|

|
|

MANAGING THE ALGAL BLOOM IN A EUTROPHIC LAKE USING SELECTIVE HERBIVOROUS FISHES - B. B. Jana and S. Datta-Saha1

|

|
|


 ABSTRACT
ABSTRACT
 INTRODUCTION
INTRODUCTION
 BIOMANIPULATION
BIOMANIPULATION
 GRAZING ACTIVITY
GRAZING ACTIVITY
 GRAZING IN SITU
GRAZING IN SITU
 GRAZING IN LABORATORY CONDITIONS
GRAZING IN LABORATORY CONDITIONS
 SELECTIVE GRAZING
SELECTIVE GRAZING
 DEFECATION
DEFECATION
 MICROCYSTIS DIGESTIBILITY
MICROCYSTIS DIGESTIBILITY
 GROWTH EFFICIENCY
GROWTH EFFICIENCY
 ICHTHYO-EUTROPHICATION
ICHTHYO-EUTROPHICATION
 CONCLUSION
CONCLUSION
 REFERENCES
REFERENCES
 FIGURE-1
FIGURE-1
 FIGURE-2
FIGURE-2
 FIGURE-3
FIGURE-3
 FIGURE-4
FIGURE-4
| ABSTRACT: |




|
Cyanabactrial blooms is a severe problem in many tropical inland waters due to increasingly high rates of eutrophication in recent years. Algal blooms disturb oxygen balance; secrete toxins of low molecular weight harmful to many aquatic animals and cause gill clogging of some surface feeding fishes. Cyanobacteria comprise an important source of food for some herbivorous fishes. The present paper attempts to highlight the use of herbivorous fishes in controlling algal bloom in terms of biomanipulation, grazing activity in situ and laboratory, selective grazing, defecation, Microcystis digestibility, growth efficiency, ichthyoeutrophication. It is concluded that silver carp is suitable for cleaning Microcystis in the long term, whereas Tilapia may be used for short-term clearance of Microcystis from small ponds.
| INTRODUCTION: |




|
Eutrophication is a growing global problem in large number of inland waters. A common symptom of lake eutrophication is the appearance of algal blooms caused by cyanobacterial species such as Microcystis, Anabaena, Oscillatoria, Nodularia, Nostoc, etc. (Skulberg et al., 1984; Codd et al., 1989; Carmichael, 1992). Such algal blooms in fish ponds are not desirable as they disturb diurnal oxygen balance of the fish ponds and cause gill clogging of some surface feeding fishes. Cyanobacterial blooms also secrete toxins of low molecular weight, which are harmful to both aquatic animals and other phytoplanktons used as source of food for fishes and other aquatic animals (Phillips et al., 1985; Rabergh et al., 1991).
Though several chemical methods are employed to control algal blooms in tropical water bodies (Jhingran, 1995; Yin et al., 1989), they are either expensive or have some residual effects in the aquatic food chain in the long run. About 70% of India's surface water are highly polluted with excessive nutrients with appearance of cyanobacterial blooms, and thereby remain unutilized for aquaculture. Bio-control of algae through introduction of suitable herbivorous fishes is the most environmentally sound management proposition in many tropical and temperate lakes. The present paper attempts to highlight the efficiency of some herbivorous fishes to control Microcystis bloom in some tropical ponds.
| BIOMANIPULATION: |




|
The biomanipulation and top-down or consumer controlled forces refer to the control of natural aquatic communities aimed at water quality improvement instead of through nutrient management (Shapiro et al., 1975). In essence, it is a method of biological engineering in which organisms are selectively removed or encouraged to alleviate the symptoms of eutrophication (Moss, 1992). The rationale of using certain herbivorous fishes is that some fishes are efficient grazers on algae; their populations are easily manipulated by removal, stocking or changing species composition; fishes are economically important and are thus cost-effective.
There are two biomanipulation approaches for controlling algal bloom through fishes; reduction of algal biomass by direct grazing or indirect repression of algal bloom through fish-derived alterations in zooplankton communities and nutrient cycling. The first one is more productive-efficient than others because of shorter food chain and less energy consumption. Many whole lake biomanipulation experiments have been performed in many developed countries to improve water quality in lakes and reservoirs. However, data on tropical lakes are hardly available for comparison.
| GRAZING ACTIVITY |




