|

|
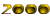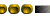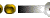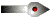
|

COMPARATIVE STUDY OF PHENOTYPE AND GENOTYPE OF THE OYSTER CRASSOSTREA MADRASENSIS IN THREE VARIABLY POLLUTED WATER BODIES OF KERALA
Ajay Narendra
|

|
|

 ABSTRACT
ABSTRACT
 MATERIALS AND METHODS
MATERIALS AND METHODS
 OBSERVATIONS AND DISCUSSIONS
OBSERVATIONS AND DISCUSSIONS
 HEAVY METAL CONTENT IN THE SEDIMENTS
HEAVY METAL CONTENT IN THE SEDIMENTS
 BIOACCUMULATION ANALYSIS
BIOACCUMULATION ANALYSIS
 EXPERIMENTS CONDUCTED ON DIFFERENT PARTS OF THE OYSTER FOR PROPER CHROMOSOMAL SPREADS
EXPERIMENTS CONDUCTED ON DIFFERENT PARTS OF THE OYSTER FOR PROPER CHROMOSOMAL SPREADS
 RHINGERMAN AND BLOOM (1977) MODIFIED KARYOTYPING PROCEDURE
RHINGERMAN AND BLOOM (1977) MODIFIED KARYOTYPING PROCEDURE
 CORRELATION INDEX BETWEEN THE PHENOTYPE AND GENOTYPE
CORRELATION INDEX BETWEEN THE PHENOTYPE AND GENOTYPE
 ACKNOWLEDGEMENT
ACKNOWLEDGEMENT
 REFERENCES
REFERENCES
 FIGURES 1,2&3
FIGURES 1,2&3
| ABSTRACT: |




|
The amount of pollution in two of the three water bodies selected in Kerala has been recorded in this study. Three different phenotypes of the same species of oyster were observed. On conducting karyotype analysis on these three phenotypes, 19, 20 and 21 chromosomes were recorded respectively. This observed chromosomal variation was recorded only with specifically associated phenotypes, giving rise to thoughts of a probable variation or mutation due to excessive pollution of lakes, which results in bioaccumulation. Higher level of pollution is substantiated by the presence of a large amount of heavy metals in the tissue of the edible oyster Crassostrea madrasensis, which was used as the indicator.




Although technological optimists may dream of living someday in an artificial environment, human life so far is extricably and intricately linked to ecological services provided by other organisms. The earth's ecosystem represents the culmination of historic evolutionary processes of immense antiquity and majesty. As man does not understand the complex inter relationships of organisms, he is often surprised and dismayed at the repercussions caused by removing seemingly insignificant members of biological communities. Extinction is a normal process of the natural world, where species die out and are replaced by others as part of the evolutionary change. In undisturbed ecosystems, the rate of extinction appears to be one species per decade. In this century, however, human impact on population and ecosystems has accelerated the rate, causing hundreds or even thousands of species, subspecies and varieties to become extinct every year. Wilson estimates that 20,000 species are pushed towards extinction every year.
Water bodies in the recent past have been forced to act as sinks to a variety of wastes produced by both the urban and rural sections of the society. Water pollution is a major threat to all organisms living in or depending on it. Human impacts on this system have come in many forms. Nutrient loading from agricultural runoff and sewage discharge, toxic chemical inputs from industry and agriculture have degraded the functioning of the system. Further, the variability of major ecological parameters has also had a major impact on the presence and the degree of effect of the pollutant on both the organism and the water body.
| MATERIALS AND METHODS: |




