 2002, Pala and Tokat, 2002, Harris et al., 2001, Ho et al., 2001, Bagane and Guiza, 2000, Ramakrishna and Viraraghavan, 1997, Kacha et al., 1997, El-Geundi, 1997, Kahr and Madsen, 1995 and Gupta et al., 1992). Clay minerals exhibit a strong affinity for both heteroatomic cationic and anionic dyes (Table 6). However, the sorption capacity for basic dye is much higher than for acid dye because of the ionic charges on the dyes and character of the clay. The adsorption of dyes on clay minerals is mainly dominated by ion-exchange processes. This means that the sorption capacity can vary strongly with pH. Al-Ghouti et al. (2003) showed that the mechanism of adsorption of dye onto diatomite is due to physical adsorption (depending on the particle size) and the presence of electrostatic interactions (depending on the pH used).
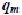 (mg/g) for natural materials
(mg/g) for natural materials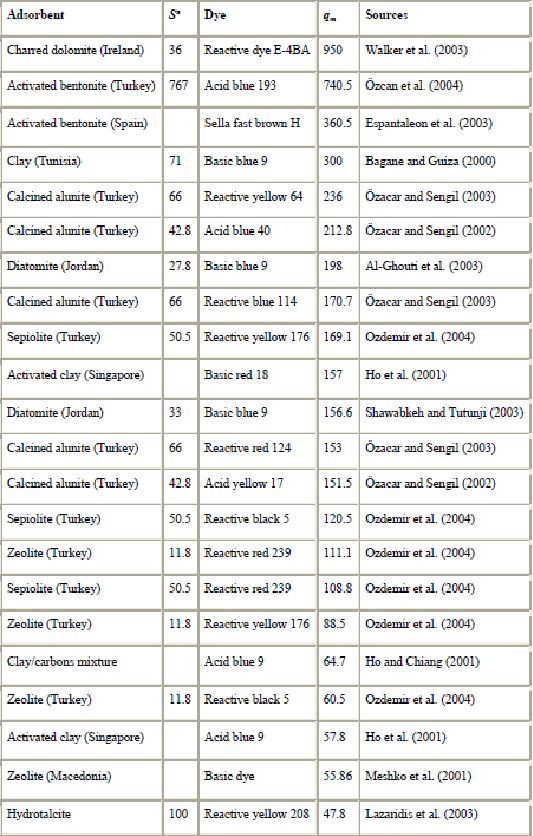
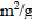 ).
).Good removal capability of clay materials to take up dye has been demonstrated. Espantaleon et al. (2003) reported that an adsorption capacity of 360.5 mg of dye/g bentonite was achieved. Due to its high surface area, it was suggested that bentonite is a good adsorbent for (basic) dye removal. Similar results have been published by Bagane and Guiza (2000). It was found that 1 g bentonite could adsorb 300 mg of basic blue 9. The adsorption of dyes on kaolinite was also studied. The adsorption to kaolinite was about 20 times greater than to alumina (Harris et al., 2001). The removal performances of Fuller’s earth and CAC for basic blue 9 were compared by Atun et al. (2003). They showed that the adsorption capacity is greater on Fuller’s earth than on CAC. Moreover, Fuller’s earth is an interesting sorbent since its average price is US$ 0.04/kg whereas CAC costs US$ 20/kg. Shawabkeh and Tutunji (2003) studied the adsorption of basic blue 9 onto diatomaceous earth (diatomite). They showed that this naturally occurring material could substitute for activated carbon as an adsorbent due to its availability and low cost, and its good sorption properties. The adsorption isotherms revealed that adsorption equilibrium was reached within 10 min. The feasibility of using diatomite for the removal of the problematic reactive dyes was also investigated by Al-Ghouti et al. (2003). The sorption of acid blue 9 onto mixed sorbent (activated clay and activated carbon 12:1) has been studied by Ho and Chiang (2001).
As with other materials, clay materials can be modified to improve their sorption capacity. Ozdemir et al. (2004) investigated modified sepiolite as an adsorbent for a variety of azo-reactive dyes. They showed that the adsorption capacities are substantially improved upon modifying their surfaces with quaternary amines. The adsorption capacity of kaolinite can be improved by purification and by treatment with NaOH solution (Ghosh and Bhattacharyya, 2002). The acid-treated bentonite showed a higher adsorption capacity than non-modified bentonite (Espantaleon et al., 2003). Similar results have been published by Özcan et al. (2004).
The results presented above show that clay materials may be promising adsorbents for environmental and purification purposes.
b) Siliceous materials : The use of natural siliceous sorbents such as silica beads, glasses, alunite, perlite and dolomite for wastewater is increasing because of their abundance, availability and low price. Among inorganic materials, silica beads deserve particular attention (Krysztafkiewicz et al., 2002, Crini and Morcellet, 2002, Woolard et al., 2002, Harris et al., 2001 and Phan et al., 2000), considering chemical reactivity of their hydrophilic surface, resulting from the presence of silanol groups. Their porous texture, high surface area and mechanical stability also make them attractive as sorbents for decontamination applications. However, due to their low resistance toward alkaline solutions their usage is limited to media of pH less than 8 (Ahmed and Ram, 1992). Moreover, the surface of siliceous materials contains acidic silanol (among other surface groups) which causes a strong and often irreversible non-specific adsorption. For that reason, it is necessary to eliminate the negative features of these sorbents. In order to promote their interaction with dyes, the silica surface can be modified using silane coupling agents with the amino functional group (Krysztafkiewicz et al., 2002). Phan et al. (2000) also showed that modified silica beads have a better potential for the removal of acid dyes from colored effluents.
