| Research Journal Of Chemistry And Environment | Biosorption of Heavy Metals | Vol.7(4) Dec(2003) Res.J.Chem.Environ |
N. Ahalya, T.V. Ramachandra* l and R.D. Kanamadi 2
1. Centre for Ecological Sciences, Indian Institute of Science, Bangalore 560 012, INDIA.
2. Department of Zoology, Karnataka University, Dharwad, INDIA.
CONTENTS |
||
The discharge of heavy metals into aquatic ecosystems has become a matter of concern in India over the last few decades. These pollutants are introduced into the aquatic systems significantly as a result of various industrial operations. Industrialization in India gained a momentum with initiation of five year developmental plan in the early 50's. The pollutants of concern include lead, chromium, mercury, uranium, selenium, zinc, arsenic, cadmium, gold, silver, copper and nickel. These toxic materials may be derived from mining operations, refining ores, sludge disposal, fly ash from incinerators, the processing of radioactive materials, metal plating, or the manufacture of electrical equipment, paints, alloys, batteries, pesticides or preservatives. Heavy metals such as zinc, lead and chromium have a number of applications in basic engineering works, paper and pulp industries, leather tanning, organochemicals, petrochemicals fertlisers, etc. Major lead pollution is through automobiles and battery manufacturers. For zinc and chromium the major application is in fertliser and leather tanning respectively (Trivedi, 1989). Over the few decades, several methods have been devised for the treatment and removal of heavy metals.
The commonly used procedures for removing metal ions from aqueous streams include chemical precipitation, lime coagulation, ion exchange, reverse osmosis and solvent extraction (Rich and Cherry, 1987). The process description of each method is presented below.
Reverse Osmosis: It is a process in which heavy metals are separated by a semi-permeable membrane at a pressure greater than osmotic pressure caused by the dissolved solids in wastewater. The disadvantage of this method is that it is expensive.
Electrodialysis: In this process, the ionic components (heavy metals) are separated through the use of semi-permeable ionselective membranes. Application of an electrical potential between the two electrodes causes a migration of cations and anions towards respective electrodes. Because of the alternate spacing of cation and anion permeable membranes, cells of concentrated and dilute salts are formed. The disadvantage is the formation of metal hydroxides, which clog the membrane.
Ultrafiltration: They are pressure driven membrane operations that use porous membranes for the removal of heavy metals. The main disadvantage of this process is the generation of sludge.
Ion-exchange: In this process, metal ions from dilute solutions are exchanged with ions held by electrostatic forces on the exchange resin. The disadvantages include: high cost and partial removal of certain ions.
Chemical Precipitation: Precipitation of metals is achieved by the addition of coagulants such as alum, lime, iron salts and other organic polymers. The large amount of sludge containing toxic compounds produced during the process is the main disadvantage.
Phytoremediation: Phytoremediation is the use of certain plants to clean up soil, sediment, and water contaminated with metals. The disadvantages include that it takes a long time for removal of metals and the regeneration of the plant for further biosorption is difficult.
Hence the disadvantages like incomplete metal removal, high reagent and energy requirements, generation of toxic sludge or other waste products that require careful disposal has made it imperative for a cost-effective treatment method that is capable of removing heavy metals from aqueous effluents.
| BIOSORPTION |
The search for new technologies involving the removal of toxic metals from wastewaters has directed attention to biosorption, based on metal binding capacities of various biological materials. Biosorption can be defined as the ability of biological materials to accumulate heavy metals from wastewater through metabolically mediated or physico-chemical pathways of uptake (Fourest and Roux, 1992). Algae, bacteria and fungi and yeasts have proved to be potential metal biosorbents (Volesky, 1986). The major advantages of biosorption over conventional treatment methods include (Kratochvil and Volesky, 1998 a):
• Low cost;
• High efficiency;
• Minimisation of chemical and lor biological sludge;
• No additional nutrient requirement;
• Regeneration of biosorbent; and
• Possibility of metal recovery.
The biosorption process involves a solid phase (sorbent or biosorbent; biological material) and a liquid phase (solvent, normally water) containing a dissolved species to be sorbed (sorbate, metal ions). Due to higher affinity of the sorbent for the sorbate species, the latter is attracted and bound there by different mechanisms. The process continues till equilibrium is established between the amount of solid-bound sorbate species and its portion remaining in the solution. The degree of sorbent affinity for the sorbate determines its distribution between the solid and liquid phases.
