

| Simple Methods for the Treatment of Drinking Water |
| Technologies |
1 Mechanisms of Coagulation
2 Coagulants
2.1 Chemicals
2.2 Materials of Soil Origin
2.3 Coagulants of Plant Origin
2.4 Other Natural Coagulants
3 Jar Test for Assessment of Proper Dosage of Coagulants
4 Application
4.1 Procedure for Alum and Iron Salts
4.2 Coagulation on the Household Level With Materials of Plant and Mineral Origin
Finely dispersed suspended and colloidal particles producing turbidity and color of the water cannot be removed sufficiently by the ordinary sedimentation process. Adding a coagulant and mixing and stirring the water causes the formation of settleable particles. These flocs are large enough to settle rapidly under the influence of gravity, and may be removed from suspension by filtration. It must be noted that this treatment unit process, although routinely applied in modern water treatment, requires more complex technical equipment and experienced operating personnel. The choice and dose rates of coagulants will depend on the characteristics of the water to be treated and must be determined from laboratory experiments. The chemicals must be readily available and their application must be closely monitored.
At the same time, on the household level, coagulation by means of natural coagulants of plant and soil origin and simple devices has been practiced traditionally by many peoples in developing countries.
Colloidal particles generally carry a negative electrical charge. Their diameter may range between 10-4 to 10-6 mm. They are surrounded by an electrical double layer (due to attachment of positively charged ions from the ambient solution) and thus inhibit the close approach of each other. They remain finely divided and don't agglomerate. Due to their low specific gravity, they don't settle out.
A coagulant (generally positively charged) causes compression of the double layer and thus the neutralization of the electrostatic surface potential of the particles. The resulting destabilized particles stick sufficiently together when contact is made. Rapid mixing (a few seconds) is important at this stage to obtain uniform dispersion of the chemical and to increase the opportunity for particle-to-particle contact. Subsequent gentle and prolonged (several minutes) mixing cements the still microscopic coagulated particles into larger floes. These floes then are able to aggregate with suspended polluting matter. When increased sufficiently in size and weight, the particles settle to the bottom.
It is common practice to use aluminium and iron salts (see Table 8). Both salts hydrolyse when added to water. They form insoluble material -aluminium and ferric hydroxides -when reacting with calcium and mangenese hydrogen carbonates, which are almost always present in water (alkalinity and hard-ness of the water). If those carbonates are not present in sufficient concentration (soft water) hydrated lime Ca(OH)2 or sodium carbonate Na2CO3 may be added also. In the case of aluminium sulphate, these reactions can be represented as follows:
Al2(SO4)3 + 3 Ca(HCO3)2 = 2 Al(OH)3 + 3 CaSO4 + 6 CO2
Al2(SO4)3 + 3 Ca(OH)2 = 2 Al(OH)3 + 3 CaSO4
Al2(SO4)3 + 3 Na2CO3 + 3 H2O = 2 Al(OH)3 + 3 Na2SO4 + 3 CO2
The formation of the insoluble hydroxides depends on the ph: it has been shown that aluminium sulphate coagulates best in a ph range between 4.4 and 6. At higher ph values, higher rates of soluble aluminate ions form.
Sodium aluminate is generally used at medium ph values (6.5 to 8). Irons salts have the advantage of being effective over a wide range of ph values (except for values between 7 and 8.5).
Whereas turbidity is best removed within a ph range of 5.7 to 8.0, color removal is generally obtained at acid ph's of about 4.4 to 6.0. To improve the coagulation and flocculation process and to reduce the dose of coagulants, flocculation aids may be used. The most commonly used material is activated silica. Yet diatome (kieselgur), activated carbon in powder form, bentonite and certain other types of adsorbtive clays, organic substances and cellulose derived materials are also used.
It was mentioned that mineral substances are used as flocculation aids in modern water treatment. A dose of 10 mg/l of bentonite, for instance, together with 10 mg/l of aluminium sulphate, yield significantly better results than a higher dose of aluminum sulphate alone.
In rural households in developing countries, however, various naturally occuring materials are traditionally used as coagulants (see [62,65]): e.g., fluvial clays from rivers and wadis (in Sudanese Arabic called "rauwaq", clarifier), clarifying rock material from desert regions, earth from termite hills. Their main constituents are quartz, montmorillonite, kaolinite, calcite and feldspar; their coagulating mechanisms differ greatly from those of metal salts. The processes and reactions which occur upon the addition of these various mineral coagulants to waters of different quality are not yet sufficiently known. This makes it difficult to specify optimal application procedures and conditions. Case by case examinations are required.