Another sorbent from siliceous materials to adsorb dye is alunite (Özacar and Sengil, 2002, Özacar and Sengil, 2003 and Dill, 2001). Alunite is one of the minerals of the jarosite group and contains approximately 50% SiO2. Characteristics of alunite can be found in the review by Dill (2001). However, untreated alunite does not have good adsorbent properties (Özacar and Sengil, 2003). After a suitable process, alunite-type layered compounds are useful as adsorbents for removing color. An investigation on the use of modified alunite for removing acid dyes from wastewater was conducted by Özacar and Sengil, 2002 and Özacar and Sengil, 2003. It was observed that an adsorption capacity of 57.47 mg of acid blue 40 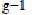 commercial activated carbon was achieved. Under the same experimental conditions, it was found that the adsorption capacity of acid blue 40 was greater on calcined alunite (212.8 mg/g) than on CAC. Alunite is so cheap that regeneration is not necessary. The surface charge on the sorbent and the pH play a significant role in influencing the capacity of alunite towards dyes.
commercial activated carbon was achieved. Under the same experimental conditions, it was found that the adsorption capacity of acid blue 40 was greater on calcined alunite (212.8 mg/g) than on CAC. Alunite is so cheap that regeneration is not necessary. The surface charge on the sorbent and the pH play a significant role in influencing the capacity of alunite towards dyes.
Other siliceous materials such as dolomite, perlite and glass have been proposed for dye removal (Table 5). Dolomite is both a mineral and a rock. Outstanding removal capability of dolomite for dye uptake has been demonstrated (Walker et al., 2003). Charred dolomite has a higher equilibrium capacity for reactive dye removal than activated carbon, with a capacity of 950 mg/g of adsorbent for dolomite compared to 650 mg dye adsorbed per g of adsorbent for CAC. However, the mechanism was not clear (probably a combination of precipitation and adsorption). Perlite is a glassy volcanic rock and has a high silica content, usually greater than 70%. It is inexpensive and easily available in many countries. The use of perlite as a low-cost adsorbent for the removal of dyes has been investigated for the first time by Alkan and co-workers (Dogan et al., 2004, Dogan and Alkan, 2003a, Dogan and Alkan, 2003b and Demirbas et al., 2002). It was suggested that dyes are physically adsorbed onto the perlite. Perlite is a good adsorbent for decontamination purposes. However, perlites of different types (expanded and unexpanded) and of different origins have different properties because of the differences in composition.
c). Zeolites : Zeolites are highly porous aluminosilicates with different cavity structures. Their structures consist of a three dimensional framework, having a negatively charged lattice. The negative charge is balanced by cations which are exchangeable with certain cations in solutions. Zeolites consist of a wide variety of species, more than 40 natural species. However, the most abundant and frequently studied zeolite is clinoptilolite, a mineral of the heulandite group. Its characteristic tabular morphology shows an open reticular structure of easy access, formed by open channels of 8–10 membered rings. Clinoptilolite applications of zeolites have been reviewed by Ghobarkar et al. (1999). High ion-exchange capacity and relatively high specific surface areas, and more importantly their relatively cheap prices, make zeolites attractive adsorbents. Their price is about US$ 0.03–0.12/kg, depending on the quality of the mineral (Babel and Kurniawan, 2003). Another advantage of zeolites over resins is their ion selectivities generated by their rigid porous structures.
Zeolites are becoming widely used as alternative materials in areas where sorptive applications are required. They have been intensively studied recently because of their applicability in removing trace quantities of pollutants such as heavy metal ions and phenols thanks to their cage-like structures suitable for ion exchange. Zeolites also appear as suitable sorbents for dyes (Table 5). Several studies have been conducted on the sorbent behavior of natural zeolites (Ozdemir et al., 2004, Armagan et al., 2004, Meshko et al., 2001, Calzaferri et al., 2000, Ghobarkar et al., 1999 and El-Geundi, 1997). However, raw clinoptilolite was not suitable for the removal of reactive dyes due to extremely low sorption capacities (Armagan et al., 2004 and Karcher et al., 2001). Similar conclusions have been found by Ozdemir et al. (2004) and Benkli et al. (2005). These authors suggested chemical modification with quaternary amines as a means of increasing sorption. In spite of the promising results, the real applicability of these natural materials to purify dye waste waters is still quite unknown. Another problem of zeolites is their low permeability and this requires an artificial support when used in column operations. The sorption mechanism on zeolite particles is complex because of their porous structure, inner and outer charged surfaces, mineralogical heterogeneity and other imperfections on the surface (Calzaferri et al., 2000 and Altin et al., 1998). However, it is recognized that, like clay, the adsorption properties of zeolites result mainly from their ion-exchange capabilities. Although the removal efficiency of zeolites for dyes may not be as good as that of clay materials, their easy availability and low cost may compensate for the associated drawbacks.
| 1.3.3 Biosorbents |
 |
The accumulation and concentration of pollutants from aqueous solutions by the use of biological materials is termed biosorption. In this instance, biological materials, such as chitin, chitosan, peat, yeasts, fungi or bacterial biomass, are used as chelating and complexing sorbents in order to concentrate and to remove dyes from solutions. These biosorbents and their derivatives contain a variety of functional groups which can complex dyes. The biosorbents are often much more selective than traditional ion-exchange resins and commercial activated carbons, and can reduce dye concentration to ppb levels. Biosorption is a novel approach, competitive, effective and cheap.
a) Chitin and chitosan : The sorption of dyes using biopolymers such as chitin and chitosan is one of the reported emerging biosorption methods for the removal of dyes, even at low concentration (ppm or ppb levels). Chitin and chitosan are abundant, renewable and biodegradable resources. Chitin, a naturally occurring mucopolysaccharide, has been found in a wide range of natural sources such as crustaceans, fungi, insects, annelids and molluscs. However, chitin and chitosan are only commercially extracted from crustaceans (crab, krill, crayfish) primarily because a large amount of the crustacean’s exoskeleton is available as a by-product of food processing. The annual worldwide crustacean shells production has been estimated to be 1.2 × 106 tonnes, and the recovery of chitin and protein from this waste is an additional source of revenue (Teng et al., 2001). Utilization of industrial solid wastes for the treatment of wastewater from another industry could be helpful not only to the environment in solving the solid waste disposal problem, but also to the economy.