Biosorbent material: Strong biosorbent behaviour of certain micro-organisms towards metallic ions is a function of the chemical make-up of the microbial cells. This type of biosorbent consists of dead and metabolically inactive cells.
Some types of biosorbents would be broad range, binding and collecting the majority of heavy metals with no specific activity, while others are specific for certain metals. Some laboratories have used easily available biomass whereas others have isolated specific strains of microorganisms and some have also processed the existing raw biomass to a certain degree to improve their biosorption properties;
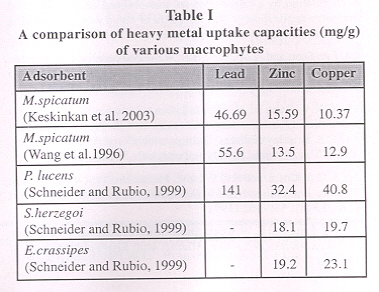
Recent biosorption experiments have focused attention on waste materials, which are by-products or the waste materials from large-scale industrial operations. For e.g. the waste mycelia available from fermentation processes, olive mill solid residues (Pagnanelli, et al 2002), activated sludge from sewage treatment plants (Hammaini et aI. 2003), biosolids (Norton et al 2003), aquatic macrophytes (Keskinkan et aI. 2003), etc.
Norton et aI. 2003, used dewatered waste activated sludge from a sewage treatment plant for the biosorption of zinc from aqueous solutions. The adsorption capacity was determined to be 0.564 mM/g of biosolids. The use of biosolids for zinc adsorption was favourable compared to the bioadsorption rate of 0.299 mM/g by the seaweed Durvillea potatorum (Aderhold et aI. 1996). Keskinkan et al. 2003 studied the adsorption characteristics of copper, zinc and lead on submerged aquatic plant Myriophyllum spicatum. The adsorption capacities were 46.69 mg/g for lead, 15.59 mg/g for zinc and 10.37 mg/g for copper. Table 1 gives a comparison of heavy metal uptakes of various macrophytes.
Pagnanelli, et al 2002 have carried out a preliminary study on the 'Use of oli ve mill residues as hea vy metal sorbent material The results revealed that copper was maximally adsorbed in the range of 5.0 to 13.5 mg/g under different operating conditions.
The simultaneous biosorption capacity of copper, cadmium and zinc on dried activated sludge (Hammaini et al. 2003) were 0.32 mmoI/g for metal system such as CuCd; 0.29 mmoI/g for Cu-Zn and 0.32 mmoI/g for Cd-Zn. The results showed that the biomass had a net preference for copper followed by cadmium and zinc.
Another inexpensive source of biomass where it is available in copious quantities is in oceans as seaweeds, representing many different types of marine macro-algae. However most of the contributions studying the uptake of toxic metals by live marine and to a lesser extent freshwater algae focused on the toxicological aspects, metal accumulation, and pollution indicators by live, metabolically active biomass. Focus on the technological aspects of metal removal by algal biomass has been rare.
Although abundant natural materials of cellulosic nature have been suggested as biosorbents, very less work has been actually done in that respect.
The mechanism of biosorption is complex, mainly ion exchange, chelation, adsorption by physical forces, entrapment in inter and intrafibrilliar capillaries and spaces of the structural polysaccharide network as a result of the concentration gradient and diffusion through cell walls and membranes.
There are several chemical groups that would attract and sequester the metals in biomass: acetamido groups of chitin, structural polysaccharides of fungi, amino and phosphate groups in nucleic acids, amido, amino, sulphhydryl and carboxyl groups in proteins, hydroxyls in polysaccharide and mainly carboxyls and sulphates in polysaccharides of marine algae that belong to the divisions Phaeophyta, Rhodophyta and Chlorophyta. However, it does not necessarily mean that the presence of some functional group guarantees biosorption, perhaps due to steric, conformational or other barriers.
Choice of metal for biosorption process: The appropriate selection of metals for biosorption studies is dependent on the angle of interest and the impact of different metals, on the basis of which they would be divided into four major categories: (i) toxic heavy metals (ii) strategic metals (iii) precious metals and (iv) radio nuclides. In terms of environmental threats, it is mainly categories (i) and (iv) that are of interest for removal from the environment and/or from point source effluent discharges.
