

| Biomass |


| Biomass |
Gasification
Ole Elmose, folk high school teacher, Viborgegnen's Energy- and Environment Office
With the increasing interest in utilizing biomass for energy supply - and preferably for combined heat and power production - gasification calls for renewed interest.
Historical Background
Gasification has been an important part of energy supply earlier; namely at coal gasifications plants wherefrom the gas was distributed through gas pipes and used in gas cookers, and the residue product, coke, was used for heating in solid fuel stoves. During World War II wood by means of a gasifier was used as petrol for cars.
Like this, gasification is not a new phenomenon, but the technology was forgotten for many years because of cheaper and more handy ways of getting energy.
The renewed interest in gasification of biomass, first of all straw and wood/chips, is due to the wish to utilize the residue products from agriculture and forestry, and encourage a more environmental conscious energy use. Here is mainly thought of the CO2 impact in the atmosphere.
As part of the new direction was made a study, at the beginning of 1992, on how far the development of gasifications plants is abroad 11/. A few companies in Europe and North America have developed gasifiers; but the study shows that it is impossible to buy fully automatic and reliable gasifiers designed for cogeneration. Another characteristic of the examined plants is that none of them are capable of utilizing straw, and there is no technology to utilize the waste heat. Up till now the technology development abroad has mainly been directed towards third world applications, where abundant labour is available, and heat production is not that interesting.
So in fact Danish technological development is not in a bad position as Danish straw gasifiers have been developed, both of 1 MW size at Kyndby Power Plant, and in smaller scale, 50 kW, at the Technical University of Denmark. It is especially interesting that it is gasification of straw, as straw is considered to be more difficult to handle than wood/chips utilization
At the moment there are around 100 district heating plants in Denmark using wood (pills, chips, bark) or straw as fuel. Moreover, some communities and institutions - like boarding schools - have started to use wood chips, straw, or wood pills for heat supply; this is a development that is expected to continue in the coming years. Furthermore there are of the order of 14,000 individual straw furnaces, mainly on farms. At last it is estimated that there are appr. 350,000 fireplaces and woodbuming stoves in private homes.
For reasons mentioned in the chapter on planning (chapter 5), it is important to change from heat supply to combined heat and power supply as far as possible. It is in this connection, gasification becomes interesting - as gas can be used in gas engines for combined heat and power production.
If we leave fireplaces and woodburning stoves out of account, there are anyway among the above mentioned heating plants a large market both in number and power for cogeneration plants based on gasification. Concurrently with the development of gasification technology, district heating plants, communities, institutions, and many farms will replace heat supply with combined heat and power supply (cf. chapter 12).
Gasification
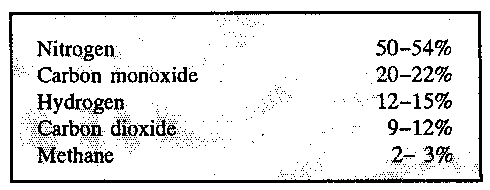
The gasification process is often called thermal gasification, because the biomass is heated in a chamber with controlled air supply. It is important to control the air supply; otherwise, there may easily appear a complete combustion without gas production. During the heating, that usually is part of the process, the volatile gasses, which make up the main part of energy in straw and wood, are liberated. The most interesting part of the gas according to energy are carbon monoxide, hydrogen, and methane. It is a very poor gas (the heating value is lower) compared to natural gas, figure 11.6.
Gasification Technologies
The chemical reactions, the fuel has to undergo before it is gasified, are: drying, pyrolysis, combustion (oxidation), and reduction.
The Countercurrent Gasifier
Figure 11.7 shows a schematic picture of a countercurrent gasifier including the most important reaction zones, temperature levels, main chemical reactions, and flow.
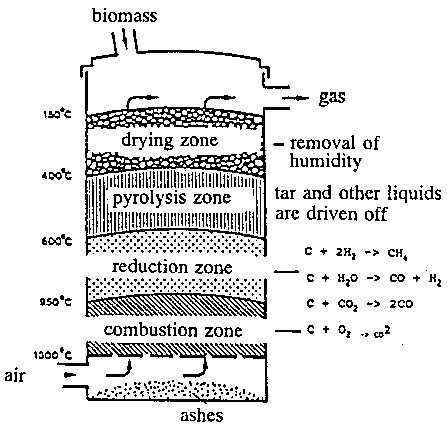
In the combustion (oxidation) zone carbon from the fuel bums and forms carbon dioxide with the oxygen in the air. Heat is emitted during the reaction and the temperature rises until a balance between heat supply and heat loss occurs.
Reaction: C + O2 -> CO2 + Q (393,800 kJ/kmol)
After the oxidation zone the hot gas passes through the reduction zone. There is no free oxygen in this zone which causes that carbon dioxide - an inflammable gas - reacts with the carbon in the fuel and forms carbon monoxide which is a flammable gas. This reaction is endoterm (demands heat) and does not happen before the temperature exceeds 900›C. Carbon monoxide is the most important flammable compound in the produced gas.