|
The non-toxic strains of cyanobacterial blooms comprise an important source of the diet in a number of cyprinid and cichlid fishes (Hofer and Schiemer, 1983; Adamek and Spittler, 1984; Drenner et al., 1984; Spataru and Gophen, 1985) as they are reported to contain >50% protein (DeMoor and Scott, 1985). Among the cyprinids, silver carp has been demonstrated to be the most promising and, therefore, has been extensively used to control algal blooms in lakes and reservoirs.
More than 40% of the increase in aquaculture production in 1990s was due to herbivorous fishes that are exclusively dependent upon micro algae (Huntley, 1996). It is well known that silver carp (Hypophthalmichthys molitrix) feeds selectively on globular colonial algae >30 µm (Kajak et al., 1977) like Microcystis, owing to their 20-25 µm gill-racker spacings (Voropaev, 1968). Microcystis comprised about 65-80% of the gut contents in 20 g fingerlings of silver carp in Dong Hu Lake, China (Iwata et al. 1986). Introduction of silver carp and bighead (Aristichthys nobilis) into a temperate eutrophic lake at high densities caused drastic reduction of Microcystis population, but caused increase in the abundance of small green algae <10 µm (Miura, 1990).
| GRAZING IN SITU |




|
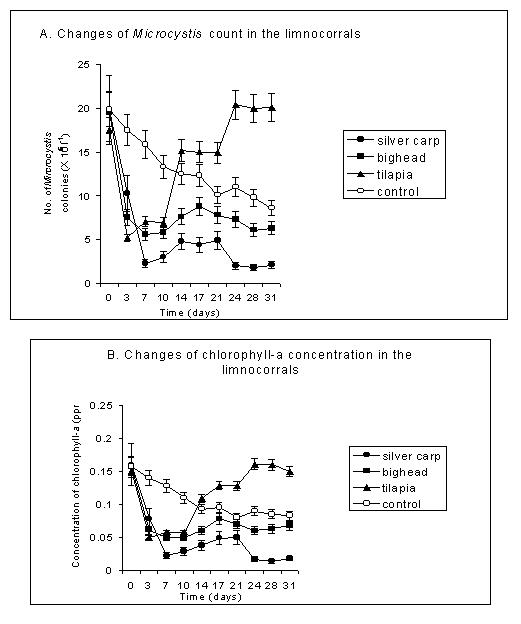
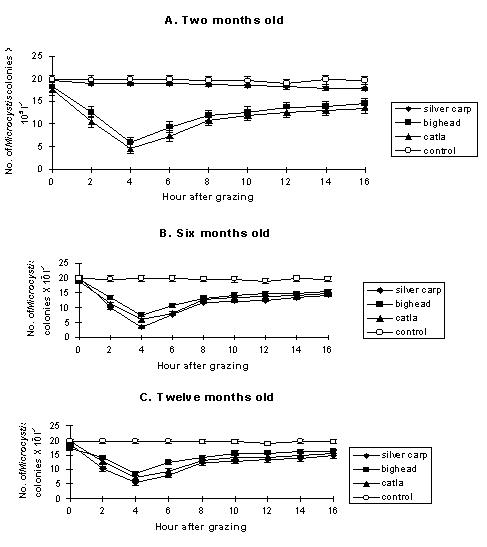
Introduction of fish, regardless of species, reduced the Microcystis population by 60-93% of the initial counts of lake water (Datta-Saha and Jana, 1998). The decline was maximal on day 3 or 7, but the counts increased thereafter. The rate of increase was several folds higher, even exceeding the counts of the control, in the case of tilapia (Fig.1 A). The responses of chlorophyll-a content of phytoplankton followed the trend as that of Microcystis population (Fig. 1 B). The results of a tropical eutrophic lake experiment using silver carp, big head and catla showed that Microcystis formed the major food item contributing 90-96% of the total gut contents of 2, 6 and 12 months old fishes (Datta-Saha and Jana, 2000). Microcystis grazing by silver carp, however, varies with the age of fishes because the 2 month old silver carp had negative selectivity for Microcystis, whereas 6 and 12 month old silver carp consumed Microcystis as usual (Fig. 2).
| GRAZING IN LABORATORY CONDITIONS |