Apart from toxicological criteria, the interest in specific metals may also be based on how representative their behaviour may be in terms of eventual generalization of results of studying their biosorbent uptake. The toxicity and interesting solution chemistry of elements such as chromium, arsenic and selenium make them interesting to study. Strategic and precious metals though not environmentally threatening are important from their recovery point of view.
| MECHANISMS |
Biosorption Mechanisms: The complex structure of microorganisms implies that there are many ways for the metal to be taken up by the microbial cell. The biosorption mechanisms are various and are not fully understood. They may be classified according to various criteria.
According to the dependence on the cell's metabolism, biosorption mechanisms can be divided into:
According to the location where the metal removed from solution is found, biosorption can be classified as
Transport of the metal across the cell membrane yields intracellular accumulation, which is dependent on the cell's metabolism. This means that this kind of biosorption may take place only with viable cells. It is often associated with an active defense system of the microorganism, which reacts in the presence of toxic metal.
During non-metabolism dependent biosorption, metal uptake is by physico-chemical interaction between the metal and the functional groups present on the microbial cell surface. This is based on physical adsorption, ion exchange and chemical sorption, which is not dependent on the cells' metabolism. Cell walls of microbial biomass, mainly composed of polysaccharides, proteins and lipids have abundant metal binding groups such as carboxyl, sulphate, phosphate and amino groups. This type of biosorption, i.e., non-metabolism dependent is relatively rapid and can be reversible (Kuyucak and Volesky, 1988).
In the case of precipitation, the metal uptake may take place both in the solution and on the cell surface (Ercole, et al. 1994). Further, it may be dependent on the cell's' metabolism if, in the presence of toxic metals, the microorganism produces compounds that favour the precipitation process. Precipitation may not be dependent on the cells' metabolism, if it occurs after a chemical interaction between the metal and cell surface.
Transport across cell membrane: Heavy metal transport across microbial cell membranes may be mediated by the same mechanism used to convey metabolically important ions such as potassium, magnesium and sodium. The metal transport systems may become confused by the presence of heavy metal ions of the same charge and ionic radius associated with essential ions. This kind of mechanism is not associated with metabolic activity. Basically biosorption by living organisms comprises of two steps. First, a metabolism independent binding where the metals are bound to the cell walls and second, metabolism dependent intracellular uptake, whereby metal ions are transported across the cell membrane. ( Costa, et.al., 1990, Gadd et.al., 1988, Ghourdon et.al., 1990, Huang et.al., 1990., Nourbaksh et.al., 1994)
Physical adsorption: In this category, physical adsorption takes place with the help of van der Waals' forces. Kuyucak and Volesky 1988, hypothesized that uranium, cadmium, zinc, copper and cobalt biosorption by dead biomasses of algae, fungi and yeasts takes place through electrostatic interactions between the metal ions in solutions and cell walls of microbial cells. Electrostatic interactions have been demonstrated to be responsible for copper biosorption by bacterium Zoogloea ramigera and alga Chiarella vulgaris (Aksu et al. 1992), for chromium biosorption by fungi Ganoderma lucidum and Aspergillus niger .
Ion Exchange: Cell walls of microorganisms contain polysaccharides and bivalent metal ions exchange with the counter ions of the polysaccharides. For example, the alginates of marine algae occur as salts of K+, Na+, Ca2+, and Mg2+. These ions can exchange with counter ions such as CO2+, Cu2+, Cd2+ and Zn2+ resulting in the biosorptive uptake of heavy metals (Kuyucak and Volesky 1988). The biosorption of copper by fungi Ganoderma lucidium (Muraleedharan and Venkobachr, 1990) and Aspergillus niger was also up taken by ion exchange mechanism.
Complexation: The metal removal from solution may also take place by complex formation on the cell surface after the interaction between the metal and the active groups. Aksu et al. 1992 hypothesized that biosorption of copper by C. vulgaris and Z. ramigera takes place through both adsorption and formation of coordination bonds between metals and amino and carboxyl groups of cell wall polysaccharides. Complexation was found to be the only mechanism responsible for calcium, magnesium, cadmium, zinc, copper and mercury accumulation by Pseudomonas syringae. Microorganisms may also produce organic acids (e.g., citric, oxalic, gluonic, fumaric, lactic and malic acids), which may chelate toxic metals resulting in the formation of metallo-organic molecules. These organic acids help in the solubilisation of metal compounds and their leaching from their surfaces. Metals may be biosorbed or complexed by carboxyl groups found in microbial polysaccharides and other polymers.