Chitin contains 2-acetamido-2-deoxy-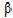 -d-glucose through a
-d-glucose through a 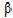 (1→ 4) linkage. This waste product is second only to cellulose in terms of abundance in nature. Chitosan contains 2-acetamido-2-deoxy-
(1→ 4) linkage. This waste product is second only to cellulose in terms of abundance in nature. Chitosan contains 2-acetamido-2-deoxy-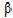 -d-glucopyranose and 2-amino-2-deoxy-
-d-glucopyranose and 2-amino-2-deoxy-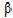 -d-glucopyranose residues. Chitosan has drawn particular attention as a complexing agent due to its low cost compared to activated carbon and its high contents of amino and hydroxy functional groups showing high potential for adsorption of a wide range of molecules, including phenolic compounds, dyes and metal ions (Guibal, 2004, Varma et al., 2004 and Ravi Kumar, 2000). This biopolymer represents an attractive alternative to other biomaterials because of its physico-chemical characteristics, chemical stability, high reactivity, excellent chelation behavior and high selectivity toward pollutants (Guibal, 2004, Varma et al., 2004 and Ravi Kumar, 2000).
-d-glucopyranose residues. Chitosan has drawn particular attention as a complexing agent due to its low cost compared to activated carbon and its high contents of amino and hydroxy functional groups showing high potential for adsorption of a wide range of molecules, including phenolic compounds, dyes and metal ions (Guibal, 2004, Varma et al., 2004 and Ravi Kumar, 2000). This biopolymer represents an attractive alternative to other biomaterials because of its physico-chemical characteristics, chemical stability, high reactivity, excellent chelation behavior and high selectivity toward pollutants (Guibal, 2004, Varma et al., 2004 and Ravi Kumar, 2000).
Various studies on chitin and chitosan have been conducted in recent years (Wong et al., 2004, Chao et al., 2004, Chiou et al., 2004, Chiou and Li, 2002, Chiou and Li, 2003, Juang et al., 1996, Juang et al., 1997, Juang et al., 2002b, Vachoud et al., 2001, Wu et al., 2000, Wu et al., 2001a, Wu et al., 2001b, Annadurai et al., 1999 and Annadurai and Krishnan, 1997). These studies demonstrated that chitosan-based biosorbents are efficient materials and have an extremely high affinity for many classes of dyes (Table 7). They are also versatile materials. This versatility allows the sorbent to be used in different forms, from flake-types to gels, bead-types or fibers.
 (mg/g) for chitosan and chitosan-based biosorbents
(mg/g) for chitosan and chitosan-based biosorbents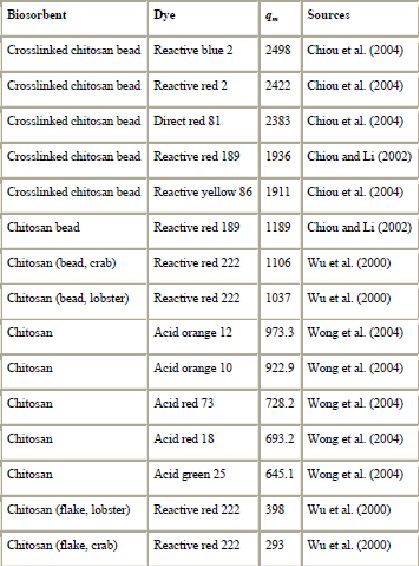
The performance of chitosan as an adsorbent to remove acid dyes has been demonstrated by Wong et al. (2004). They found that the maximum adsorption capacities of chitosan for acid orange 12, acid orange 10, acid red 73 and acid red 18 were 973.3, 922.9, 728.2, and 693.2 mg/g, respectively. Wu et al. (2000) also reported the usefulness of chitosan for the removal of reactive dyes. However, the bead type of chitosan gives a higher capacity for dye than the flake type by a factor of 2–4 depending on the source of fishery wastes. For example, a comparison of the maximum adsorption capacity for reactive red 222 by chitosan flakes and beads showed 293 mg/g for flakes and 1103 mg/g for beads. This can be explained by the fact that the beads possessed a greater surface area than the flakes.
Both batch contacting and column processes are available for chitosan materials with solution containing dyestuffs (McKay et al., 1989). In sorption columns, chitin and chitosan are often used as powder or flake forms. This technique usually causes a significant pressure drop in the column. Moreover, another limitation of chitosan is that it is soluble in acidic media and therefore cannot be used as an insoluble sorbent under these conditions, except after physical and chemical modification. To avoid these problems, crosslinked beads have been developed. Chitosan-based biosorbents are easy to prepare with relatively inexpensive reagents. These materials are insoluble in acidic and alkaline media as well as in organic solvents and become more resistant to high temperature and low pH compared to their parent biopolymer. After crosslinking, they maintain their properties and original characteristics. Chemical modifications of chitosan have also been made to improve its removal performance and selectivity for dyes, to control its diffusion properties and to decrease the sensitivity of sorption to environmental conditions.
