FACTORS RESPONSIBLE FOR FISH MORTALITY
Role of various factors responsible for fish kill are discussed next.
-
Temperature: Higher temperature increases the oxygen demand and rate of biochemical activity of the micro biota as well as plant respiratory rate. Higher temperature decreases solubility of oxygen and also increases level of ammonia in water that can build up to dangerously high levels affecting fish health (Bhatnagar and Devi, 2013). Fish affected by thermal stress (cold or warm temperatures shock) reduces its resistance to diseases thereby could be susceptible to bacterial and fungal infections that eventually kill them. Weather related disturbances or turnovers could bring anoxic (lack of oxygen) bottom water and decaying materials of lakes into the water column and release large quantities of hydrogen sulphide (H2S).
-
Untreated waste: Sewage brings in large quantities of carbon (C), nitrogen (N) and phosphorus (P) that are trapped within the lake ecosystem (Mahapatra et al., 2011). The discharge of untreated industrial effluents and sewage into lakes and rivers causes oxygen depletion, increases the level of chlorophyll ‘a’, and alkalinity (pH 9.2) which in turn causes large scale mortality of fishes (Zutshi and Prasad, 2008). The suspended particles reduce the amount of sunlight penetrating the water, disrupting the growth of photosynthetic algae/plants and micro-organisms, thus hampering the normal functioning of aquatic ecosystems. Organic waste entering lakes may overload a natural system causing a serious depletion of oxygen supply in the water that in turn leads to fish kill (Murthy et al., 2014).
Microorganisms in water feed on biodegradable substances and their population depends on the quantity of organic matter/biodegradable material in water. When sewage enters a lake, micro-organisms begin to decompose the organic materials. The decomposition activities cause oxygen depletion and thus, aerobic microorganisms begin to die and anaerobic microorganisms begin to thrive. Some anaerobic microorganisms attack the organic matter and produces harmful toxins such as methane, ammonia and sulfides (Mahapatra et al., 2011). When organic compounds decompose in the absence of oxygen, it gives rise to the undesirable odours usually associated with foul-smelling, septic or putrid conditions. Heterotrophic bacteria consume oxygen and release carbon dioxide while oxidizing organic matter, whereas the autotrophic nitrifying and sulphur bacteria consume oxygen and carbon-dioxide while oxidising ammonium, nitrite or sulphide, respectively (Moriarty, 1997).
- Dissolved oxygen: The major source of dissolved oxygen (DO) in the water is through photosynthesis by phytoplankton and aquatic plants, but some also enters the water directly from the atmosphere by diffusion. At the same time, however, oxygen is being removed from the water by the respiration (breathing) of fish, plants, and other underwater inhabitants. Decomposition of plant and animal matter in the water also consumes oxygen. During night, the photosynthesis ceases, and the algae, sediment and fish consume oxygen (respiration), producing fluctuating patterns of dissolved oxygen concentration. The competition between plants, bacteria and animals particularly at night and in dull weather can lead to lack of oxygen, causing fish suffocation and subsequent mortality. A decline in DO has serious implications on the health of the aquatic system, as hypoxic and anoxic conditions reduce or eliminate sensitive native fish and invertebrate species (Mahapatra et al., 2011). Fish usually require a minimum of 5 milligrams per liter (mg/L) of DO for optimum health. Most fish can tolerate DO below 2 mg/L for short periods, but starts dying when DO drops below 1 mg/l. Under normal conditions, the DO in a water body is lowest in the morning just before the sunrise.
Oxygen depletion in water bodies can occur due to algae die-offs, weather related ‘turnovers’ of lakes water, surface run-off of organic materials into water bodies, disturbance of sediments containing large quantities of aquatic vegetation or with excess nutrient loads, low water levels, and high temperatures etc. During the summer, water holds less oxygen and warm temperatures make bacteria grow faster resulting in faster oxygen depletion. Temperature influences water chemistry, e.g. DO, solubility, density, pH, conductivity etc. Water holds lesser oxygen at higher temperatures. Some compounds are more toxic to aquatic organisms at higher temperatures (Ramachandra and Solanki, 2007). During cloudy days, the rate of photosynthesis as well as oxygen production by algae will decrease and enough oxygen will not be available for bacterial respiration. In addition, fish experience a faster metabolic rate as water temperature increases; therefore, their requirement for oxygen increases. The fish are therefore more likely to be stressed during the warmer months. The possible signs of fish kills due to oxygen depletion are fish gasping at the surface, sluggish movement, larger fish die earlier than smaller fish of the same species, kill occur at night or in the early morning.
- Carbondioxide: Decomposition of the dead plants will also raise carbon dioxide and total ammonia concentrations. When carbon dioxide concentrations are high, the fish will have difficulty in reducing internal carbon dioxide concentrations, resulting in its accumulation in fish blood. This accumulation inhibits the ability of hemoglobin, the oxygen-carrying molecule in fish blood, to bind oxygen, and may cause the fish to feel stress similar to suffocation.