Precipitation: Precipitation may be either dependent on the cellular metabolism or independent of it. In the former case, the metal removal from solution is often associated with active defense system of the microorganisms. They react in the presence of a toxic metal producing compounds, which favour the precipitation process. In the case of precipitation not dependent on the cellular metabolism, it may be a consequence of the chemical interaction between the metal and the cell surface. The various biosorption mechanisms mentioned above can take place simultaneously.
Use of Recombinant bacteria for metal removal: Metal removal by adsorbents from water and wastewater is strongly influenced by physico-chemical parameters such as ionic strength, pH and the concentration of competing organic and inorganic compounds. Recombinant bacteria are being investigated for removing specific metals from contaminated water. For example a genetically engineered E.coli, which expresses Hg2+ transport system and metallothionin (a metal binding protein) was able to selectively accumulate 8 mmole Hg2+/g cell dry weight. The presence of chelating agents Na+, Mg2+ and Ca2+ did not affect bioaccumulation.
Factors affecting Biosorption : The investigation of the efficacy of the metal uptake by the microbial biomass is essential for the industrial application of biosorption, as it gives information about the equilibrium of the process which is necessary for the design of the equipment.
The metal uptake is usually measured by the parameter 'q' which indicates the milligrams of metal accumulated per gram of biosorbent material and 'qH' is reported as a function of metal accumulated, sorbent material used and operating conditions.
The following factors affect the biosorption process:
1. Temperature seems not to influence the biosorption performances in the range of 20-35 0C (Aksu et al. 1992)
2. pH seems to be the most important parameter in the biosorptive process: it affects the solution chemistry of the metals, the activity of the functional groups in the biomass and the competition of metallic ions (Friis and Myers-Keith, 1986, Galun et al. 1987)
3. Biomass concentration in solution seems to influence the specific uptake: for lower values of biomass concentrations there is an increase in the specific uptake (Fourest and Roux, 1992; Gadd et al. 1988). Gadd et al. 1988 suggested that an increase in biomass concentration leads to interference between the binding sites. Fourest and Roux, 1992 invalidated this hypothesis attributing the responsibility of the specific uptake decrease to metal concentration shortage in solution. Hence this factor needs to be taken into consideration in any application of microbial biomass as biosorbent.
4. Biosorption is mainly used to treat wastewater where more than one type of metal ions would be present; the removal of one metal ion may be influenced by the presence of other metal ions. For example: Uranium uptake by biomass of bacteria, fungi and yeasts was not affected by the presence of manganese, cobalt, copper, cadmium, mercury and lead in solution (Sakaguchi and Nakajima, 1991). In contrast, the presence of Fe2+ and Zn2+ was found to influence uranium uptake by Rhizopus arrhizus (Tsezos and Volesky, 1982) and cobalt uptake by different microorganisms seemed to be completely inhibited by the presence of uranium, lead, mercury and copper (Sakaguchi and Nakajima, 1991).
Biosorption equilibrium models - Assessment of sorption performance: Examination and preliminary testing of solidliquid sorption system are based on two types of investigations: (a) equilibrium batch sorption tests and (b) dynamic continuous flow sorption studies.
The equilibrium of the biosorption process is often described by fitting the experimental points with models (Gadd, et al. 1988) usually used for the representation of isotherm adsorption equilibrium. The two widely accepted and linearised equilibrium adsorption isotherm models for single solute system are given by the following:
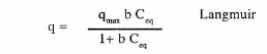
where q is milligrams of metal accumulated per gram of the biosorbent material; Ceq is the metal residual concentration in solution; qmax is the maximum specific uptake corresponding to the site saturation and b is the ratio of adsorption and desorption rates. This is a theoretical model for monolayer adsorption.
Another empirical model for monolayer adsorption is
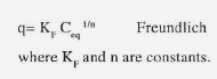
These models can be applied at a constant pH. These models are used in literature for modeling of biosorption equilibrium in the presence of one metal. These values are plotted in a 2D line where the specific uptake q is reported as a function of the metal concentration Ceq
But the above said adsorption isotherms may exhibit an irregular pattern due to the complex nature of both the sorbent material and its varied multiple active sites, as well as the complex solution chemistry of some metallic compounds (Volesky and Holan, 1995). Evaluation of equilibrium sorption performance needs to be supplemented by process-oriented studies of its kinetics and eventually by dynamic continuous flow tests.
Biosorption by immobilized cells: Microbial biomass consists of small particles with low density, poor mechanical strength and little rigidity. The immobilization of the biomass in solid structures Qeates a material with the right size, mechanical strength and rigidity and porosity necessary for metal accumulation. Immobilisation can also yield beads and granules that can be stripped of metals, reactivated and reused in a manner similar to ion exchange resins and activated carbon.