|
Kaalamukke in Cochin (9o97`N 76o23`E) acts as a major sink for domestic sewage and industrial effluents. There is a large amount of pollution caused by the offshore and onshore loading of ships. A large amount of oil is also let out during cleaning of the ships. Ashtamudi lake in Kollam (8o53` - 9o52`N and 76o31` - 76o41`E) is the second largest estuarine system in Kerala. Kollam recorded a major amount of oil leakage in 1997 killing fishes and disrupting the food chain. Munamakalle in Chetua is a small island in Kerala, which is nearly uninhabited. Ships do not travel in these waters, making the place comparatively less polluted. Field investigations were carried out in these three places.
Regular fortnightly grab sampling was carried out from May to July 1999. Surface water sampling was done using 1000-ml glass bottle. The bottle was washed in ambient water before sampling. Sampling was done by carefully dipping the bottles inside, preventing entry of air bubbles. A water sampler was used to take water samples from the bottom of the water bodies. Physico-chemical parameters - pH, temperature, turbidity, salinity, chlorinity, dissolved oxygen, ammonia, phosphorus, nitrates and nitrites and heavy metals - mercury, lead, cadmium, copper, iron, manganese and zinc were analyzed. Oysters were used as indicators as they posses certain physiological adaptation that enables them to tolerate a fairly wide range of variation in hydrographic factors. Oysters are also scientifically the best-known marine animals in the world (Beaumont, A. R and L.L.D. Gruffyd., 1974). The species selected for the study was the Indian backwater oyster Crassostrea madrasensis Preston. The tissues of this species (an edible oyster having high export value) were checked for heavy metal content. Ten animals of similar size were washed and kept in filtered sea water system to purge the gut content. Whole body tissues of animals were removed, washed with de-ionized water and brought to the laboratory in frozen condition and stored in the deep freezer at -20oC for further analysis. At the time of analysis the tissues were washed and dried in an oven at 95oC to constant weight and powdered. For the estimation of trace metals, 50g of the sample were digested with nitric acid-sulfuric acid mixture. Samples were analyzed for iron, copper, zinc, lead, manganese and cadmium by acid digestion and quantified using atomic absorption spectrophotometer. Sample digestion for the determination of mercury was done according to the AOAC method 25.112 (5) and estimation of mercury was carried out by the cold vapor atomic absorption spectrophotometric technique in a Mercury analyzer.
Experiments were carried out by varying the concentration of chemicals and also the time duration of exposure to the chemicals. When the best results (chromosome spreads) were obtained, it was tried upon the kidney, mantle and gill tissues of the organism and tabulated. The method used for karyotyping was a modified version of Rhingerman and Bloom (1977).
After the standardization values were decided, the oysters were karyotyped. Ten oysters of this species were collected from Cochin, Chetua and Kollam.
The oyster was placed in a trough filled with 500 ml of seawater (collected from the habitat) and 500 ml of micro algae. To this, 0.05% of colchicine was added. Colchicine was used as it arrests the cell division at metaphase. The oyster was kept in the trough for 6 hours. The gill tissues were then removed and stored in a hypotonic solution for 20 - 30 minutes. Then the tissue was kept in a vial containing a fixative, methyl alcohol and methyl acetate in the ratio of 3:1. Changes were made in the vial every 10, 15 and 20 minutes. The gill tissue became a bit hard after this process. The vial was then kept in the refrigerator overnight. The tissue was later taken out of the vial and minced and the large fragments that remained after mincing were removed. The minced tissue appeared as a solution. Glass slides were warmed to a temperature of 45 - 50oC. The minced tissue was dropped on the warm slides using a Pasteur pipette and immediately sucked back. A dry ring appeared on the slide. The slide was stained with Giemsa stain with a phosphate buffer (pH = 6.8) for 20 minutes and washed with distilled water. The slides were dried and viewed under the microscope for the chromosome spreads.
| OBSERVATIONS AND DISCUSSIONS: |