Application of-clay as a coagulant yields the following results:
- reduction of turbidy;
- no effect on ph value;
- an initial mineral taste, later on normal;
- no effect on bacteria count (more conclusive research is not available).
Potential health hazards:
- Clays contain traces of heavy metals (mostly chromium and manganese). High intakes of these metals may have toxic effects;
- viruses survive in the settled sludge.
2.3 Coagulants of Plant Origin
Such substances are widely used in developing countries to purify water. Usually the plants are not cultivated. Rather, according to passed on experience, certain substances are gathered, prepared and added to the water that is to be purified; seeds, leaves, pieces of bark, roots, fruit extracts and plant ashes. Some examples of traditionally used coagulants and coagulant aids as described in the literature [61-65] are:
- seeds from the Indian Nirmali tree (strychnos potatorum);
- seeds of the trees of the family of the Moringaceae: Moringa Olifeira, occurring in India, Senegal, Sudan (Behenus tree) and Moringa Stenopetala, Kenya;
- sap from the stem of the tuna cactus (opuntia ficus indica) occurring in Peru and Chile: two commercially available extracts are Tunaflex A and B,
- the bark of the south American tree Schinopsis Quebracho-Colorado? which contains tannin: it is known commercially as "Floccatan ;"
- potato starch.
For most of these plant materials, it is not known which particular substance actually triggers the coagulation. Neither is it known whether there are toxic side effects from frequent use.
To obtain the optimal dose for various substances and raw water qualities, coagulation experiments must be carried out; generally this dose is smaller than that of aluminum sulphate.
For coagulation with Moringa Olifeira seeds, the following effects can be obtained,
- significant reduction of turbidity;
- pleasant taste;
- unchanged pH value;
- initial reduction of the bacteria count, followed by a secondary rise after only 24 hours, reaching or even surpassing the initial concentration;
- antibiotic effect on various bacteria and fungi.
Nirmali seeds and Tunaflex as natural coagulants and aid substances combined with alum salts have been successfully used in municipal water treatment. It was shown that substantial savings in primary coagulants could be achieved which, in turn, reduced the overall cost considerably.
- Algae-derived substances;
- Chitosan, acting faster than any known coagulant from plant materials (produced from the shells of shrimp and lobster);
- dough from millet bread (Sudan) or curds (thin layers).
3 Jar Test for Assessment of Proper Dosage of Coagulants
Coagulation and flocculation processes are dependent on a multitude of variable interrelated factors: temperature! turbidity, color, pH-value, alkalinity, nature of coagulant and intensity and duration of stirring during mixing and flocculation. The optimal dose of the coagulant cannot be found by analyzing the raw water. Rather, it must be determined by an experiment on laboratory scale (approximation of real conditions). Such a test ought to follow this procedure:
1. Measurement of color, turbidity, pH-value and alkalinity of raw water.
2. Addition of the coagulant in different dosages to six samples of 1000 ml each (e.g. 10, 20, 30, 40, 50, 60 mg/1 of a 1% aluminum sulphate solution).
3. High speed stirring initially for 2 minutes and low speed stirring for some 20 minutes using a laboratory mixer.
4. Allow the water to settle (up to 1 hour).
5. Measurement of color and turbidity of the clarified water. Identify samples showing optimal result as regards dosage of coagulant.
A second test can be carried out for the optimal ph-value for flocculation. The same procedure is followed as before. This time however, different amounts of calcium hydroxide or sodium carbonate are added together with the optimal dose of the coagulant as found in the first test. The resulting range of pH values should extend from 4.5 to 8.5. After stirring, flocculation and sedimentation, the optimal pH-value is determined from the samples.
In this paragraph coagulation in larger scale treatment operations is described, outlining simple techniques. Also coagulation by means of natural materials at the household level is discussed.
4.1 Procedure for Alum and Iron Salts
1. In a test the required dose of the coagulant is determined. The pH-value is adjusted.
2. The coagulant solution is prepared. Usually the coagulant is introduced in a solution or suspension of known concentration (3-7%). Jars made of resistant material are to be used (see Table 8). Addition of the coagulant in solid form is also possible.

3. Constant dosing of the coagulant by means of an adequate closer. A dosing apparatus should be used (such as those for chlorine dosing) which delivers a constant yet adjustable dose rate. A simple example is exhibited in Fig. 10.