Various applications are available for biomass immobilization. The principal techniques that are available in literature for the application of biosorption are based on adsorption on inert supports, on entrapment in polymeric matrix, on covalent bonds in vector compounds, or on cell cross-linking.
Adsorption on inert supports: Support materials are introduced prior to sterilization and inoculation with starter culture and are left inside the continuous culture for a period oftime, after which a film of microorganisms is apparent on the support surfaces. This technique has been used by Zhou and Kiff, 1991 for the immobilization of Rhizopus arrhizus fungal biomass in reticulated foam biomass support particles; Macaskie et al. 1987, immobilised the bacterium Citrobacter sp. by this technique. Scott and Karanjakar 1992, used activated carbon as a support for Enterobacter aerogens biofilm. Bai and Abraham, 2003 immobilized Rhizopus nigricans on polyurethane foam cubes and coconut fibres.
Entrapment in polymeric matrices: The polymers used are calcium alginate (Babu et al. 1993, Costa and Leite, 1991, Peng and Koon, 1993, Gulay Bayramoglu et al. 2002), polyacrylamide (Macaskie et aI., 1987, Michel et al. 1986, Sakaguchi and Nakajima et al. 1991, Wong and Kwok, 1992), polysulfone (Jeffers et al. 1991, Bai and Abraham, 2003) and polyethylenimine (Brierley and Brierley, 1993). The materials obtained from immobilization in calcium alginate and polyacrylamide are in the form of gel particles. Those obtained from immobilization in polysulfone and polyethyleneimine are the strongest.
Covalent bonds to vector compounds: The most common vector compound (carrier) is silica gel. The material obtained is in the form of gel particles. This technique is mainly used for algal immobilization (Holan et al. 1993, Mah:!mn and Holocombe, 1992).
Cross-linking: The addition of the cross-linker leads to the formation of stable cellular aggregates. This technique was found useful for the immobilization of algae (Holan et al. 1993). The most common cross linkers are: formaldehyde, glutaric dialdehyde, divinylsulfone and formaldehyde - urea mixtures.
Desorption: If the biosorption process were to be used as an alternative to the wastewater treatment scheme, the regeneration of the biosorbent may be crucially important for keeping the process costs down and in opening the possibility of recovering the metals extracted from the liquid phase. For this purpose it is desirable to desorb the sorbed metals and to regenerate the biosorbent material for another cycle of application. The desorption process should:
• yield the metals in a concentrated form;
• restore the biosorbent to close to the original condition for effective reuse with undiminished metal uptake and
• no physical changes or damage to the biosorbent.
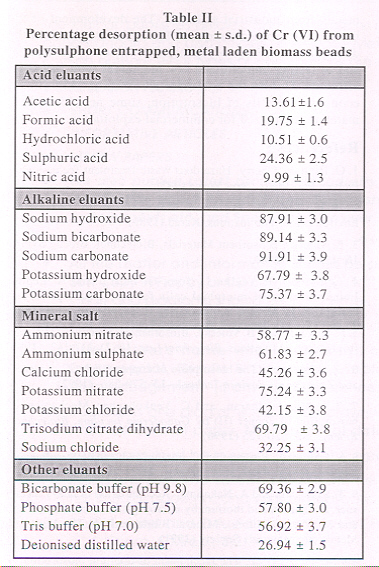
While the regeneration of the biosorbent may be accomplished by washing the metal- laden biosorbent with an appropriate solution, the type and strength of this solution would depend on the extent of binding of the deposited metal. Dilute solutions of mineral acids like hydrochloric acid, sulphuric acid, acetic acid and nitric acid can be used for metal desorption from the biomass (de Rome and Gadd, 1987, Zhou and Kiff, 1991, Luef et.al. 1991, Holan et.al. 1993, Pagnanelli etal. 2002, Baiand Abraham, 2003).
Polysulphone immobilized Rhizopus nigricans were subjected to Cr (VI) recovery experiments using 0.01 N solutions of mineral acids, salt solutions, alkalies, deionised distilled water and buffer solutions. The percentage desorption by various eluants is given in Table 2.
A few experiments were conducted to desorb the metal ions from the loaded waste fungal biomass of Aspergillus species (Chandrashekar et al. 1998) as a function of HCl concentration in the case of iron, calcium and nickel. The results revealed that with increase in HCl concentrations, the desorption of the metal ions increased and at 5M HCI, complete removal of calcium and iron would be achieved while about 78% Nickel would be desorbed.