The interaction between crosslinked chitosan and dyes has been intensively investigated by Chiou et al. (Chiou et al., 2004, Chiou and Li, 2002 and Chiou and Li, 2003). Several crosslinked biomaterials were prepared by using a procedure described by Zeng and Ruckenstein (1996). Chitosan beads were crosslinked with glutaraldehyde (GLA), epichlorohydrin (EPI) or ethylene glycol diglycidyl ether (EGDE). Chiou and Li (2003) showed that the chitosan-EPI beads presented a higher adsorption capacity than GLA and EGDE resins. It was found that 1 g chitosan adsorbed 2498 mg of reactive blue 2. Chitosan-based biosorbents have also demonstrated outstanding removal capabilities for direct dyes. In comparison with commercial activated carbon, the beads exhibited excellent performance for adsorption of anionic dyes: the adsorption values were 3–15 times higher at the same pH (Chiou et al., 2004). Hence chitosan chelation is the procedure of choice for dye removal from aqueous solution. However, it is known that chitosan has a low affinity for cationic (basic) dyes. Chao et al. (2004) suggested enzymatic grafting of carboxyl groups onto chitosan as a means to confer the ability to adsorb basic dyes on beads. The presence of new functional groups on the surface of beads results in increases in surface polarity and the density of sorption sites, and this improves the sorption selectivity for the target dye.
There are, of course, disadvantages of using chitosan in wastewater treatment. Its adsorption properties depend on the different sources of chitin, the degree of N-acetylation, molecular weight and solution properties, and vary with crystallinity, affinity for water, percent deacetylation and amino group content (Guibal, 2004, Varma et al., 2004 and Ravi Kumar, 2000). These parameters, determined by the conditions selected during preparation, control swelling and diffusion properties of the biopolymer and influence its characteristics. Performance is dependent on the type of material used and the efficiency of adsorption depends on the accessibility of sorption sites. The uptake is strongly pH-dependent. Dye molecules have many different and complicated structures. This is one of the most important factors influencing adsorption (Wong et al., 2004). There is, as yet, little information in the literature on this topic. The traditional and commercial source of chitin is from shells of crab, shrimp and krill that are wastes from the processing of marine food products. However, this traditional method of extraction of chitin creates its own environmental problems as it generates large quantities of waste and the production of chitosan also involves a chemical deacetylation process. These problems can explain why it is difficult to develop chitosan-based materials as adsorbents at an industrial scale.
Despite the large number of papers dedicated to the removal of dyes by chitosan-based materials, most of them focus on the evaluation of sorption performances and only a few of them aim at gaining a better understanding of sorption mechanisms. This can perhaps be explained by the fact that different kinds of interactions, such as ion-exchange interactions, hydrophobic attraction, physical adsorption, etc., can be acting simultaneously. Wide ranges of chemical structures, pH, salt concentrations and presence of ligands often add to the complication. Wu et al. (2000) showed that intraparticle diffusion plays an important role in the sorption mechanism. The uptake of dyes on chitosan may also proceed through ion-exchange mechanisms. The major adsorption site of chitosan is a primary amine group which is easily protonated to form Click to view the MathML source in acidic solutions. The strong electrostatic interaction between the Click to view the MathML source groups and dye anions can be used to explain the sorption mechanism (Chiou et al., 2004). The difference in the degree of adsorption may also be attributed to the chemical structure of each dye (Wong et al., 2004).
The results presented above show that chitosan-based materials may be promising biosorbents for adsorption processes since they demonstrated outstanding removal capabilities for dyes.
b) Peat : Peat is a porous and rather complex soil material with organic matter in various stages of decomposition. Based on the nature of parent materials, peat is classified into four groups, namely moss peat, herbaceous peat, woody peat and sedimentary peat. This natural material is a plentiful, relatively inexpensive and widely available biosorbent, which has adsorption capabilities for a variety of pollutants. Raw peat contains lignin, cellulose, fulvic and humic acid as major constituents. These constituents, especially lignin and humic acid, bear polar functional groups, such as alcohols, aldehydes, ketones, carboxylic acids, phenolic hydroxides and ethers that can be involved in chemical bonding.
Because of its polar character, peat can effectively remove dyes from solution (Allen et al., 2004, Ho and McKay, 1998b, Ho and McKay, 2003, Sun and Yang, 2003, Ramakrishna and Viraraghavan, 1997 and Poots et al., 1976). Peat adsorption capacities are reported in Table 8. The use of peat to remove dyes was investigated by Allen et al., 1988a, Allen et al., 1988b, Allen et al., 1994, Allen et al., 2004, Ho and McKay, 1998b and Ho and McKay, 2003 and Ramakrishna and Viraraghavan (1997). It was observed that peat tends to have a high cation exchange capacity, and is an effective sorbent for the removal of dyes. For acid and basic dyes, the removal performance was comparable with that of activated carbon, while for disperse dyes, the performance was much better.