|
The ecological parameters and heavy metal content were analyzed in all the 3 areas and the results have been tabulated below as S - surface and B - bottom values.
COCHIN
|
PARAMETERS |
MAY |
JUNE |
JULY |
|
pH |
S7.8 B7.9 |
S7.7 B7.8 |
S7.8 B8.1 |
|
TEMPERATURE (oC) |
S27.9 B28.2 |
S27.9 B28.1 |
S27.8 B28.0 |
|
SALINITY (ppt) |
S2 B11 |
S2 B12 |
S3 B14 |
|
SILICATES (m mol/l) |
S219.5 B140.2 |
S219.15 B280.2 |
S219.05 B280 |
|
NITRATES (m mol/l) |
S6.15 B6.40 |
S8.65 B8.90 |
S8.98 B9.15 |
|
NITRITES (m mol/l) |
S7.79 B7.85 |
S7.84 B7.89 |
S7.90 B7.93 |
|
PHOSPHATES (m mol/l) |
S27.5 B22.7 |
S27.7 B22.6 |
S27.8 B22.7 |
|
AMMONIA (m mol/l) |
S7.8 B8.1 |
S10.0 B11.2 |
S11.3 B12.6 |
|
TURBIDITY (JTU) |
S31 B33 |
S33 B32 |
S32 B33 |
|
DISSOLVED OXYGEN (mg/l) |
S3.6 B3.1 |
S2.9 B2.7 |
S3.9 B3.2 |
|
CHLORINITY (m mol/l) |
S1.107 B6.088 |
S1.107 B6.642 |
S1.66 B7.74 |
KOLLAM
|
PARAMETERS |
MAY |
JUNE |
JULY |
|
pH |
S7.86 B7.88 |
S8.09 B8.13 |
S8.26 B8.29 |
|
TEMPERATURE (oC) |
S30.2 B29.8 |
S28.7 B28.3 |
S29.1 B28.6 |
|
SALINITY (ppt) |
S31.8 B31.9 |
S28.9 B29.5 |
S16.9 B30.0 |
|
SILICATES (m mol/l) |
S38.40 B43.60 |
S38.60 B43.80 |
S37.90 B42.20 |
|
NITRATES (m mol/l) |
S3.15 B3.43 |
S4.69 B4.95 |
S5.97 B6.24 |
|
NITRITES (m mol/l) |
S1.45 B2.15 |
S1.51 B2.45 |
S2.21 B2.70 |
|
PHOSPHATES (m mol/l) |
S5.78 B7.45 |
S4.62 B5.86 |
S5.13 B6.29 |
|
AMMONIA (m mol/l) |
S7.8 B8.7 |
S10.2 B11.5 |
S11.3 B12.2 |
|
TURBIDITY (JTU) |
S43.0 B47.0 |
S45.0 B46.0 |
S44.0 B47.0 |
|
DISSOLVED OXYGEN (mg/l) |
S3.99 B3.59 |
S4.07 B3.68 |
S5.03 B4.43 |
|
CHLORINITY (m mol/l) |
S17.60 B17.65 |
S15.99 B16.32 |
S9.35 B16.60 |
CHETUA
|
PARAMETERS |
MAY |
JUNE |
JULY |
|
pH |
S7.60 B7.71 |
S7.22 B7.36 |
S7.80 B 7.86 |
|
TEMPERATURE (oC) |
S32.4 B31.7 |
S31.0 B29.1 |
S31.7 B30.1 |
|
SALINITY (ppt) |
S00.72 B06.00 |
S00.72 B07.00 |
S00.79 B10.00 |
|
SILICATES (m mol/l) |
S33.3 B39.60 |
S33.6 B39.90 |
S32.4 B38.40 |
|
NITRATES (m mol/l) |
S18.55 B19.10 |
S30.51 B30.83 |
S30.69 B30.85 |
|
NITRITES (m mol/l) |
S3.75 B4.10 |
S4.40 B4.78 |
S5.64 B6.12 |
|
PHOSPHATES (m mol/l) |
S15.50 B15.73 |
S13.90 B14.13 |
S14.80 B15.10 |
|
AMMONIA (m mol/l) |
S3.30 B3.40 |
S5.10 B5.50 |
S5.90 B6.70 |
|
TURBIDITY (JTU) |
S16.0 B18.0 |
S19.0 B20.0 |
S19.0 B18.0 |
|
DISSOLVED OXYGEN (mg/l) |
S4.47 B4.29 |
S4.68 B4.51 |
S5.69 B5.50 |
|
CHLORINITY (m mol/l) |
S0.398 B3.321 |
S0.4025 B3.870 |
S0.437 B5.535 |
Availability of space, competition, predation and tolerance range to environmental variation are some of the factors that control the zonation of bivalves. Temperature rather than salinity has been suggested to be limiting in the distribution of temperate bivalves (Brown J R and Hartwick E B. 1988 a, b). However, in tropical conditions where monsoon plays an important role, salinity has been considered as the main factor influencing the distribution of bivalves. The venerid clams Meritrix meritrix and Katelysia opima coexist in estuaries of Maharashtra. However, due to its more tolerant nature, M. meritrix has been found to dominate the typical estuarine zone while K. opima is more marine. In the Vembanad lake of Kollam, zonation has also been observed in the distribution of clams. Sunetta scripta, a stenohaline species dominates the bar mouth and open coast. M.casta, a typical estuarine form, found in the area towards the estuary side close to the bar mouth region, followed by a zone in the upper reaches where the typical euryhaline species Villorita cyprinoides, which can tolerate a very broad range of salinity, dominates. This zonation (similar to the oyster zonation observed in the present study) is primarily due to the difference in the lower threshold of their salinity tolerance (Brown J, 1988).
Environmental changes connected with south west monsoon in the west coast such as heavy flooding and silting have known to cause mortality in the clam species Villorita cyprinoides in Karnataka (Joseph and Joseph, 1985), and Paphia malabarica and Crassostrea madrasensis. One of the major factors affecting the distribution of edible oysters in the coastal estuaries of the west coast appeared to be siltation. In Ashtamudi lake, the reduction in population density can be attributed to the reduction in salinity (during Aug - Oct) due to southwest monsoon and river discharge. Experiments have shown that Sacostrea cucculata can tolerate salinity up to 8.6 ppt. Salinity of the water was considered as the primary factor in controlling the biological activities and the occurrence, distribution and survival of sedentary organism in estuarine regions of tropics (Gopakumar G. 1992). Continued submergence in salinity conditions beyond the optimum range a species can tolerate would affect its growth and survival. Though oyster density declines considerably, complete mortality was not observed.
The tolerance capacity of both the oyster species is highlighted by their survival during the monsoon period in the areas of their distribution. Depressed salinity due to southwest monsoon and river discharge would have created physiological stress on the oysters. During such stressful conditions, the animals react by reducing the intensity of feeding and instead utilize the stored energy for survival. The gonadial development also becomes slow. Reduced food intake due to decreased salinity has been observed in Mytilus edulis. In the American oyster Crassostrea virginica (Davis H.C.1941), similar low intake of food was noted under decreased saline conditions. Salinity zonation has been found to be the main reason for the zonation pattern displayed by Crassostrea madrasensis and the variation in the associated fauna of oyster beds (Velayudhan et. al., 1995). The low saline conditions during monsoon cause mortality among the oysters resulting in their low density and biomass. Similar variation in the oyster population due to salinity has been recorded on the west and the east coast. However, in the temperate regions, the variations in temperature can be as high as 15 - 20oC. In such regions, temperature plays a major role in the distribution of bivalves and survival, hindering their activities. Inhibition of spawning; byssus formation in mussels and pectinids; delayed larval development and setting are some factors, which have been reported due to thermal stress (Rao K Virabhadra. 1956).
| HEAVY METAL CONTENT IN THE SEDIMENTS |