The desorption of the adsorbed Hg (II) from the biosorbent - immobilized and heat inactivated Trametes versicolor and Pleurotus sajur-caju were studied in a batch system (Arica et al. 2003). Hg (II) ions adsorbed onto the biosorbents were eluted with 10 mmol dm-3 HCl and the results showed that more than 97% of the adsorbed Hg (II) ions were desorbed from the biosorbents.
Effect of Pre-treatment on the biosorption of heavy metals: Metal affinity to the biomass can be manipulated by pretreating the biomass with alkalies, acids, detergents and heat, which may increase the amount of the metal sorbed. The bioadsorption capacity of autoclaved Mucor rouxii decreased as compared to the live fungus, attributed to the loss of intracellular uptake (Yan and Viraraghavan, 2000). Whistler and Daniel (1985) reported that the heat treatment could cause a loss of amino-functional groups on the fungal surface through the non-enzymic browning reaction. Aminofunctional groups in the polysaccharides contribute to the binding of heavy metals (Loaec et al., 1997). However, Galun et al., 1987 reported that Pencillium biomass pretreatment at 100°C for 5 minutes increased the biadsorption of lead, cadmium, nickel and zinc and the increase was attributed to the exposure of latent binding sites after pre-treatment.
In the case of alkali pre-treatment, bioadsorption capacity of Mucor rouxii biomass was significantly enhanced in comparison with autoclaving (Yan and Viraraghavan, 2000). In a study by Galun et al. (1987), NaOH treated Pencillium digitatum also showed enhancement of cadmium, nickel and zinc biosorption. Removal of surface impurities, rupture of cell-membrane and exposure of available binding sites for metal bioadsorption after pre-treatment may be the reason for the increase in metal bioadsorption. McGahren et al. (1984), Brierly et.al (1985) and Muraleedharan and Venkobachar (1990) showed that alkali treatment of biomass may destroy autolytic enzymes that cause putrefaction of biomass and remove lipids and proteins that mask reactive sites. The cell wall of Mucor rouxii was ruptured by NaOH treatment. Besides, the pre-treatment could release polymers such as polysaccharides that have a high affinity towards certain metal ions (Mittelman and Geesey, 1985; Loaec et.al. 1997).
Acid pretreatment of Mucor rouxii significantly decreased the bioadsorption of heavy metals (Yan and Viraraghavan, 2000), which is in agreement with the observation of Kapoor and Viraraghvan (1998) in the case of A.niger. This is attributed to the binding ofH+ ions to the biomass after acid treatment may be responsible for the reduction in adsorption of heavy metals. The polymeric structure of biomass surface exhibits a negative charge due to the ionization of organic and inorganic groups (Hughes and Poole, 1989). Bux and Kasan (1994) suggested that the higher the biomass electronegativity, the greater the attraction and adsorption of heavy metal cations. Thus the remaining H+ions on the acidic pretreated M.rouxii biomass may change the biomass electronegativity, resulting in a reduction in bioadsorption capacity.
However, Huang and Huang (1996) reported that acid pretreatment can strongly enhance the adsorption capacity of Aspergillus.oryzae mycelia. In case of A.oryzae, live biomass after acid pre-treatment was directly used in bioadsorption of heavy metals instead of being autoc1aved and dried. The difference in results after a specific pretreatment may be attributed to the different strains of fungi used and whether the biomass was live or stead when it is used in biosorption of metal ions. For example; pre-treatment of A.oryzae by HClO4 increased the bioadsorption of lead, cadmium and nickel, but it was not the case for the species of R.oryzae (Huang and Huang, 1996). When non-viable biomass is used in the removal of heavy metals, alkali pretreatment is an effective method to improve the bioadsorption capacity for metal ions (Yan and Viraraghavan, 2000). Hence, the bioadsorption efficiency of dead biomass may be greater, equivalent to, or less than that of live biomass depending on the pre-treatment method applied. It is necessary to carry out more detailed studies to understand why enhancement or reduction in adsorption capacity occurs under specific pre-treatment conditions.