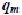 (mg/g) for peat
(mg/g) for peat 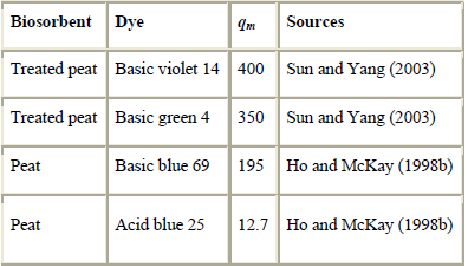
However, when raw peat is used directly as an adsorbent, there are many limitations: Natural peat has a low mechanical strength, a high affinity for water, poor chemical stability, a tendency to shrink and/or swell, and to leach fulvic acid (Couillard, 1994 and Smith et al., 1977). Chemical pretreatment and the development of immobilized biomass beads can produce a more robust medium. As with other sorbents, chemical processes are also used for improving sorption properties and selectivity. For example, Sun and Yang (2003) prepared modified peat-resin by mixing oxidizing peat with polyvinylalcohol and formaldehyde. These materials possess a macroreticular porous structure with enhanced physical characteristics. Their studies demonstrated that modified peat can be used for the removal of a variety of basic dyes. The maximum adsorption capacities for basic violet 14 and basic green 4 were 400 and 350 mg/g treated peat, respectively.
The mechanism by which dyes are adsorbed onto peat has been a matter of considerable debate. Different studies have reached different conclusions. Various pollutant-binding mechanisms are thought to be involved in the biosorption process, including physical adsorption, ion-exchange, complexation, adsorption–complexation and chemisorption (Brown et al., 2000). Variations in peat type and sorbent preparation also make the comparison of results difficult. However, it is now recognized that ion-exchange is the most prevalent mechanism.
c). Biomass : Decolorization and/or bioadsorption of dye wastewater by (dead or living) biomass, white-rot fungi and other microbial cultures was the subject of many studies reviewed in several recent papers (Aksu, 2005, Pearce et al., 2003, McMullan et al., 2001, Fu and Viraraghavan, 2001a, Stolz, 2001 and Robinson et al., 2001). In particular, these studies demonstrated that biosorbents derived from suitable microbial biomass can be used for the effective removal of dyes from solutions since certain dyes have a particular affinity for binding with microbial species (Robinson et al., 2001, Fu and Viraraghavan, 2001a, Bustard et al., 1998 and Nigam et al., 1996). The use of biomass for wastewater is increasing because of its availability in large quantities and at low price. Microbial biomass is produced in fermentation processes to synthesize valuable products such as antibiotics and enzymes. In such processes, a large amount of by-products is generated, which can be used in biosorption of pollutants. Biomass has a high potential as a sorbent due to its physico-chemical characteristics. A wide variety of microorganisms including algae, yeasts, bacteria and fungi are capable of decolorizing a wide range of dyes with a high efficiency (Swamy and Ramsay, 1999, Nigam et al., 1996, Jinqi and Houtian, 1992 and Mou et al., 1991).
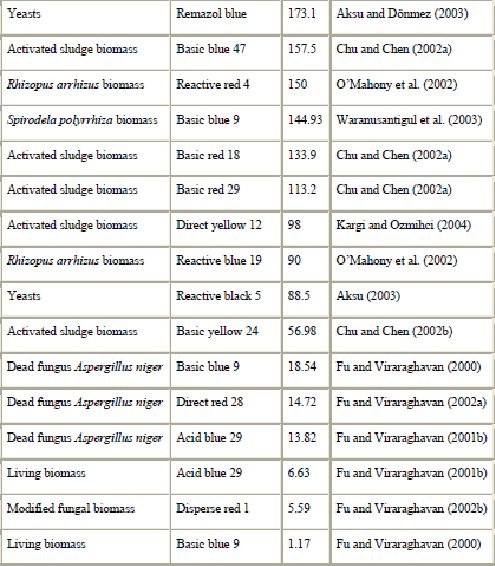
In fungal decolorization, fungi can be classified into two kinds according to their life state: living cells to biodegrade and biosorb dyes, and dead cells (fungal biomass) to adsorb dye (Fu and Viraraghavan, 2001a). Most of the studies concentrated on living fungi for biosorption of the dyes. There are few studies on dye removal using dead fungal biomass, except in recent years. Table 9 shows some of the adsorption capacities reported in the literature. Removal of dyes has recently been studied with strains of Aspergillus niger (Fu and Viraraghavan, 2002a and Fu and Viraraghavan, 2002b) and Rhizopus arrhizus (O’Mahony et al., 2002 and Aksu and Tezer, 2000). Fu and Viraraghavan, 2000, Fu and Viraraghavan, 2001b, Fu and Viraraghavan, 2002a and Fu and Viraraghavan, 2002b demonstrated that, compared with commercial activated carbon, dead fungal biomass of Aspergillus niger is a promising biosorbent for dye removal. Aksu and Tezer (2000) demonstrated uptake of 588.2 mg of reactive black 5 per g using Rhizopus arrhizus biomass. Waranusantigul et al. (2003) and Chu and Chen, 2002a and Chu and Chen, 2002b also reported the usefulness of biomass for the removal of basic dyes. The biosorption capacity of fungal biomass could be increased by some pretreatment (by autoclaving or by reacting with chemicals) (Fu and Viraraghavan, 2001a). Other types of biomass such as yeasts have been studied for their dye uptake capacities. Yeasts are extensively used in a variety of large-scale industrial fermentation processes and waste biomass from these processes is a potential source of cheap adsorbent material. The performance of yeasts as a low-cost adsorbent to remove dyes has been demonstrated by Aksu and coworkers (Aksu and Dönmez, 2003 and Aksu, 2003). They found that the maximum adsorption capacities of yeasts for remazol blue and reactive black 5 were 173.1 and 88.5 mg/g, respectively.