- Turbidity: Lakes with higher silt transport in the catchment (due to removal of vegetation cover) will have more turbidity, restricting the penetration of sunlight and reducing the photosynthetic activity, which in turn influence the productivity of water (Chandrasekhar, 2002). The higher turbidity shows the presence of higher concentration of organic and non-biodegradable components in the lake water that require higher amount of oxygen for their decomposition (Poodari et al., 2014), which in turn affects fish population. Turbidity thus, affects the productivity of an aquatic ecosystem. In highly turbid waters, gill surfaces of fishes will be clogged with suspended matter.
- Nitrates, Phosphates and Biochemical Oxygen Demand (BOD): Nitrates are the end product of the aerobic decomposition of organic nitrogenous matter (Sincy et al., 2012). The significant sources of nitrates are chemical fertilizers from cultivated lands, drainage from livestock feeds, as well as domestic and industrial effluents (Ramachandra and Ahalya, 2001). Phosphates occur in natural or wastewaters as orthophosphates, condensed phosphates and naturally found phosphates. Their presence in water is due to detergents, used boiler waters, fertilizers, biological processes and occurs in detritus. They are essential for the growth of organisms and is a nutrient that limits the primary productivity of the water body (Ramachandra and Ahalya, 2001). Nitrates and phosphates in water can contribute to high BOD levels. Nitrates and phosphates allow algae to grow rapidly. The death of algae contributes to organic waste in the water, which is then decomposed by bacteria contributing to high BOD levels. When BOD levels increases, dissolved oxygen (DO) level decreases as the oxygen available in the water will be consumed by different bacteria.
- Phytoplankton bloom: Algae tend to grow very quickly under high nutrient availability but are short-lived. The high nutrient levels in lakes can even produce dense “blooms” of phytoplankton. The entire algae population dies, sinks and becomes food for the bacteria. The available oxygen will be rapidly consumed by the bacteria, but oxygen cannot be replenished soon as a result of the decreased algal population and minimal photosynthetic activity. Thus, a sudden phytoplankton die-off and the decomposition of dead plankton can reduce DO to levels lethal to fish. Also, the blooms can reduce or block sunlight penetrating the water, stressing or killing aquatic plants.
- Ammonia: Ammonia poisoning is another primary cause of the fish kills. The various forms of nitrogen influent in sewage are organic N (protein N), urea, ammonia, nitrites and nitrates through processes like nitrification, denitrification and ammonification. Autotrophic nitrification consists of two consecutive aerobic reactions (figure 1), the conversion of ammonia to nitrite by Nitrosomonas and then from nitrite to nitrate by Nitrobacter (Mahapatra et al., 2011).
Nitrogen such as un-ionised ammonia (NH3) is highly toxic to fish. Potential sources of ammonia are organic pollution, fertilizers, overcrowding of fish and industrial effluents. A higher level of ammonia exposes fish to higher incidences of bacterial gill disease. Ammonia enters the aquatic ecosystem via anthropogenic sources such as sewage entry, agricultural runoff, nitrogen fixation and the excretion of nitrogenous wastes from animals (USEPA, 2013). One of the main sources of ammonia in lake water is through fish excretion. The excrement rate is directly related to the feeding rate and the protein level in the feed being used. Fish digest the protein in their feed and excrete ammonia through their gills and in their feces. Another main source of ammonia is the diffusion from the lake sediments itself. Large amounts of organic matter are produced by algae or added to lakes as feed. Fecal solids and dead algae settle to the lake bottom and begin the process of decomposition and produces ammonia that diffuses from the sediment bottom into the water column. In water, ammonia is present in a molecular form (NH3) and in the form of ammonium ions (NH4+). The ratio between these two forms depends on the pH and temperature of the water. The cell walls of the organisms are comparatively impermeable to the ammonia ion (NH4+), but molecular ammonia (NH3) can readily diffuse across the tissue barriers where a concentration gradient exists and is therefore the potentially toxic form to fish (Patil et al., 2015).
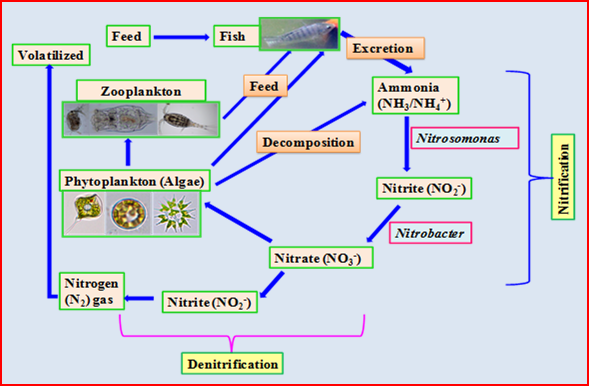
Figure 1: Role of ammonia in aquatic ecosystem