Biosorption is being demonstrated as a useful alternative to conventional systems for the removal of toxic metals from industrial effluents. The development of the biosorption processes requires further investigation in the direction of modeling, of regeneration of biosorbent material and of testing immobilized raw biomasses with industrial effluents. Due to the extensive research and significant economic benefits of biosorption, some new biosorbent materials are poised for commercial exploitation.
| REFERENCES |
1. G. Rich, K. Cherry, Hazardous Waste Treatment Technologies, Pudvan Publishers, New york (1987)
2. R.K. Trivedi, Pollution Management in Industries, Environmental Publications, Karad (1989)
3. B. Vole sky, Biosorbent Materials, Biotechnoi. Bioeng Symp., 16: 121-126 (1986)
4. C. Ercole, F. Veglio, L. Toro, G. Ficara and A. Lepidi, Immobilisation of microbial cells for metal adsorption and desorption. In: Mineral Bioprocessing II. Snowboard. Utah (1994)
5. N. Kuyucak and B. Vole sky, Biosorbents forrecovery of metals from industrial solutions. Biotechnol Left., 10 (2), 137-142 (1988)
6. Z. Aksu et aI., The biosorption of copper (II) by C. vulgaris and Zramigera. Environ Technol., 13: 579-586 (1992)
7. T.R. Muraleedharan and C. Venkobachar, Mechanism of biosorption of copper (II) by Ganoderma lucidum. Biotechnol. Bioeng., 35: 320-325 (1990)
8. N. Friis et aI., Biosorption of uranium and lead by Streptomyces longwoodensis. Biotechnol. Bioeng., 28:21-28 (1998)
9. T. Sakaguchi and A. Nakajima, Accumulation of heavy metals such as uranium and thorium by microorganisms In : R.W. Smith and M. Misra (Editors), Mineral Bioprocessing. The Minerals, Metals and Materials Society (1991)
10. M. Tsezos et aI., The mechanism of uranium biosorption by Rhizopus arrhizus. Biotechnol. Bioeng., 24 : 385-401 (1982)
11. G.M. Gadd et aI., Heavy metal and radionuclide by fungi and yeasts. In: P.R. Norris and D.P. Kelly (Editors), Biohydrometallurgy. A. Rowe, Chippenham, Wilts., U.K. (1988)
12. J.L Zhou and R.J. Kiff, . The uptake of copper from aqueous solution by immobilized fungal biomass. J. Chern Technol. Biotechnol. 52, 317-330 (1991)
13. LE. Macaskie, J.M. Wates and A.C.R. Dean, Cadmium accumulation by a citrobacter sp. immobilized on gel and solid supports: applicability to the treatment of liquid wastes containing heavy metal cations. Biotechnol. Bioeng. 30:66-73 (1987)
14. J .A. Scott and A.M. Karanjkar, Repeated cadmium biosorption by regenerated Enterobacter aerogenes biofilm attached to activated carbon. Biotechnol. Left., 14,8:737-740 (1992)
15. G.R.Y. Babu etal., Degradation Of inorganic cyanides by immobilized Pseudomonas putida cells. In A.E. Torma., M.L Apel and C.L Brierley (Editors). Biohydrometallurgical Technologies, Vol. II The minerals, metals and materials Society (1993)
16. A.C.A. Costa and S.G.F. Leite, Metals Biosorption by sodium alginate immobilized ChIarella homosphaera cells. Biotechnol Left., 13 (8): 55_-562 (1991)
17. T.Y. Peng and K.W Koon, Biosorption of cadmium and copper by Sacchromyces cerevisiae. Microb. Util. Renewable resour., 8: 494-504 (1993)
18.LJ. Michel et aI., Cadmium accumulation by immobilized cells of a Citrobacter sp. using various phosphate donors. Biotechnol. Bioeng., 28: 1358-1365 (1986)
19. P.K. Wong and S.c. Kwok, Accumulation of nickel ion by immobilized cells of Enterobacter species, Biotechnol. Left., 14(7): 629 - 634 (1992)
20. T.H. Jeffers et aI., Biosorption of metal contaminants from acidic mine waters. In: R.W Smith, M. Misra. (Editors) Mineral Bioprocessing. The Minerals, Metal and Materials Society (1991)
21. c.L Brierley et aI., Immobilisation of Biomass for industrial application of Biosorption. In: A.E. Torma., M.L Apel and C.L Brierley (Editors), Biohydrometallurgical Technologies.Vol II. The Minerals, Metal and Materials Society (1993)
22. Z.R. Holan et aI., Biosorption of cadmium by biomass of marine algae. Biotechnol. Bioeng., 41: 819-825 (1993)
23. C.A. Mahan and J.A. Holcombe, Immobilisation of algae cells on silica gel and their characterization for trace metal preconcentration. Anal. Chem., 64: 1933-1939 (1992)
24. C.L Brierley, Bioremediation of metal contaminated surface and Groundwater. Geomicrobiol. J. 8:201-223 (1990)
25. G.M. Gadd, Fungi and Yeasts for metal accumulation. In: c.L Ehrlich, Brierly, (Eds), Microbial Mineral Recovery. McGrawHill, New York, 249-276(1990)
26. Bai R. Sudha and Emilia Abraham., Studies on chromium (VI) adsorption-desorption using immobilized fungal biomass. Bioresource Technology, 87, 17-26 (2003)
27. Francesca Pagnanelli, Luigi Toro and Francesco Veglio, Olive mill solid residues as heavy metal sorbent material: a preliminary study. Waste Management 22,901-907 (2002)
28. Yan Guangyu et aI., Effect of pretreatment on the bioadsorption of heavy metals on Mucor rouxii. Water SA. 26: 1 (2000)
29. F. Bux and H.c. Kasan, Comparision of selected methods for relative assessment of surface charge on waste sludge biomass. Water SA, 20 (1). 73-76 (1994)
30. M. Galun et aI., Removal of metal ions from aqueous solutions by Pencillium biomass: Kinetic and uptake parameters, Water, Air and Soil Pollution. 33: 359-371 (1987) .