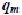 (mg/g) for biomass
(mg/g) for biomass 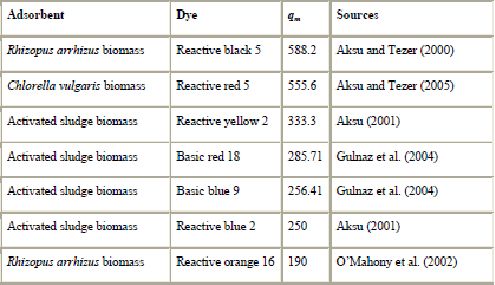
The major advantages of biosorption technology are its effectiveness in reducing the concentration of dyes to very low levels and the use of inexpensive biosorbent material. Fungal biomass can be produced cheaply using relatively simple fermentation techniques and inexpensive growth media (Fu and Viraraghavan, 2002a and Mittal and Gupta, 1996). The use of biomass is especially interesting when the dye-containing effluent is very toxic. Biosorption is also an emerging technology that attempts to overcome the selectivity disadvantage of conventional adsorption processes. The use of dead rather than live biomass eliminates the problems of waste toxicity and nutrient requirements. Biomass adsorption is effective when conditions are not always favorable for the growth and maintenance of the microbial population.
In spite of good sorption properties and high selectivity, some problems can occur. The sorption process is slow: in the case of biomass of Aspergillus niger equilibrium was reached in 42 h. Another problem is that the initial pH of the dye solution strongly influenced the biosorption (Aksu and Dönmez, 2003). Biosorption was also influenced by the functional groups in the fungal biomass and its specific surface properties (Kargi and Ozmihci, 2004). Biosorption performance depends on some external factors such as salts and ions in solution which may be in competition. Other limitations of the technology include the fact that the method has only been tested for limited practical applications since biomass is not appropriate for the treatment of effluents using column systems, due to the clogging effect. Because of major limitations regarding its efficient utilization in a column reactor, there is a need for it to be immobilized. This step forms a major cost factor of the process.
Dyes vary greatly in their chemistries and their interactions with microorganisms depend on the chemistry of a particular dye. There is also limited information available on the interactions between biomass and dyes (Chu and Chen, 2002b and Banks and Parinson, 1992). This can be explained by the fact that decolorization by living and dead cells involves several complex mechanisms such as surface adsorption, ion-exchange, complexation (coordination), complexation–chelation and micro-precipitation. Cell walls consisting mainly of polysaccharides, proteins and lipids offer many functional groups. The dyes can interact with these active groups on the cell surface in a different manner. The accumulation of dyes by biomass may involve a combination of active, metabolism-dependent and passive transport mechanisms starting with the diffusion of the adsorbed solute to the surface of the microbial cell (O’Mahony et al., 2002, Aksu and Tezer, 2000 and Veglio and Beolchini, 1997). Once the dye has diffused to the surface, it will bind to sites on the cell surface. The precise binding mechanisms may range from physical (i.e. electrostatic or Van der Waal forces) to chemical binding (i.e. ionic and covalent). However, it is now recognized that the efficiency and the selectivity of adsorption by biomass are due to ion-exchange mechanisms.
Biosorption processes are particularly suitable for the treatment of solutions containing dilute (toxic) dye concentration. Biosorption is a promising potential alternative to conventional processes for the removal of dyes (Aksu, 2005, Aksu and Tezer, 2005, Fu and Viraraghavan, 2002a, Fu and Viraraghavan, 2001a and Robinson et al., 2001). However, these technologies are still being developed and much more work is required.
| 4.4. Miscellaneous sorbents |
 |
Other materials have been studied as low-cost sorbents, such as starch (Delval et al., 2001, Delval et al., 2002 and Delval et al., 2003) and cyclodextrins (Crini, 2003, Crini and Morcellet, 2002, Crini et al., 1999, Crini et al., 2002a, Crini et al., 2002b, Martel et al., 2001 and Shao et al., 1996). Adsorption capacities are reported in Table 10. Next to cellulose, starch is the most abundant carbohydrate in the world and is present in living plants as an energy storage material. Starches are mixtures of two polyglucans, amylopectin and amylose, but they contain only a single type of carbohydrate, glucose. They are composed of a-d-glucose units linked together in 1,4-position. Amylose is nearly unbranched, while amylopectin is highly branched with the branches connected via the a-1,6-position of the anhydroglucose unit. Starch is used mostly in food applications, but there is a growing interest in its utilization as a renewable raw material for non-food industrial applications. Starches are unique raw materials in that they are very abundant natural polymers, inexpensive and widely available in many countries. They possess several other advantages that make them excellent materials for industrial use. They have biological and chemical properties such as hydrophilicity, biodegradability, polyfunctionality, high chemical reactivity and adsorption capacities. However, the hydrophilic nature of starch is a major constraint that seriously limits the development of starch based-materials. Chemical derivatisation has been proposed as a way to solve this problem and to produce water resistant sorbents.