|
COCHIN
|
HEAVY METALS (ppm) |
MAY |
JUNE |
JULY |
|
CADMIUM |
20.20 |
17.60 |
16.20 |
|
COPPER |
89.40 |
85.30 |
84.20 |
|
MANGANESE |
401.0 |
406.0 |
419.0 |
|
ZINC |
334.3 |
336.7 |
341.2 |
|
LEAD |
73.00 |
66.00 |
52.00 |
|
MERCURY |
0.201 |
0.205 |
0.206 |
KOLLAM
|
HEAVY METALS |
MAY |
JUNE |
JULY |
|
CADMIUM |
22.60 |
16.50 |
16.20 |
|
COPPER |
62.60 |
57.80 |
57.50 |
|
MANGANESE |
434.0 |
431.0 |
427.0 |
|
ZINC |
290.7 |
292.8 |
295.2 |
|
LEAD |
86.00 |
79.00 |
73.00 |
|
MERCURY |
0.171 |
0.168 |
0.169 |
CHETUA
|
HEAVY METALS |
MAY |
JUNE |
JULY |
|
CADMIUM |
11.20 |
10.80 |
10.90 |
|
COPPER |
51.60 |
49.20 |
44.70 |
|
MANGANESE |
275.0 |
278.0 |
283.0 |
|
ZINC |
279.1 |
282.4 |
285.8 |
|
LEAD |
41.00 |
36.00 |
34.00 |
|
MERCURY |
0.025 |
0.028 |
0.036 |
| BIOACCUMULATION ANALYSIS |




