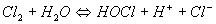


| Simple Methods for the Treatment of Drinking Water |
| Technologies |
1 Chlorination
1.1 The Action of Chloride and its Range of Application
1.2 Chemicals
1.3 Determination of Chlorine Dose
1.4 Practical Application
2 Iodine
3 Ozonation
4 Potassium Permanganate
5 Disinfection by Silver
6 Boiling
7 Ultra-violate Radiation
It is essential that drinking water be free of pathogenic organisms. Storage, sedimentation, coagulation, flocculation and filtration of water both individually and jointly reduce the contents of bacteria in water to a certain extent. None of these methods can guarantee the complete removal of germs. Disinfection is needed at the end. Water with low turbidity may even be disinfected without any additional treatment for bacteria removal.
Groundwater abstracted from deep wells is usually free of bacteria. Surface water and water obtained from shallow wells and open dug wells generally need to be disinfected.
Water disinfection processes are designed to destroy diseaseproducing organisms by means of disinfectants. The degree or efficiency of disinfection depends on the method employed and on the following factors influencing the process:
- kind and concentration of microorganisms in the water,
- other constituents of the water which may impede disinfection or render it impossible,
- contact time provided (important for chemical disinfectants, since their effect is not instantaneous, a time of contact is necessary),
- temperature of the water (higher temperatures speed up chemical reactions).
Water disinfection can be accomplished by several means:
- physical treatment: removal of bacteria through slow sand filtration, straining of macroorganisms by means of microscreening , application of heat (boiling), storage, etc.
- irradiation, such as UV-light,
- metal ions, such as silver (and copper),
- chemical treatment, use of oxidants (halogens and halogen compounds -chlorine, iodine, bromine -, ozone, potassium permanganate, hydrogen peroxide, etc.).
A good chemical disinfectant should have the following abilities:
- destroy all organisms present in the waterwithin reasonable contact time, the range of water temperature encountered, and the fluctuation in composition, concentration and condition of the water to be treated;
- accomplish disinfection without rendering the water toxic or carcinogenic;
- permit simple and quick measurement of strength and concentration in the water,
- persist in residual concentration as a safeguard against recontamination;
- allow safe and simple handling, application and monitoring;
- ready and dependable availability at reasonable cost.
Just as important as the proper choice of the disinfectant, applying the foregoing criteria, is that of the type of device to be used to add the agent to the water in a safe and controllable fashion.
It cannot be emphasized strongly enough that there are potential hazards for the human organism associated with prolonged ingestion of chemicals. Nevertheless, the application of chlorine and its compounds for the purpose of water disinfection is the best and most tested compromise when evaluated according to the aforementioned criteria. It is therefore discussed here in detail. The other methods differ significantly from each other in terms of their effect, the technological level and particularly in their applicability. They are introduced only briefly.
Chlorination is the most widely used method for drinking water disinfection. It is effective and economical. Its use requires some knowledge about the complex processes that take place during chlorination. Those processes will be briefly summarized in the following paragraphs.
1.1 The Action of Chloride and its Range of Application
Chlorination is known as the addition of chlorine gas or some other oxidizing chlorine compound (sodium or calcium hypochlorite, chlorinated lime, chlorine dioxide) to the water to be treated. The actual agent is hypochlorous acid (HOCl) which forms when chlorine is added to water:
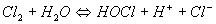
Hypochlorous acid also forms subsequent to dissociation, when chlorinated lime or hypochlorites are added:
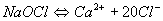
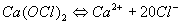
The following chemical equilibrium
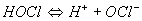
depends on pH and temperature. At pH levels between 3 and 6, hypochlorous acid dissociates poorly. Chlorination is most effective in that range of pH. At pH levels greater than 8, hypochlorite ions predominate or exist almost exclusively. Hence the disinfecting effect drops off rapidly as the pH level increases.
Simultaneously with the dissociation, hypochlorous acid partly breaks up, forming monatomic oxygen, which contributes to the oxidizing effect:
HOCl ® HCl + 0
The fraction that becomes effective as an oxidizing agent when chlorine or some of its compounds is added to raw water is called "free available" or "active" chlorine.