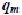 (mg/g) for other sorbents
(mg/g) for other sorbents 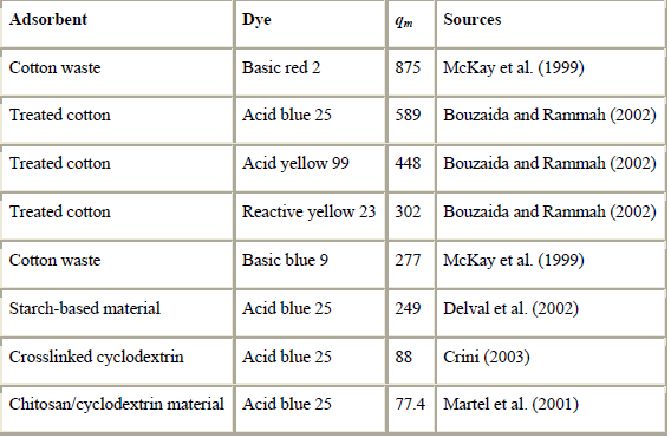
More important than starch is its cyclic derivative, cyclodextrin. Cyclodextrins (CDs) are torus-shaped cyclic oligosaccharides containing six to twelve glucose units. The CD molecules are natural macrocyclic polymers, formed by the action of an enzyme on starch (Szejtli, 1998). Beta-cyclodextrins containing seven glucose units are available commercially at a low cost. The most characteristic feature of CDs is the ability to form inclusion compounds with various aromatic molecules, including dyes. CDs possess a hydrophobic cavity in which a pollutant can be trapped. A review of cyclodextrin-based materials can be found in a recent review by Crini and Morcellet (2002).
Like other polysaccharides, starches and cyclodextrins can be crosslinked by a reaction between the hydroxyl groups of the chains with a coupling agent to form water-insoluble crosslinked networks. Due to the hydrophilic nature of their crosslinking units, crosslinked starches also possess a remarkably high swelling capacity in water, and consequently their networks are sufficiently expanded to allow a fast diffusion process for the pollutants. Crosslinked cyclodextrin polymers also have interesting diffusion properties and possess an amphiphilic character. It is precisely this character of these sorbents what makes them so appealing, since they are hydrophilic enough to swell considerably in water allowing fast diffusion processes for the dyes, while at the same time they possess highly hydrophobic sites which trap non-polar dyes efficiently. It is well known that synthetic resins have a poor contact with aqueous solutions and their modification is necessary for enhanced water wettability. Activated carbons adsorb some hydrophilic substances poorly.
In spite of varied characteristics and properties, a limited number of dye adsorption studies have been carried out on starch-based derivatives. Crini et al. (Crini, 2003, Crini and Morcellet, 2002, Crini et al., 1999, Crini et al., 2002a and Crini et al., 2002b) demonstrated that efficient extraction of dyes is achieved using crosslinked cyclodextrin gels. The presence of CD molecules in the polymer network permits an increase in its sorption properties. Delval et al., 2001, Delval et al., 2002 and Delval et al., 2003 proposed crosslinked starch polymers containing amine groups. The polymers were prepared by crosslinking an agroalimentary by-product. The sorption results showed that the adsorption rate was high and the sorption capacities were significant. Several hundred ppm of dyes could be removed from water effectively in a few minutes using column experiments. The control of the crosslinking reaction allows control of the sorption properties of the material. However, due to the protonation of the amine groups on the surface of the sorbent, its adsorption behavior is strongly influenced by the pH values. Another interesting idea is to combine the properties of two biopolymers. Attempts were made to prepare adsorbents by coupling chitosan and cyclodextrin (CD) via several spacer arms without affecting the selectivity of the two polymers. The novel biosorbents containing both cyclodextrin and chitosan are in general more hydrophilic than commercial synthetic resins. Decontamination of water containing textile dyes was carried out with these sorbents (Martel et al., 2001). The results showed excellent sorption properties toward different classes of dyes. The chitosan beads containing CD are also characterized by a rate of sorption and an efficiency superior to that of the parent chitosan bead without CD and of the crosslinking cyclodextrin-epichlorohydrin gels. The maximum adsorption capacities of starch-based material, crosslinked cyclodextrin and chitosan/cyclodextrin mixed sorbents for acid blue 25 were 249, 88 and 77.4 mg/g, respectively. There are several disadvantages of using starch-based materials for dye removal. The efficiency of adsorption depends strongly on the control or particle size and the expansion of the polymer network (Crini, 2003). Performance is also dependent on the type of material used. Another problem with these materials is that they are non-porous and possess low surface area. Adsorption by starch-based materials occurs by physical adsorption, complexation and ion-exchange interactions (Delval et al., 2003 and Crini, 2003).
Other materials used to adsorb dyes are cotton waste (Sawada and Ueda, 2003, Bouzaida and Rammah, 2002 and McKay et al., 1999) and alumina (Harris et al., 2001 and Desai et al., 1997). Adsorption capacities are reported in Table 10. Cotton is the most abundant of all naturally occurring organic substrates and is widely used. This material characteristically exhibits excellent physical and chemical properties in terms of stability, water absorbency and dye removal ability. The performance of treated cotton in a continuous system has been demonstrated by Bouzaida and Rammah (2002). They found that the adsorption capacities of cotton for acid blue 25, acid yellow 99 and reactive yellow 23 were 589, 448 and 302 mg/g, respectively. McKay et al. (1999) also evaluated the performance of cotton waste for dye removal. It was found that this waste had the potential to adsorb 875 and 277 mg of basic red 2 and basic blue 9/g, respectively.
1.3.4 Comparison of sorption performanceGenerally, a suitable non-conventional low-cost adsorbent for dye adsorption should meet several requirements: (i) efficient for removal of a wide variety of dyes; (ii) high capacity and rate of adsorption; (iii) high selectivity for different concentrations; and (iv) tolerant of a wide range of wastewater parameters.