|
COCHIN
|
HEAVY METALS (ppm) |
MAY |
JUNE |
JULY |
|
CADMIUM |
3.80 |
4.60 |
4.35 |
|
COPPER |
31.60 |
33.90 |
30.50 |
|
MANGANESE |
1.25 |
1.35 |
1.55 |
|
IRON |
29.5 |
45.5 |
53.3 |
|
ZINC (mg/g) |
0.699 |
0.984 |
0.914 |
|
LEAD |
4.65 |
4.55 |
5.30 |
|
MERCURY (ppb) |
17.65 |
19.13 |
24.86 |
KOLLAM
|
HEAVY METALS |
MAY |
JUNE |
JULY |
|
CADMIUM |
3.45 |
4.45 |
3.97 |
|
COPPER |
32.60 |
34.40 |
31.50 |
|
MANGANESE |
3.15 |
3.10 |
3.40 |
|
IRON |
41.30 |
38.20 |
53.10 |
|
ZINC |
0.910 |
1.150 |
0.975 |
|
LEAD |
5.48 |
5.45 |
5.25 |
|
MERCURY |
31.31 |
36.60 |
47.25 |
CHETUA
|
HEAVY METALS |
MAY |
JUNE |
JULY |
|
CADMIUM |
1.18 |
1.45 |
1.37 |
|
COPPER |
28.40 |
33.60 |
27.75 |
|
MANGANESE |
0.60 |
0.65 |
0.70 |
|
IRON |
10.0 |
19.00 |
23.0 |
|
ZINC |
0.331 |
0.680 |
0.588 |
|
LEAD |
1.95 |
1.80 |
2.15 |
|
MERCURY |
9.05 |
9.85 |
11.28 |
The interactions of heavy metals in aquatic systems are complicated because of the possible changes due to many dissolved and particulate components and non-equilibrium conditions. Often heavy metals experience a change in the chemical form or speciation as a result of these processes and their distribution, subsequently affecting the bioavailability and other interactions in the environment. For example, the speciation of heavy metals is controlled not only by their chemical properties but also environmental variables like pH, redox potential, dissolved oxygen, ionic strength, temperature, salinity, alkalinity, hardness, concentration and nature of inorganic ions such as carbonate, bicarbonate, sulfate, sulfides, chlorides, concentration and nature of dissolved organic chelating agents such as organic acids, humic materials, peptides and polyamino carboxylates. As environmental factors change the chemical reactions and speciation of heavy metals, they influence not only the mobilization, transport and bioavailability, but also the toxicity of heavy metal ions towards biota in both freshwater and marine ecosystems.
The factors affecting the toxicity and bioaccumulation of heavy metals by aquatic organisms include:
The extent to which most of the methylated metals are bioaccumulated and/or biomagnified is limited by the chemical and biological conditions and how readily the methylated metal is metabolized by an organism. Despite these adverse effects at very low levels, heavy metals have some essential physiological roles as micronutrients. Heavy metals such as chromium, manganese, iron, cobalt, molybdenum, nickel, copper and selenium are required in small amounts to perform important biochemical functions in plant and animal systems. In higher concentrations they can be toxic, but usually some biological regulatory mechanism is available by means of which animals can speed up their excretion or retard their uptake of excessive quantities. In contrast, non-essential heavy metals are of primary concern in terrestrial and aquatic systems as they are toxic and persistent. Metal ions commonly bond with sulfhydryl and carboxyl acid groups in amino acids, which are components of proteins or polypeptides. This increases their bioaccumulation and inhibits excretion. For example, heavy metals such as lead, cadmium and mercury bind strongly with -SH and SCH3 groups in cysteine and methionine and so inhibit the metabolism of the bound enzymes. In addition, other heavy metals may replace an essential element, decreasing its availability and causing symptoms of deficiency.
| EXPERIMENTS CONDUCTED ON DIFFERENT PARTS OF THE OYSTER FOR PROPER CHROMOSOMAL SPREADS |




