3.2 Ammonia toxicity: Ammonia causes negative effects on fish like reduced growth rates, poor feed conversion and reduced disease resistance. Excess ammonia (NH4) levels accumulate in organisms and cause alteration of metabolic activities or increases in body pH harming the aquatic life. At extreme ammonia levels, fish may experience convulsions, coma and death. A short-term exposure to toxic un-ionized ammonia at about 0.6 mg/L (ppm) is capable of killing fish over a few days. But, chronic exposure to toxic un-ionized ammonia as low as 0.06 mg/L (ppm) can cause gill and kidney damage, growth reduction, brain malfunctioning and reduction in the oxygen-carrying capacity of the fish (Durborow et al., 1997; Hargreaves and Tucker, 2004; Waqar K et al., 2013). Ammonia in the range >0.1 mg/L tends to cause gill damage, destroy mucous producing membranes, “sub-lethal” effects like reduced growth, poor feed conversion and reduced disease resistance at concentrations that are lower than lethal concentrations, osmoregulatory imbalance, kidney failure. Fish suffering from ammonia poisoning generally appear sluggish or often at the surface gasping for air (Bhatnagar and Devi, 2013). The maximum admissible ammonia concentration is 0.05 mg/L, where as the ammonia level in the Taj boudi (of Bijapur) water was 2.71 mg/L, which is lethal to fish (Patil et al., 2015).
3.3 Detergents: Detergents are commonly used for cleaning purposes in households. The extensive use of the detergents pollutes the aquatic ecosystem as they are made up of surface-active agents, builders and fillers. In addition, they contain additives such as anti re-deposition agents, optical fibre brighteners (whitening agents), bluing agents, bleaching agents, foam regulators, organic sequestering agents, enzymes, perfumes and substances that regulate density and assure the crispness of the material they are used on. Phosphates act as a builder in laundry detergents and automatic dishwasher detergents. They make the water soft and slightly alkaline and dissolve dirt and keep it in suspension during washing and thus, increase the performance of the detergent. The presence of detergent in water accelerates the corrosive action, impedes the filtering, sedimentation and coagulation processes, increases the saturation of water with oxygen and also deteriorates the taste properties of water (Vasanthi et al., 2013; Ramachandra et al., 2015).
The ‘after wash’ of detergents is either drained into the aquatic environment such as ponds, lakes, rivers, streams etc. or they find their way into the aquatic environment through sewage line connected to lakes. Fish has been used as model organism to detect the level of toxicity of different chemicals drained/contaminated in aquatic environment. Linear Alkyl Benzene Sulphonate (LABS) detergent was found to have acute toxic and severe histopathological effects on the gill of Puntius ticto fish. When fishes were exposed to LABS (Henko) in graded concentrations (20 - 28 mg/L, except 22 mg/L) for 24, 48, 72 and 96 hr duration, it was found that all the 30 fishes (100%) died in Henko concentration of 28 mg/L at 24 hr, indicating that the acute toxicity of LABS is dose dependent. The LC50 of Henko was found to be 25.5 mg/L. The histopathological examination of fishes exposed to 2 mg/L Henko for one month revealed severe changes in the epithelial lining of gill arch, gill rakers and gill filaments, suggesting that LABS may induce abnormal cellular structure in gills. As the dose/concentration of LABS increases, the mortality percentage of Puntius ticto fingerlings also increases. Thus, the drainage of ‘after wash’ of this detergent besides other chemicals into the aquatic environment should be strictly prohibited (Varsha et al., 2011). A significant decrease in protein, carbohydrate and cholesterol content in the tissues of fish species Cirrhinus mrigala was observed when the fishes were exposed to sub-lethal concentration of detergent Tide i.e., 3.6 mg/L for 24, 48 and 72 hrs respectively. The kidney showed the highest percent decrease in carbohydrate (77.27%), in protein (76.42%) and in cholesterol (80.03%) content (Vasanthi et al., 2013). The fishes belonging to species Cirrhinus mrigala of mean body weight (1.25g±0.12) were exposed to three concentrations of detergent, Surf excel easy wash i.e, 0.5 mg/L, 1 mg/L, 3 mg/L respectively. Eventhough the test fish exhibited no signs of deformity; the fishes showed marked increase in protease activity with increasing concentration of Surf excel easy wash than the control group (Rani and Kaushik, 2014). The oxygen consumption in the freshwater fish, Mystus montanus increased with 1/3rd sublethal concentration of detergents like Surf and Nirma powderwith increase in time (Chandanshive, 2014).