Small amounts of chlorine, due to its ability to penetrate cells of microorganisms, are sufficient to destroy many different strains of bacteria. Similarly, many types of viruses and macro-organisms such as schistosoma larvae can be killed. A contact time of at least 30 minutes is required, at the end of which the residual chlorine concentration in the water must still be between 0.1 and 0.5 mg/l (= ppm). Amoebic cysts and spores with resistant cell membranes require higher doses and longer contact times.
Chlorine also reacts with many other oxidizable water constituents such as iron and manganese compounds, ammonia, and compounds thereof (forming chloramines), as well as numerous types of organic particles. The presence of these substances reduces the germicidal effect considerably. Sufficient chlorine must be added to the water to make sure that there is a residual concentration to prevent recontamination.
It is advisable to remove or reduce prior to chlorination, those substances by means of sedimentation and/or filtration which would impede disinfection. Through such pretreatment, helminth eggs (parasitic worms) can be removed which are insensitive to chlorination.
In recent times, it was found that through chlorination, certain undesirable side effects may occur. Particularly in industrialized areas, synthetic organic compounds may enter the hydrologic cycle in high concentrations. The presence of chlorine enhances the danger of the formation of carcinogenic compounds (e.g., chloroform and other trihalomethanes).
Chlorine gas and chlorine dioxide are widely used in water treatment on account of their high efficiency and ease of application. Handling and transport, however, are considered too demanding and hazardous for the purposes described in this manual (explosive, toxic).
Several chlorine compounds which have various active chlorine contents (cf. Table 13) are more easily applicable. In some form or another they are available virtually anywhere.
Table 13: Strength of Various Chlorine Preparations
| Name |
% Active Chlorine | Amount for Preparation of 1 l of 1% Solution |
| Sodium Hypochlorite | 14 (10-15) | 71 g |
Household Bleach | 5 (3-5) | 200 g |
| Javelle Water | ca. 1 | 1000 g |
| Chlorinated Lime | 30 (25-37) | 40 g |
| HTH |
70 (60-70) |
15 g |
Sodium hypochlorite (NaOCl), commonly known as bleach or Javelle water:
This is generally available in dissolved form. Its commercial strength in terms of active chlorine is between 1 and 15%. It is stored in dark glass or plastic bottles. The solution loses some of its strength during storage. Prior to use, the active chlorine content should be tested. Sunlight and high temperatures accelerate the deterioration of the solution. The containers therefore should be stored in cool darkened areas. The stability of the solution decreases with increasing contents of available chlorine. A 1% solution is relatively stable. But it is not economical to store. Even though hypochlorite solutions are less hazardous than chlorine gas, every precaution should be taken to avoid skin contact and to protect containers against physical damage.
Chlorinated Lime or Bleaching Powder (CaO · 3 CaOCl2 · 3 H2O)
In general, the powder is readily available and inexpensive. It is stored in corrosion resistant cans. When fresh, it contains 35% active chlorine. Exposed to air, it quickly loses its effectiveness. It is usually applied in solution form which is prepared by adding the powder to a small amount of water to form a soft cream. Stirring prevents lumping when more water is added. When the desired volume of the solution has been prepared, it is allowed to settle before decanting. Solutions should have concentrations between 5 and 1% of free chlorine, the latter being the most stable solution. Some 10% of the chlorine remains in the settled sludge. The same precautions for the NaOClNaomi solution pertain also to the storage of dissolved chlorinated lime.
High Test Hypochlorite (HTH) is a stabilized version of calcium hypochlorite (Ca(OCI)2) containing between 60% and 70% available chlorine. Under normal storage conditions, commercial preparations will maintain their initial strength with little loss. Even though HTH is expensive, it may be economical, thanks to its properties. It is available in tablet or granular form (commercial names: Stabo-Chlor, Caporit or Para-Caporit).
These chemicals must be handled with great caution. They are caustic, corrosive and sensitive to light. They should be stored in tightly closed containers and in darkened spaces, accessible only to authorized personnel. When handling the material, contacts with skin, eyes and other body tissues must be avoided. Chlorine corrodes metal and to a less extent, wood and some synthetic materials. Metal parts which come in contact with the chemicals should be resistant.