|
|
TISSUE |
COLCHICINE |
COLCHICINE EXPOSURE |
HYPOTONIC TREATMENT |
HYPOTONIC TREATMENT |
|
Mantle |
0.05% |
30 mins |
0.9% |
1 hr |
|
Kidney |
0.05% |
30 mins |
0.9% |
1 hr |
|
Gills |
0.05% |
30 mins |
0.9% |
1 hr |
|
ACETIC ACID SUSPENSION |
STAIN |
FIXATIVE |
STORAGE |
RESULT |
|
50% |
Giemsa |
CH3OH: CH3COOH |
Methanol |
- |
|
50% |
Giemsa |
CH3OH: CH3COOH |
Methanol |
- |
|
50% |
Giemsa |
CH3OH: CH3COOH |
Methanol |
+ |
| RHINGERMAN AND BLOOM (1977) MODIFIED KARYOTYPING PROCEDURE |




|
|
TISSUE |
COLCHICINE |
COLCHICINE EXPOSURE |
HYPOTONIC TREATMENT |
HYPOTONIC TREATMENT |
|
Gills |
0.05% |
6 hours |
25% - 50% sea water |
30 min |
|
FIXATIVE |
ACETIC ACID SUSPENSION |
STAIN |
RESULT |
|
CH3OH: CH3COOH 3:1 |
45% |
Giemsa pH – 6.8 |
+ |
Ten oysters (Crassostrea madrasensis) were analyzed for chromosomes in Cochin, Kollam and Chetua respectively. The analysis revealed the following.
| CORRELATION INDEX BETWEEN THE PHENOTYPE AND GENOTYPE |




|
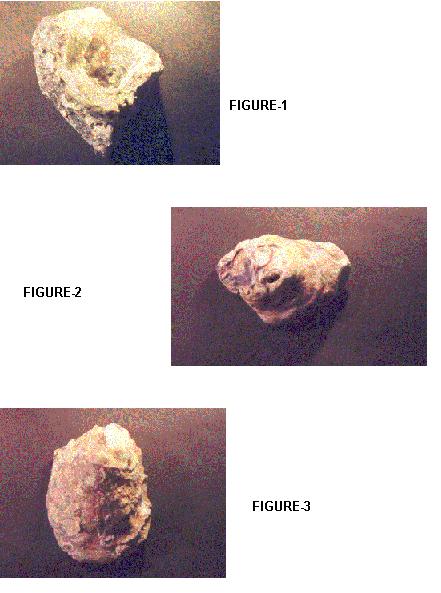
This correlation index helps in knowing the variations exhibited morphologically by the oysters, which have three different diploid numbers.
The study of karyotype and chromosome behaviour in relation to the pattern of inheritance of chromosomal genes through reproduction is known as cytogenetics. This has a central role in phylogenetic studies of plants and animals and in investigations of the biology of natural populations. Cytogenetic studies of molluscs include those concerned with hybrid study, polyploidy, chromosome morphology and behaviour, karyotype analysis, sex chromosomes and supernumerary chromosome.
The oyster Crassostrea madrasensis has been used as an indicator for this study as it is known to be extremely tough and resistant. There has been a huge heavy metal content present in the sediments of two of the selected areas. Large amounts of heavy metals have also been present in the tissues of the organisms. These heavy metals could probably have an antagonistic effect, though a synergistic effect on them is also definitely possible. These organisms are affected by ecological variations they have adapted to. This study has also seen variations in chromosomal numbers within the species. This kind of a variation could be due to a deletion or translocation of the chromosomes. But the correlation seen between the chromosomal numbers and the phenotype could just be more than a coincidence.
| ACKNOWLEDGEMENT: |




|
I would like to thank St Joseph's College, Bangalore for having given me an opportunity to undertake this study, Mrs. Geetha Viswanathan, Department of Zoology, St Joseph's College, for her assistance and Dr. Kripa and Dr. Thomas from Central Marine Fisheries Research Institute, Cochin, for extending their unbound help for the completion of this study.
| REFERENCES: |




|
| FIGURES-1,2&3 |




|
| Address: |


|
1.) Undergraduate student,
Department of Zoology,
St. Joseph's College,
Bangalore - 560 025,
Karnataka,
India
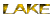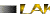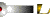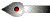
|
|