31. C. Huang and C.P. Huang, Application of Aspergillus oryzae and Rhizopus oryzaefor Cu (II) removal. Water Res. 9: 1985-1990 (1996)
32. A. Kapoor and T. Viraraghvan, Biosorption of heavy metals on Aspergillus niger: Effect of pretreatment. Bioresour. Technol 63: 109-113 (1998)
33. A. Kapoor A and T. Viraraghvan, Fungal Biosorption: An alternative treatment option for heavy metal bearing wastewater: A review. Bioresour. Technol. 53: 195-206 (1995)
34. M. Loaec et aI., Uptake of lead, cadmium and Zinc by a novel bacterial exopolysaccharide. Water Res. 31 (5): 1171-1179 (1997)
35. T.R. Muraleedharan and C. Venkobachar, Mechanism of cobalt biosorption. Biotechnol. Bioeng. 33: 823-831 (1990)
36. R. Whistler and T.R. Daniel, Carbohydrates. In Owen R Fennema (ed.) Food Chemistry. 96-105 (1985)
37. W.I. McGahren et aI., Chitosan by fermentation. Process Biochem. 19: 88-90 (1984)
38. I.A. Brierley et aI., European patent application No. 85112810, Publication No. 0181497 (1985)
39. M.W. Mittleman and G.G.Geesey, Copper binding characteristics of exopolymers from a freshwater sediment bacterium. Appl. Environ. Microbiol. 49: 846-851 (1985)
40. B.Volesky and Z.R. Holan, Biosorption of heavy metals. Biotechnol Prog. 11 : 235-250 (1995)
41. A. Hammaini et aI., Simultaneous uptake of metals by activated sludge. Minerals Engineering. 16 : 723-729 (2003)
42. Lisa Norton et aI., Biosorption of zinc from aqueous solutions using biosolids. Advances in Environmental Research (2003)
43. O. Keskinan et aI., Heavy metal adsorption characteristics of a submerged aquatic plant (Myriophyllum spicatum). Process Biochemistry. 1-5 (2003)
44. Gulay Bayramoglu et aI., Entrapment of Lentinus sajor-caju into Ca-alginate gel beads for removal of Cd (II) ions from aqueous solution: preparation and biosorption kinetic analysis. Microchemical Journal. 72: 63-76 (2002)
45. T.c. Wang et aI., Parameters for removal of toxic heavy metals by water milfoil Bull Environ. Contam. Toxicol. 57:779- 786 (1996)
46. IAH Schneider et aI., Sorption of heavy metal ions by the non-living biomass of freshwater macrophytes. Environ Sci. Technol. 33:2213-7 (1999)
47. D. Kratochivl et aI., Water Res. 32,2760-2768 (1998a)
48. K.Chandra Shekar et aI., Removal of metal ions using an industrial biomass with reference to environmental control. Int. J. Miner. Process. 53: 107 -120 (1998)
49. M. Y. Arica et aI., Comparative biosorption of mercuric ions from aquatic systems by immobilized live and heat inactivated Trarnetes versicolor and Pleurotus sajur-caju (2003)(Received 4th September 2003, accepted 15th November 2003)