Reaction:
C + CO2 + Q (172,600 Kj/kmol) -> 2CO
Another important endoterm reaction in the reduction zone is the reaction of water vapour and carbon to carbon monoxide and hydrogen. The reaction is often called the water gas reaction (known from the old coal gasification plants). Both gasses are flammable, and the heating value of the gas is increased.
Reaction:
C + H2O + Q (13,400 kJ/kmol) ~ CO + H2
During the endoterm reactions the gas temperature decreases and other reactions occur. One of these is the reaction between carbon and water vapour, which forms carbon dioxide and hydrogen.
Reaction:
C + 2H2O + Q (88,000 kJ/kmol) -> CO: + 2H2
If there is a surplus of water in the reduction zone, then carbon monoxide may react with water vapour and form carbon dioxide and hydrogen. This reaction is exoterm (emits heat) and decreases the heating value of the produced gas.
Reaction:
CO + H2O - Q(41,200 kJ/kmol) ~ CO2 + H2
By gasification of biomass, the water content in the fuel is usually that big, that some part of the evaporated water passes through the gasifier and forms part of the outgoing gas flow.
In the pyrolysis zone a thermal decomposition of the fuel is taking place at temperatures over 400›C. Water vapour, methane, tar, etc., are formed in the pyrolysis zone. After the pyrolysis the fuel has changed to charcoal.
In the drying zone in the upper part of the gasifier water is separated from the fuel as water vapour.
The heating value of the gas depends to a great extent on, if the oxygen needed for the gasification is supplied by the air. The nitrogen in the air (appr. 78%) passes through the gas generator and makes up the dominant compound of the produced gas. Another important compound is the water vapour that decreases the heating value of the gas. If very wet fuel is gasified, there is a risk that the produced gas is inflammable.
The countercurrent gasifier has several advantages. First of all it is very simple in its construction and function. Second it is able to gasify a material with relatively high humidity; as the processes in the reduction zone show, water/humidity exactly takes part in the process.
The drawback is that the gas contains a lot of tar which makes it impossible to use the gas directly in engines. Therefore it is necessary to remove the tar - or even better to crack it to flammable substances in order to utilize the energy as good as possible.
Parallel gasifier
Figure 11.8 shows a schematic picture of a parallel gasifier. It is mainly the same chemical reactions in this type of gasifier as described for the countercurrent gasifier.
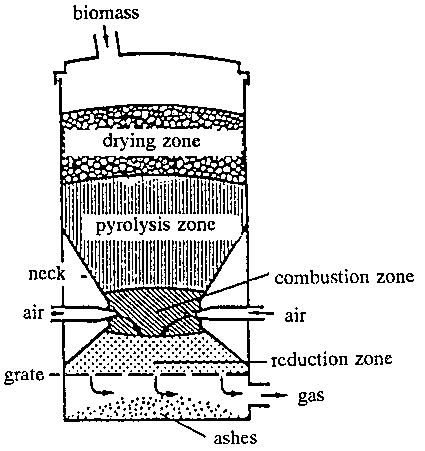
Unlike the countercurrent gasifier the gas outlet is placed at the bottom of the gasifier and the reduction zone is under the combustion (oxidation) zone. These two modifications cause that tar, etc., which is formed in the pyrolysis zone, has to pass the hot combustion zone before it leaves the gasifier. By this passage the tar takes part in the combustion or is decomposed to light hydrocarbons, and the outgoing gas is under ideal circumstances tar-free. In practice the tar content will be appr. 0.1-0.2 g/Nm.
When using this gasification technology, the gas is directly usable for running engines after soot and ash particles have been washed out. On the other hand the parallel gasifier demands that the biomass do not have too large water content.
A special kind of parallel gasifier has been developed at the Technical University of Denmark. In this, the pyrolysis is separated as a process by itself where for example a cogenerator's exhaust gasses can be used as heating source. This way profit is achieved. But the reason is first of all that the pyrolysis gas can be utilized in the further process taking place in the combustion chamber, in the cavity over the fuel. In this way, the process is optimized and a total combustion and formation of cinders are avoided.
Literature
Undersogelse af det udenlandske marked for kommercielle troforgasningsanlog til kraftvarmeprodultion (Study of the foreign market of commercial wood gasifications plants for cogeneration). dk-TEKNIK et.al., July 1992.
Forgasning - en C(L)EMT - Teknologi (Gasification - a kept (forgotten) Technology), Lars Erik Brath. VEEK- kontakt, no. 10, 1990.
Gasgenetorer - sma kraftvarmeanlog med gasgenerator (Gas generators - small cogeneration plants with gas generators). Jesper Schramm and Erik Kofoed, Laboratory for Energy Technology, April 1985.
Gengas: Svenska erfarenheter fran aren 193945 (Generator gas: Swedish experiences from 1939-45). Academy of Science, Stockholm 1950.
Articles from "Vedvarende Energi og Miljo" (Renewable Energy and Environment) and "Energi og Planlogning" (Energy and Planning).