|
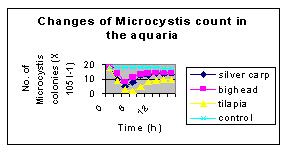
A laboratory grazing experiment quantified Microcystis colonies, consumed by silver carp, bighead and tilapia, to the tune of 10.3 to 22.1 × 105 per litre of lake water. Maximal filling of the gut occurred mostly during the fourth hour of feeding after which gut evacuation started and completed during the sixteenth hour. This implied that grazing intensity was maximum at fourth hour of their introduction followed by gradual decline (Fig. 3). Caulton (1982) reported that maximum feeding activity of tilapia was restricted to a few hours in the early morning and late afternoon. The ingestion rate of Microcystis aeruginosa by O. niloticus varied from 6.4 to 17.1 × 104 cells g-1 h-1 with fish biomass ranging from 2.3 to 22.3 g under a wide range of experimental conditions (Northcott et al., 1991; Dempster et al., 1993).
Short term changes in Microcystis grazing by silver carp, big head, catla and tilapia showed a gradual decline (50-83%) in Microcystis population until fourth hour (Datta-Saha and Jana, 1998), suggesting that maximum filling of gut in all the test fishes occurred during the fourth hour of introduction. The decline was maximal (17.3-2.0 × 103 colonies l-1) in the case of tilapia, followed by silver carp (17.5-4.6 × 105 colonies l-1) and big head (17.8-7.5 × 105 colonies l-1), implying maximal grazing intensity in the former than the other fishes.
| SELECTIVE GRAZING |




|
Feeding activity of silver carp and tilapia differ when they are exposed to toxic and non-toxic strains of Microcystis separately or in mixed populations, because of the fact that feeding of tilapia and silver carp are completely suppressed when they are exposed to only the toxic strains of M. aeruginosa (Beveridge et al., 1993). Keshavnath et al. (1994) found that the proportion of toxic strains in the mixed population of toxic and non-toxic cells of the species determine the ingestion rate of M. aeruginosa by tilapia. The food uptake in silver carp was found to be highly regulated by the quality and quantity of mucus secreted under different food conditions (Omarov and Lazareva ,1974).
| DEFECATION: |




|
Maximal gut filling at fourth hour was followed by defecation, which was much prolonged in bighead (12 h) followed by tilapia (10 h) and silver carp (6 h). Gut evacuation time was reported to be temperature dependent, being 4-10 h at 230C in silver carp and 7-13 h at 21-280C in bighead (Opuszynski and Shireman, 1995).
Microscopic examination of the fecal matter of tilapia showed the presence of a good amount (0-6.0 × 105 colonies) of intact Microcystis, which were undigested. Furthermore, the undigested cells were voided in the fecal matters in viable condition and contributed to the rise of Microcystis population of field enclosures (Fig. 1 A). DeMoor and Scot (1985) found that, as much as 25% of the algal cells ingested by O. mossambicus were undigested. Moreover, the digestibility of algal materials in the natural environment appeared to be 28.3 to 45% (Moriarty and Moriarty, 1973). This suggests that tilapia can be used for clearance of Microcystis only for a short time.
| MICROCYSTIS DIGESTIBILITY: |