4. Immediate rapid mixing: Upon the addition of the coagulant, rapid mixing and dispersion must be provided for between I and 5 minutes. In fact, hydrolisis and polymerisation occurs almost instantly. Also, the destabilization of the colloids takes very little time. Principally, there are two practical methods:
- hydraulic mixing: channels, weirs or hydraulic jumps are used to create turbulence. At appropriate points the coagulant is introduced (Fig. 11 );
- mechanical mixing: electrically driven mixers create a uniform dispersion. This requires a reliable supply of electricity and maintenance. Also, mechanical parts are susceptible to wear. This is why the former method is preferable.
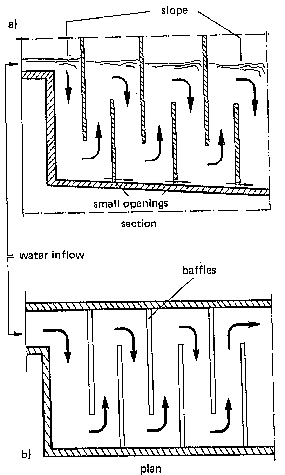
5. Flocculation: slow and even mixing allows the particles to collide and contact so as to form flocs (30-60 minutes). The efficiency of floc formations is contingent on the frequency of particle-to-particle contact. - hydraulic mixing: this can be done by routing water through a vertically or horizontally baffled flocculation basin. The resulting turbulence has a mixing effect (see Fig. 12). It must be noted that this method does not allow any adjustment or control in case of changing characteristics of the water quality.
- mechanical mixing: flocculation takes place in tanks equipped with an electrically driven stirring system. This stirring system consists of screws, paddles or blades mounted on vertically or horizontally rotating shafts.
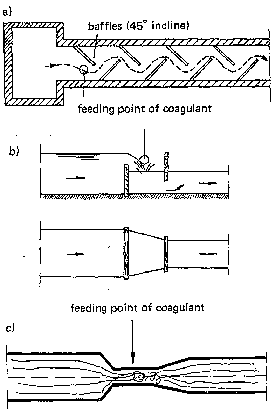
Fig. 11: Hydraulic mixing in water flow. a) channel with baffles, b) overflow weir, c) hydraulic jump.
6. In the sedimentation tank, the particles are allowed to settle. Or, alternatively, they are removed by filtration.
In order to obtain optimal coagulation and flocculation performances, a number of design considerations must be followed: after finding the required dose of coagulant through experimentation, the flocculation and mixing chamber must be hydraulically designed. Approximate speed and duration of mixing, flow velocity, hydraulic profile and detention time of the particles in the tank must be determined. This procedure, however, is more suited to the design of larger scale plants. Smaller plants usually operate without these more sophisticated engineering solutions, e.g.:
- the introduction of the coagulant may be made in the feeder pipe preceding a rapid filter;
- the addition of the coagulant may be made at the point of the inlet weir to the sedimenation basin.
4.2 Coagulation on the Household Level With Materials of Plant and Mineral Origin
The following are standard recipes for coagulation with locally available materials which may be modified according to the specific conditions.
The doses for the coagulant are best determined by experiments. Locally used jars, e.g., clay vessels, may be used for the purification process For mixing, wooden twirling sticks would be appropriate.
After the floes are settled, the supernatant water is to be transferred carefully into a clean jar. To avoid secondary pollution by unhygienic contact with a jug, the water could be scopped out with a ladle or syphoned off into a nearby vessel. The purified water should be consumed within a few hours, so as to avoid renewed contamination due to temperature-induced growth of bacteria. Even better, is to boil the water prior to consumption or to disinfect it by some other method.
The settled mud on the bottom of tile jar is to be collected carefully. It should be exposed to the sun for some time (several days) to assure that potentially existing pathogens be destroyed completely.
Coagulation with fluvial clay
Dose of coagulant: 3.5 g/l (rauswaq). For a 40 l capacity jar, this translates into 140 g (1 teaspoon of the pulverized clay corresponds to 2.5 -3 g).
1. Dried clay is pounded to powder and added to water (possibly clarified) in a small bowl.
2. The suspension is added to the turbid water.
3. Very slow stirring of the water for about 5 min.
4. Jar is covered and the water left to settle.
Coagulation with seeds of Moringa Olifeira
Dose: 150 -200 mg/l. For a jar of 40 l capacity this translates into 30 seeds.
1. After removing the seed husks, the white kernel material is crushed in a clean mortar or a stone covered with a piece of clean cloth. The powder must be prepared fresh before every use. Humidity causes deterioration.
2. The power is then dissoleved in a small amount of clarified water and a suspension is prepared.
3. The suspension is added to the raw water under short and rapid mixing (coagulating).
4. Gentle and slow stirring follows (for flocculation, 10 to 15 min).
5. Finally, the water is left covered in the jar to allow the floes to settle.