1.3 Determination of Chlorine Dose
Chlorine of any type must be added to water in closely controlled concentrations which depend on the characteristics of the water. As the use of dry chemicals doesn't always permit sufficient accuracy of dosing, solutions are preferred. Chlorine is usually added to the water for disinfection at the end of the treatment process. This allows the most effective treatment at the lowest level of chlorine application. Measurements of the chlorine demand and residual chlorine must be taken to assure that sufficient free chlorine is available to accomplish disinfection.
Water characteristics and, hence, the chlorine demand may vary due to external influences (e.g., rainy season, etc.). It is therefore necessary to monitor the water quality from time to time, at the points of consumption in cases where the chlorine dosage is fixed. The objective of disinfection via chlorination can only be obtained if the chlorine dosage is adjusted to the changed water characteristics.
In the field, the chlorine demand of water of a given quality can be determined as follows: One lifer samples of the water are taken. Chlorine solution of a known concentration is added and mixed with the water. After 30 minutes of contact time, the residual chlorine content is measured. The difference to the amount added then yields the chlorine consumption.
Chlorine demand = chlorine consumption + desired residual
Usually 1% chlorine solutions are applied. The chlorine flow is set such that a chlorine residual level of between 0.1 and 0.3 mg/l is obtained. Higher levels are recommended if rapid recontamination is likely.
Colorimetric tests are employed to determine total chlorine residuals. Chemical agents (DPD or OT method) are used which are oxidized by chlorine to produce a colored complex, the intensity of which is proportional to the amount of chlorine present. Reading the colors and matching color standards by means of a comparator and disks, gives the amount of free, available, and residual chlorine. Various simple test kits are commercially available, using permanent glass and containing DPD reagents in liquid or compressed tablet form.
Calculation of the required amount of chlorine: Given the amount or flow of water to be chlorinated, the chlorine demand and the strength of the chlorine solution to be used, the necessary amount of solution can be calculated as follows:
chlorine demand (g/m³) x amount of water to be treated (m³/h) = required amount of active chlorine per hour (g/h); required amount of chlorine solution per hour (l/h) = required active chlorine per hour (g/h) divided by active chlorine per liter of solution (g/a)
It must be noted that the manufacturers usually express the available chlorine con. tent in terms of percent weight (g/100 g). In the field, however, it is often expressed in terms of percent volume (g/100 ml of solution). Since the density of chlorine solutions is higher than that of water, the percent weight measure for a given solution is lower than the percent volume measure.
Aside from using commercially available chlorine feeder instruments, it is quite possible to make a simple dosing apparatus for a constant feed rate. The most difficult part is the setting of the proper rate of delivery. Reliable operation and regular maintenance must be provided. Sufficient contact time for the chlorine must be ensured.
Chlorination should never be performed prior to slow sand filtration (residual chlorine destroys biological agents). Sedimentation and filtration preceding chlorination enhance the disinfection effect. The lower the turbidity, the smaller the amount of chlorine necessary for effective disinfection.
The chlorine solution can either be added to a batch of water (non-continuous or diffusion chlorination) or alternatively, it can be fed continuously to a constant flow of water.
Batch Chlorination
Where tanks are used for storageof drinking water, the required amount of chlorine can be added to the tank periodically. It is advantageous to alternate between two tanks (see Fig. 30). While one tank is in use, the other one is refilled and treated with chlorine. The water can be used after a minimum of 30 minutes contact time. This procedure allows uninterrupted supply.
The amount of chlorine required for a given size tank can be calculated according to the foregoing formula. Using a 1% hypochlorite solution, the dose is:
chlorine demand x tank capacity.
If the water quality of a given source varies, the chlorine demand must be reevaluated from time to time. Before a tank is used the first time for storing water, it must be cleaned carefully and disinfected (application of between 50 and 100 ppm active chlorine). Once the water has been disinfected, recontamination must be carefully prevented. Tanks should be covered. A tap should be used to release the water so as to avoid scooping out the water with unclean jars and the like. If the water is not used immediately but left in the house for awhile, only well cleaned and covered jars should be used.
Chlorine Tablets: In certain situations, e.g., while travelling, chlorine tablets can be used. They are available from various firms. They are used for periodic chlorination of small batches of water.
Diffusion Chlorination
Open wells are often bacteriologically contaminated because of non hygienic methods for lifting the water, or due to careless use of the surroundings of the well.