|
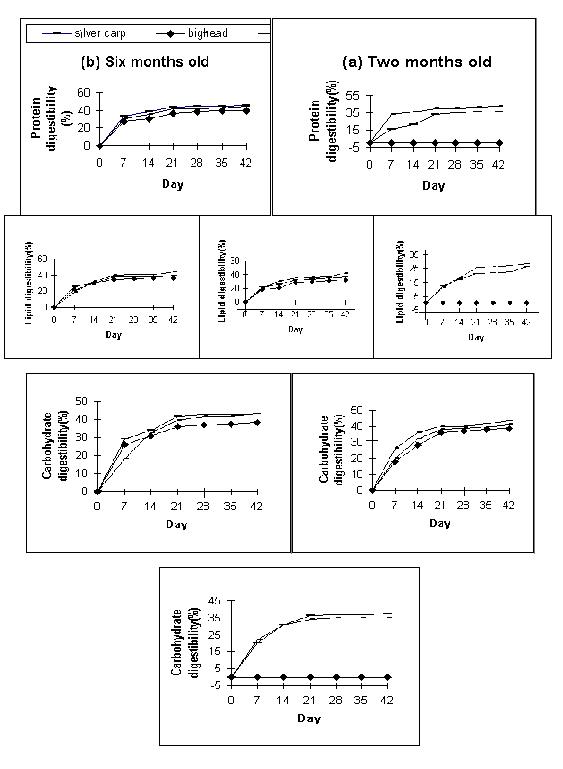
Though non-toxic strains of Microcytis is consumed well by silver carp, bighead, catla and tilapia, all the ingested cells are not digested by the fishes. Microcystis digestibility in these fishes in terms of protien, carbohydrate and lipid was 43.0-51.0%, 32.3-43.4% and 32.9-45.0% respectively (Datta-Saha and Jana, 2000). Among the three test fishes Microcystis digestibility was maximal in all age groups of silver carp except the two month old fishes (Fig.4). There are reports that silver carp of 5-10 g can digest only 60% weight of consumed algae (Ekpo and Bender, 1989).
| GROWTH EFFICIENCY: |




|
Growth studies of fish in Microcystis infested lakes revealed that the normalised biomass increase was maximum in big head (191.4 g), followed by silver carp (167.7 g) and catla (131.2 g), though the grazing intensity and Microcystis digestibility were considerably higher in silver carp compared to big head or catla (Datta-Saha and Jana, 2000). The assimilation rate of silver carp fed with M. aeruginosa was known to be 35-48% (Zhu and Deng, 1983), whereas only 1.5% of the consumed algae were utilized for its growth (Hamada et al., 1983). The cyanobacterial cells are also assimilated up to 37-49% by the juvenile big head carps (Panov et al., 1969).
The RNA: DNA ratio of the fish tissue provides information on changes in the growth rate of fish in a population (Bulow, 1970, 1987). Determination of RNA: DNA ratios of silver carp, big head and catla held in Microcystis infested lake water showed high degree of correlation (r2 = 0.90 to 0.97) with fish weight implying that fish growth was the direct function of protein synthesis.
| ICHTHYO-EUTROPHICATION: |




|
Though herbivorous fishes reduce algal population by grazing and are being used for eutrophication control, most of them may lead to ichthyo-eutrophic condition if not properly managed. Datta-Saha and Jana (1998) observed that about 6 to 180% nutrient enrichment was attributable to the defaecation and excretion of the test fishes. The potentials for enrichment among the fishes was in the order: tilapia > bighead > silver carp.
| CONCLUSION: |




|
Among the selected herbivorous fishes silver carp (of above two months age) is the most efficient grazer and is suitable for cleaning Microcystis from the lake because of its minimal ichthyo-eutrophication effect. Tilapia may be used for short term and in small ponds only, so that they may be easily removed before exerting the ichthyo-eutrophic effects. However, long-term tropical lake studies are necessary to examine the ecological consequences of these fish introductions into Microcystis bloom infested water bodies.
| REFERENCES: |




|
| Fig. 3. Short-term changes in Microcystis count due to grazing activity of silver carp, big head and tilapia under laboratory conditions. Note intensity of grazing was maximum in tilapia. |




|
| Fig. 4. Time course variability of digestibility of protein, lipid and carbohydrate of three age groups silver carp, big head and catla. Note digestibility increases with age of fish. |




|
| Address: |


|
Aquaculture and Applied Limnology Research Unit,
Department of Zoology,
University of Kalyani,
Kalyani- 741235,
West Bengal
|
|