Certain waste products, natural materials and biosorbents have been tested and proposed for dye removal. Which low-cost adsorbent is better? There is no direct answer to this question because each low-cost adsorbent has its specific physical and chemical characteristics such as porosity, surface area and physical strength, as well as inherent advantages and disadvantages in wastewater treatment. In addition, adsorption capacities of sorbents also vary, depending on the experimental conditions. Therefore, comparison of sorption performance is difficult to make. However, it is clear from the present literature survey that non-conventional adsorbents may have potential as readily available, inexpensive and effective sorbents. They also possess several other advantages that make them excellent materials for environmental purposes, such as high capacity and rate of adsorption (Table 11), high selectivity for different concentrations, and also rapid kinetics (Table 12).
 in mg/g) for commercial activated
in mg/g) for commercial activated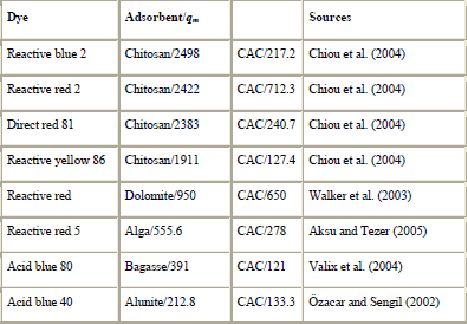
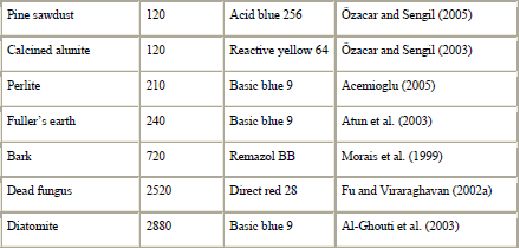
Table 11 presents a summary of some of the highest adsorption capacities reported. From the recent literature reviewed, adsorbents that stand out for high adsorption capacities are chitosan (2498, 2422, 2383, 1911 mg/g of reactive blue 2, reactive red 2, direct red 81, and reactive yellow 86, respectively), pinewood (1176 and 1119 mg/g of acid blue 264 and basic blue 25, respectively), bark (1119 mg/g of basic red 2) and corncob (1060 mg/g of acid blue 25). These adsorbents are efficient and can be used effectively for the removal of dye from aqueous solutions. In particular, chitosan has received considerable interest for dye removal due to its excellent dye-binding capacities. Since fishery wastes are abundantly available, chitosan may be produced at a low cost and it was competitive against CAC. Chitosan has demonstrated outstanding removal capabilities for certain dyes compared to activated carbon (Table 11). For example, the adsorption capacity for reactive blue 2 is much greater on chitosan (2498 mg/g) than on CAC (217.2 mg/g). Several significant sorption results have been published by Chiou et al. (Chiou et al., 2004, Chiou and Li, 2002 and Chiou and Li, 2003). They demonstrated that chitosan can be used for the decontamination of effluents. It offers both a procedure of choice for extraction processes and a lot of promising benefits for commercial purposes. Another interesting low-cost adsorbent shown to adsorb pollutants is peat. Adsorption using peat is well established. Its use in water treatment systems has received attention over the past 30 years and peat filters and biofilters currently offer an attractive method of wastewater treatment (Allen et al., 1994, Allen et al., 2004 and Brown et al., 2000).
Several recent reports have been published on the comparison of sorption performance. For example, Atun et al. (2003) demonstrated that basic blue 9 adsorption on Fuller’s earth was very fast compared to that on CAC and Fuller’s earth particles have higher adsorption capacity. Furthermore, Fuller’s earth is approximately 500 times cheaper than CAC. Espantaleon et al. (2003) reported that the capacity of sepiolite and acid-treated bentonite to adsorb anionic dyes was much greater than that of conventional adsorbents. Bagane and Guiza (2000) showed that the adsorption capacity was 20 times greater on kaolinite than on alumina. Wang et al. (2005) showed that fly ash exhibited higher capacity than red mud. Peat has excellent ion-exchange properties similar to those of natural zeolites (Allen et al., 2004). Kargi and Ozmihci (2004) reported that powdered activated sludge can be used for removal of different dyestuffs as an alternative to powdered activated carbon.
It is evident that natural materials, waste materials from industry and agriculture and biosorbents are an interesting alternative to replace activated carbons. Some promising results could be noted in the case of clays, peat and chitosan-based materials (Alkan et al., 2005, Alkan et al., 2004, Allen et al., 2004, Guibal, 2004, Varma et al., 2004, Ho and McKay, 2003 and Ravi Kumar, 2000). Several biosorption processes have also been developed, patented and introduced for application in removing contaminants from waters (Aksu, 2005, Aksu and Tezer, 2005, Pokhrel and Viraraghavan, 2004, Fu and Viraraghavan, 2002a, Fu and Viraraghavan, 2001a and Robinson et al., 2001). A comprehensive study of the application of biosorption for the removal of organic pollutants can be found in a recent review by Aksu (2005). However, despite the number of published laboratory data, non-conventional low-cost adsorbents have not been applied at an industrial scale. There are several reasons for this difficulty in transferring the process to industrial applications. They can be summarized as follows:
In this review, a wide range of non-conventional low-cost adsorbents has been presented. Inexpensive, locally available and effective materials could be used in place of commercial activated carbon for the removal of dyes from aqueous solution. Undoubtedly low-cost adsorbents offer a lot of promising benefits for commercial purposes in the future. In particular, from the recent literature reviewed, chitosan-based sorbents have demonstrated outstanding removal capabilities for certain dyes in comparison to activated carbon. However, despite a number of papers published on low-cost adsorbents, there is as yet little information containing a full study of comparison between sorbents. Although much has been accomplished in the area of low-cost sorbents, much work is necessary (i) to predict the performance of the adsorption processes for dye removal from real industrial effluents under a range of operating conditions, (ii) to better understand adsorption mechanisms and (iii) to demonstrate the use of inexpensive adsorbents at an industrial scale.
 |