CPHERI and NEERI (India) respectively, experimented with simple devices that would allow providing water in a well or in a tank with a sufficient amount of chlorine over a certain period of time (see Fig. 31 and 32).
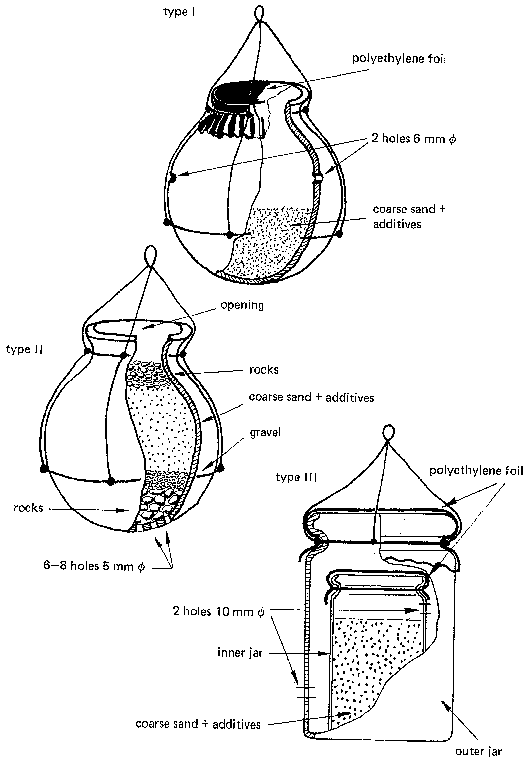
Type I is a clay jar (12 to 15 l volume), filled nearly half-way with a mix of 1.5 kg bleach powder and 3 kg coarse sand (grain size 1.4 to 1.6 mm). It has two holes above the sand surface. The jar is covered with a plastic foil. The jar is suspended approximately 1 m below the water surface in the well. The chlorine can thus diffuse through the two holes into the well water.
Range of application: Wells of 9 to 13 m³ volume of water, daily removal some 10% (0.9 to1.3 m³);
Effectiveness: 1 week at a residual chlorine content of between 0.2 and 0.8 mg/a.
Type II also consists of a clay jar (volume 7 to 10 a). It has 6 to 8 holes in the bottom. These are covered with stones on top of which a layer of gravel is placed. On top of that is put a mix of 1.5 kg bleaching powder and 3 kg of coarse sand. Stones are filled to the rim of the jar, which is then lowered into the water.
Range of application: same as before Effectiveness: Two weeks at a residual chlorine content of between 0.2 and 1.0 mg/a.
For larger wells and higher rates of water use, two jars should be used which are refilled interchangeably.
Type III: For small household wells,a double jar is recommended which releases less chlorine per time unit. The inner jar contains a mix of 1 kg bleaching powder and 2 kg coarse sand. The diffusion openings are provided as shown in Fig. 32.
Range of applications: Wells with 4.5 m³ volume of water and daily removal of between 360 to 450 l.
Effectiveness: Two to three weeks at a residual chlorine content of between 0.15 and 0.5 mg/l.
As these devices are not fit for large variations in water use, insufficient chlorination may occur at higher rates of water use.
Continuous Chlorination
Simple chlorine dosing instruments can be installed in piped water supply systems. Chlorine is fed to the water in proportion to the flow rate.
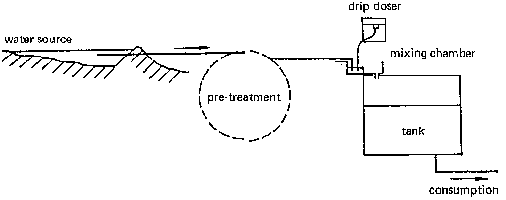
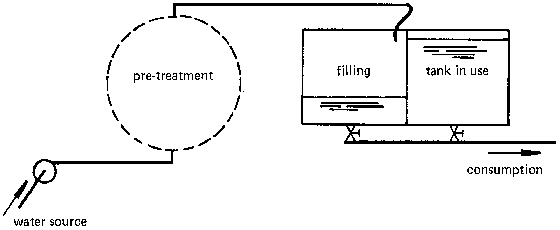
Fig. 33 shows a water supply scheme including continuous chlorination. A pipeline transmits the water from the source to the reservoir, passing through some sort of pretreatment (e.g., coagulation/flocculation and settling). Before entering the reservoir, the water is passed through a mixing chamber where a dosing apparatus introduces droplets of chlorine into the water.