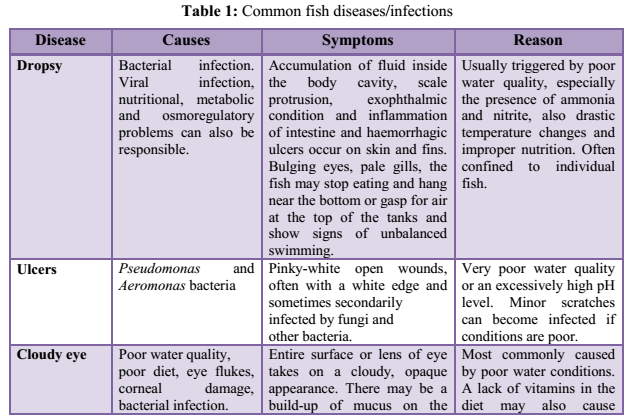
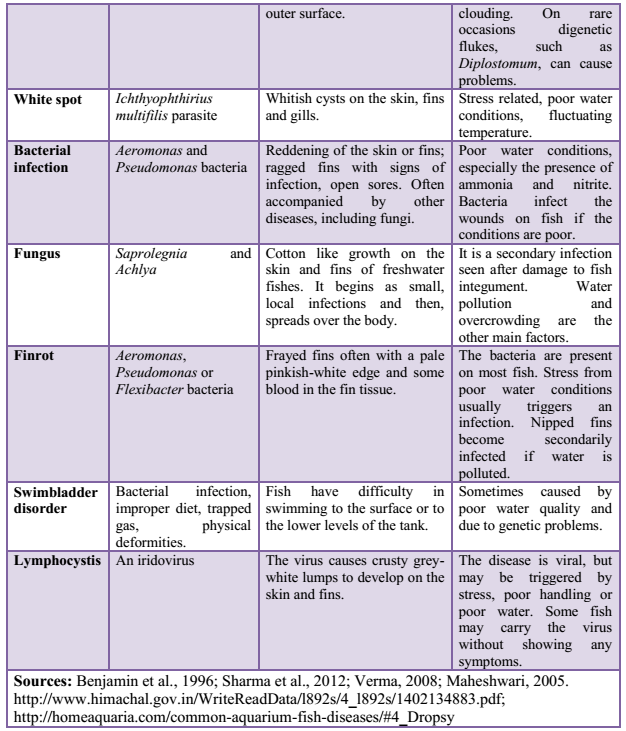
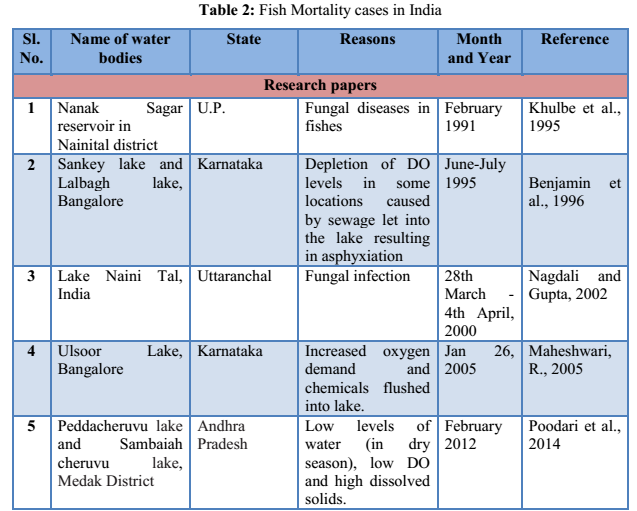
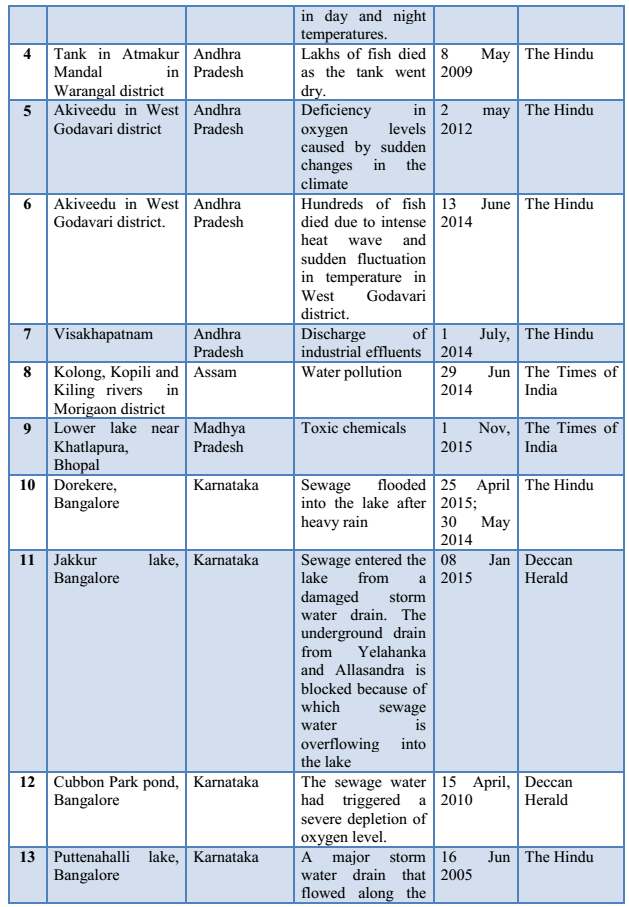
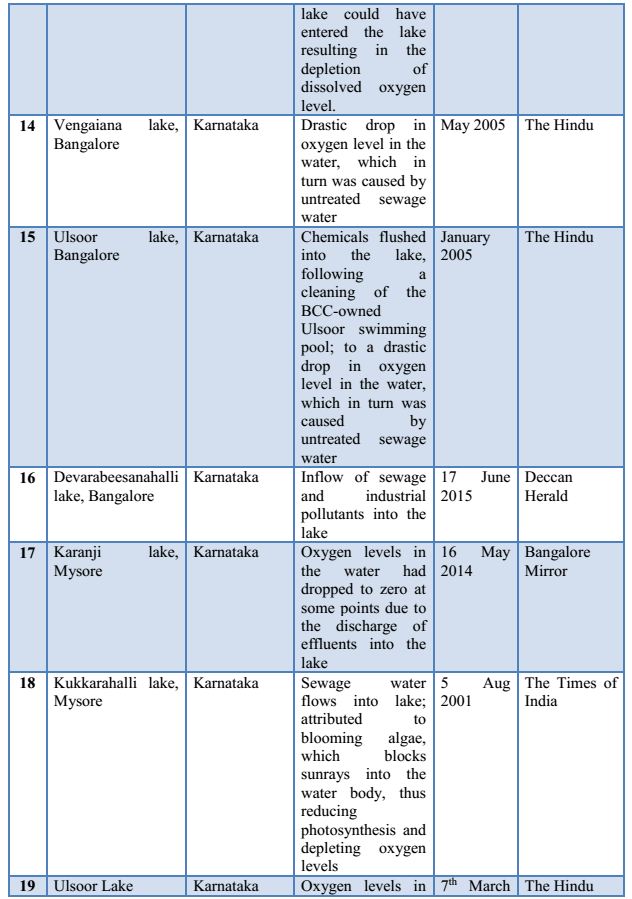
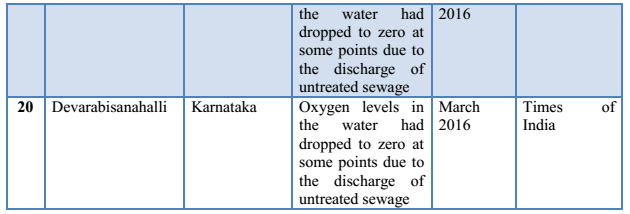
3.4 Fish Diseases/Infections: Disease is a prime agent affecting fish mortality. Fishes are exposed to different environmental pollutants, including drugs and chemicals. They carry pathogens and parasites and get infected by different pathogens, microorganisms or parasites. Some commonly found fish diseases, listed in Table 1 are gill disease, water quality induced diseases, constipation, anorexia, columnaris, ick, dropsy, tail and fin-rot, fungal infections, white spot disease, pop-eye, cloudy eye, swim bladder disease, lice and nematode worms infection, tuberculosis, lymphocytosis etc. (Sharma et al., 2012). Most fish pathogenic bacteria can reside in the environment or on/in normal fish. Thus, infections induced by some stress (e.g. overcrowding, low DO, high ammonia) upsets the natural defense of organisms (Khatun et al., 2011).