In the following paragraphs, some examples of drop dosers are discussed:
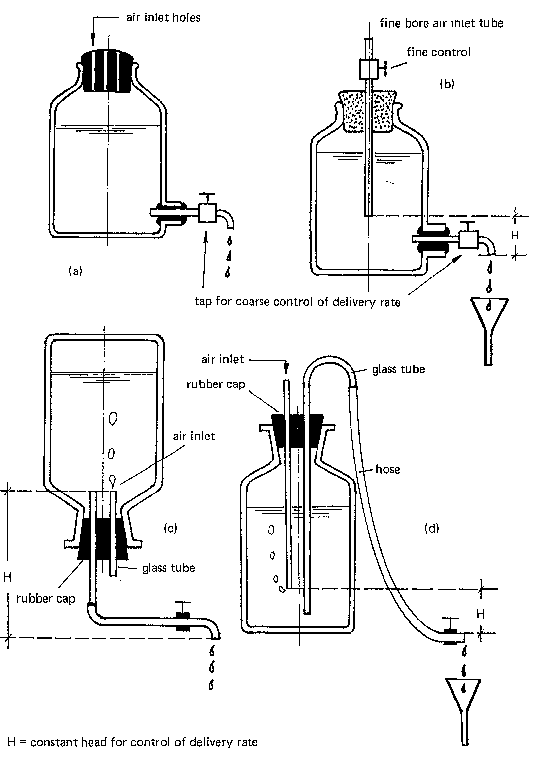
- Glass or plastic bottles. Through a tap near the bottom, the chlorine is released into the water. The tap also serves as a coarse control of the delivery rate (Fig. 34a, b). A constant head H provides fine control of the delivery rate. This head H is measured either between the faucet and the fine bore air inlet tube (b) or between the two tubes which pass through the rubber cap (c, d).
- 200 a metal drum. The drum (Fig. 35), painted inside with bituminous paint to protect the metal from corrosion, holds the hypochlorite solution. A floating bowl (plastic, glass or ceramics - two versions are shown in Fig. 35) which is anchored and stabilized in the tank, controls the delivery rate. The solution enters the bowl via a small bore glass inlet tube. From there, it leaves the bowl through a wide bore delivery tube. The flow rate is controlled by the head difference H (between the upperend of the glass tube and the level of the liquid in the tank) and the diameter of the fine bore inlet tube. The flow rate is given by the following expression (based on Bernoulli's equation):
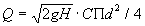
where Q = flow rate, g = gravitational acceleration, H = head difference, C = empirical discharge coefficient, approx. 0.6, d = diameter of small bore inlet tube.
From the above expression, it can be seen that the delivery rate is proportional to the second power of the tube diameter and to the square root of the head difference. That is to say, the smaller H or d, the smaller is the flow rate. Hence, the flow rate is independent of the level of the solution in the tank. As the level drops, so does the floating bowl. To stop the delivery completely, the bowl must be lifted at least a distance H, so as to stop the gravitational driving force of the closer. The outlet (wide bore tube) must not be closed or else the bowl will gradually fill up and sink to the bottom, possibly suffering damage.
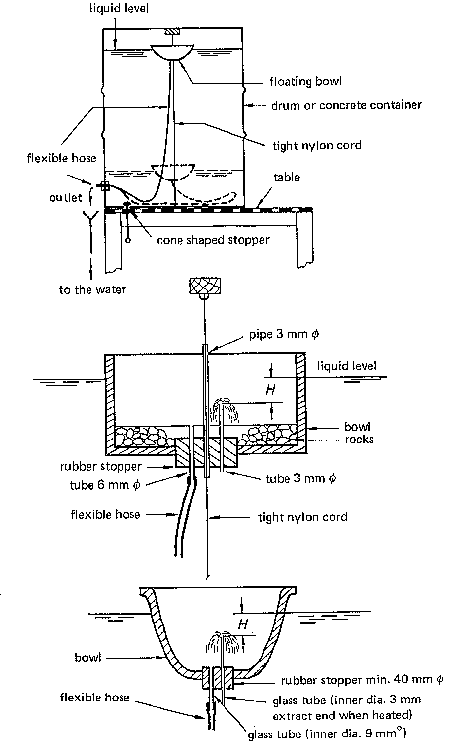
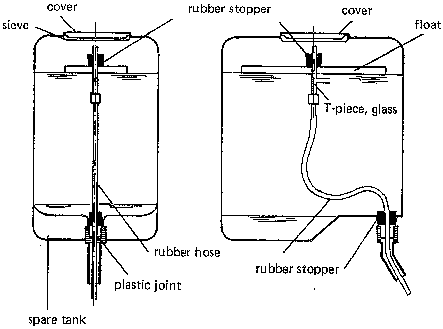
- Similar instruments of different sizes are shown in Fig. 36. The solution enters a glass or copper pipe through an inlet hole somewhat below the surface. The pipe is connected to a rubber hose which runs to the outlet. A float again provides a constant head difference between the liquid level and the inlet hold. The delivery rate is controlled by the size of the hole and its distance below the surface.
A variety of types of chlorine dosing instruments are commercially available. They range from manually controlled types to fully automated ones. Usually a unit consists of a storage tank and a diaphragm pump for feeding the hypochlorite solution. The feed rate is proportional to the water flow rate and, thus depends on the consumption. The use of these devices is limited to piped water supply systems. Installation and setting up should be carried out by professional personnel.
Iodine is an excellent disinfectant, effective against bacteria, amoeba cysts, cercerea and some viruses. It is added to the water mostly in the form of an aqueous solution. WHO recommends the application of 2 droplets per lifer of water of a 2% iodine tincture [56]. Iodine preparations are also available in tablet form.
In comparison to chlorination, the use of iodine has the following advantages:
- effectiveness over a wider range of pH values (up to pH 10), except at very low temperatures;
- amonia and organic nitrogenous compounds have little effect on germicidal efficiency because they do not form substitution compounds with iodine;
- action depends less on contact time and temperature;
- effectiveness against more pathogenic organisms within short times;
- use and handling is simpler.
Since operating costs are too high, the use of iodine is not expected to ever become an important widely applied disinfectant. The applicability is limited due to the following disadvantages:
- higher concentrations than chlorine (on a ppm basis) are necessary for effective action; -muddy or turbid water substantially affect germicidal action;
- iodine is about 20 times as expensive as chlorine per unit of germicidal effectiveness;
- taste and slight color produced by the iodine affect palatability and aesthetic quality;
- physiological effect of prolonged use of iodine (especially in children) is suspected.
Allergies were ascertained.
In view of these economic and health implications the use of iodine for disinfection is recommended only for occasional application (e.g., in case of catastrophe or while travelling). Aside from that, iodine is a highly effective and technically widely applicable disinfectant.
Ozone (O3) is one of the most effective disinfectants. As a powerful oxidant, it reduces the contents of iron, manganese, and lead, and eliminates most of the objectionable taste and odor present in water. Its effectiveness does not depend on the pH value, temperature or ammonia content of the water. Since ozone is relatively unstable, it is generated almost invariably at the point of use. Ozone is obtained by passing a current of dried and filtered air (or oxygen) through between two electrodes (plates or tubes) subjected to an alternating current potential difference. A portion of the oxygen is then converted into ozone.
This principle of ozone production has been used in Europe for a long time, since it has the advantage of being applicable under a wide range of conditions. It leaves no chemical residuals behind in the treated water. On the other hand, no lasting protection against recontamination is provided either. Capital costs for the instrumentation of ozone production and feeding, as well as operating costs due to the electrical energy requirements, are very high. Moreover, operation of ozonizers requires continuous and skilled monitoring. The operational requirements therefore exceed the resources available in rural areas of most developing countries.
Potassium permanganate (KMnO4), a powerful oxidant, is rarely applied in water treatment for the purpose of disinfection. It is sufficiently. effective against cholera bacteria, but not against other pathogenic germs. A dose of 1 to 5 ppm KMnO4 is recommended for application. It must be noted, though, that it creates a purplebrown precipitate which coats the walls of the tank. It cannot be removed easily.
In recent years, potassium permanganate has gained steadily in the application in pretreatment since it has proved effective at:
- removing objectionable odor and taste by means of oxidation of organic material, hydrogen sulfide;
- preventing algal growth;
- removing iron and manganese compounds by means of oxidation and subsequent separation by filtration.
Preparations containing silver may be used to reduce the germ count of water. The products are commercially available, either as a liquid or a powder. They are readily soluble in water and can be dosed easily.
The effectiveness of silver can be explained by the oligo-dynamic properties of silver ions (silver nitrate or salt compounds). Even minute concentrations (0.03 to 0.04 ppm) are notably effective. The silver ions curb the growth of germs. After contact of between 30 minutes and 6 hours, depending on the level of bacteriological contamination, water of a very low germ content may be obtained. Odor and taste of the water are not affected by the application of silver. Disinfection by silver is a simple and very effective method. Its major advantage is that it provides already treated water with long-lasting protection against recontamination by germs.
The effect of silver and other metals has been known to many peoples for a long time. The tradition of storing drinking water in silver vessels is still maintained in wealthier Hindu families. Although there is a tendency at present to exchange the metallic containers for plastic ones, even simple Indian villagers can still be seen fetching water from the well in brass or copper vessels. The metallic vessels are believed to have antiseptic qualities.
Silver preparations are also used in ceramic filters . Major disadvantages of silver for the purpose of disinfection are the costs of treatment (about 200 times higher than gaseous chlorine), relatively long contact periods are required, organic substances and iron, sulphur, etc., inhibit action, thus limiting the applicability of the technique. If combined with chlorine, silver preparations are more widely applicable (direct disinfection and protection against recontamination).
Boiling water is a very effective though energy-consuming method to destroy pathogenic germs: bacteria, viruses, spores, cercerea and amoeba cysts, worm eggs, etc.
The presence of turbidity or other impurities has little effect on germicidal effectiveness. If boiling is the only type of treatment available, it is recommended to let the water settle before, and decant it or filter it through a fine-meshed cloth so as to remove coarse impurities and suspended particles. The water is then brought to a strong boil which is maintained for at least five, preferably twenty minutes. For storing, it must not be transferred to a different vessel, but left in the former one and covered, so as to protect it from recontamination.
Boiling, together with the associated release of gases, especially CO2, alters the taste of water. But through stirring while boiling and by letting the water sit in the partially filled vessel for a few hours afterward, the water picks up air and loses its bland taste. To improve the taste of the water, flavoring plant materials may be added during boiling.
If done properly, boiling is a very effective and simple disinfection method. Since it requires a significant amount of energy, this method is only recommended in exceptional castes. If it is not possible for any reason to apply a different method, the most energy-efficient way of boiling should be employed.
The germicidal effect of UV rays had been known long before the first experiments were carried out to harness it for water disinfection. In principle, the effect of sunlight on surface water is imitated in a more intense and controllable way. The most commonly used source of UV-radiation is a low pressure quartz mercury vapor lamp which emits invisible light at a wavelength in the range between 200 and 300 nm with part of the energy in the spectral region of 2537 A.
The germicidal effect depends on the electric power of the lamp and on the time of exposure of the water to the radiation. It decreases with increasing distance between water and lamp. Also, many substances present even in pretreated water (e.g., small amounts of dissolved iron) absorb UV light. Other constituents (turbidity, suspended matter) inhibit or prevent the transmission of radiation. A disinfection unit is built such that the water is made to flow through a pipe in a thin film around the lamp, which is located atthe pipe's center, emitting radiation. The flow rate is adjusted as required. The water must be pre-filtered.
Disinfection by UV radiation is a "clean" process, since no chemical additives are used. Residual matter doesn't occur, and tastes and odors are neither produced in the water nor altered. Automatic devices are available which indicate when the lamp's output is not sufficient.
Due to some severe disadvantages of this type of treatment, it is not expected to find any consideration for application in the areas targeted by this manual:
- commercially available devices are relatively expensive,
- there is a dependence on steady power supply ?
- the lamp's powers of penetration are limited; thin water films are necessary,
- turbidity, and impurities reduce the effectiveness notably,
- the lamps gradually lose their radiation power, accelerated by a coating of dirt. The lamp's average life is 1000 to 5000 hours,
- disinfection occurs rather quickly and effectively (up to 99.9%), though no protection for recontamination is provided.

