Rejuvenation & Sustainable Management Of Gokarna Temple Pond - Kotiteertha
Ramachandra T.V1,2,3, Durga Madhab Mahapatra1,2, Subash Chandran M D1, Sincy V1, Asulabha K S1, Rao G R1, Vishnu D. Mukri1, Akhil C A1 |
| l |
EXECUTIVE SUMMARY:
Kotiteertha or temple pond with daily sacraments constitute the vital ecosystem linking the culture with the society in India. The sharp increase of devotees performing rituals within and around the temple premises in the last decade, and lack of regular maintenance of temple pond has led to the eutrophication. Disposal of ritual constituents’ rich in organic matters beyond the bioremediation potential has led to the enrichment of nutrients, evident from the enhanced primary productivity with rampant growth and spread of macrophytes and filamentous algae. This has posed serious threat to the sustenance of pond ecosystem. In this context, ecological investigations were carried out the Gokarna temple pond - Kotiteertha, located at Gokarna village, Kumta taluk, Uttara Kannada district, Karnataka in response to the requests from progressive youth of Gokarna temple town. The investigation included the assessment of physical and chemical parameters with the biotic components (spread and distribution of aquatic plants and algae). The results reveal of higher nutrient levels, signifying more cultural pressure due to (i) disposal of organic materials (flowers, rice balls, linseed, banana, curd, ghee, etc.) after performing rituals, (ii) bathing of large number of devotees, (iii) washing of cloth with detergents, (iv) disposal of solid waste and (iv) washing of utensils, etc. The algal diversity analyses show the proliferation of filamentous cyanophycean members and minimal suspended algae. Higher biomass growth, productivity and spread of aquatic plants Vallisneria spiralis, Nymphae nouchali, followed by Certophyllum demersum and Blyxa species highlight of nutrient enrichment. The overgrowth of such species triggers internal nutrient loading leading to further enrichment and subsequent deterioration of water quality. Therefore following action plans are recommended to regain the sanctity of the pond and to ensure pristine water in the surrounding wells.
Pollution Indicator |
Cause |
Solutions |
|
1. Profuse growth of macrophytes |
Nutrient (Carbon and Nitrogen) enrichment due to
i. The disposal of pooja materials (including rice balls – pinda, flowers, ghee, linseed, etc.) after performing rituals of paying homages to departed souls in a family
ii. Enrichment of nutrients (Phosphorous) – due to Bathing (soap, etc.)
iii. washing of cloth with detergents – introduces phosphorous into the ponds, which help in algal growth
iv. non-maintenance of the pond
v. Transport of silt and seasonal water flow into the pond
vi. Maintaining minimum water flow to the pond and also maintaining groundwater table in the region.
|
Restrictions on the disposal of organic matter (flowers, rice balls –pinda, grains, etc.) after rituals of pithru karma. Alternate arrangements for disposal of organic wastes (flower, rice balls) and an arrangements to transfer to goshala (cattleshed). This could provide nourishment and also serving to milking cattle could be the best option of offering to the lord. As this pond is also used in the temple, restrictions on the use of pond for bathing by the community visiting the temple. Appropriate signage to this effect, would help in controlling the pollutants entering the pond. Only the head priest performing pooja to the deities at Gokarna be allowed to take bath (without synthetic soap) Ban on washing of cloth and utensils in the pond
Regular partial removal of water plants (kalé) twice a year: at least before (a) Ganesha / vinayaka Chaturthi (b) Shivaratri – water plants to be removed by uprooting (about 60% - not complete removal)
Management of watershed – arrest deforestation. Planting of natives species in the catchment /watershed. This could be implemented by introducing the mandatory plant native sapling by the devotees performing rituals (with a nominal fee to cover the sapling cost) |
2. Presence of bad odour |
Enrichment of nutrients especially carbon and nitrogen. Carbon and nitrogen gets into the pond with the prevailing practice of the disposal of pooja materials (flower, banana, linseed, grains, curd, milk, ghee, cloth, etc.) and rice balls (pinda). Due to bioremediation, organisms in the pond uses the organic inputs. However, the excess quantity over threshold creates pollution of the water body, evident from bad odour, excess growth of algae and water plants (macrophytes). |
Restrictions on the disposal of organic matter (flowers, rice balls –pinda, grains, etc.). Alternate arrangements for disposal of organic wastes (flower, rice balls) and an arrangements to transfer to goshala (cattleshed). This could provide nourishment and also serving to milking cattle could be the best option of offering to the lord. Implement bio-manipulation (Labeo rohita and Catla catla– surface phytoplankton feeders, Rohu – Column zooplankton feeder, Gambusia and Guppies – larvivorous fishes for mosquito control, |
3. Algal bloom |
|
Restrictions on the disposal of organic matter (flowers, rice balls –pinda, grains, etc.). Introduce ducks (at least four pairs to begin with) , which will aid in aeration and control of water plants, algae, etc.. Implement bio-manipulation (Labeo rohita and Catla catla– surface phytoplankton feeders, Rohu – Column zooplankton feeder, Gambusia and Guppies – larvivorous fishes for mosquito control, |
4. Health problems (of people using water), turbidity in water and bad odour. |
Presence of Escherichia coli - indicates faecal contamination. This may be due to leakages from the nearby septic tanks (toilets) / community drains or use of pond after defecation. |
Plug cracks and other defects in the pond embankments. Restriction on the use of pond by all except the temple head priest who performs rituals at the temple. |
5. Un-aesthetic waste litters |
Disposal of solid waste and plastic. No provision to dispose the waste at designated site with proper bins (collection containers) |
Awareness among general public to dispose solid waste at the designated sites / locations. |
6. Irrational littering, dumping of debris, pollution of holy pond. |
Lack of personal and community hygiene. |
Environmental education among all. |
7. Silt deposition in the pond and accumulation of heavy metals (in the silt). |
Idol submersion during festival (Ganesha, etc.). |
Environment friendly Ganesha festival celebration in the temple or use of silver Ganesha / Pancha hola idols and worhip at home. |
8. Dumps of large quantity of rice balls and ritual materials in the shallow region of the pond. |
Lack of awareness among priests and also lack of suitable alternate arrangements |
Awareness among priests about the need for environment friendly option of disposal of organic materials of the ritual (as contaminating the pond water would also contaminate the nearby groundwater sources – bore well, open wells, etc.). |
9. Pollution of water body |
Lack of ‘sense of belonging’ among the local residents
No management or poor management by the municipality / Panchayath. |
Environmental awareness among the local residents about the need to protect water bodies (at least keeping the next generation in mind)
|
10. Littering and spitting |
Lack of cultural ethos |
Ensure cultural heritage through awareness programmes |
- INTRODUCTION
Wetlands (ponds, lakes, tanks, etc.) constitute the most productive ecosystems with a wide array of goods and services. These ecosystems serve as life support systems; serve as habitat for a variety of organisms including migratory birds for food and shelter. They aid in bioremediation and hence aptly known as ‘kidneys of the landscape’. Major services include flood control, wastewater treatment, arresting sediment load, drinking water, protein production, and more importantly recharging of aquifers apart from aiding as sinks and climate stabilizers. The wetlands also function as wild fauna sanctuary, with public access. These ecosystems are valuable for education and scientific endeavours due to rich biodiversity.
Harvesting of rainwater through wetlands (ponds, constructed tanks and lakes) is being practiced in India since the time immemorial. These man-made ponds have been used as an alternate source of drinking water and have been employed for domestic and irrigation purposes (Arya et al., 2011; Gupta et al, 2011; Mahapatra et al., 2011a; Mehta, 2013; Ramachandra, 2001; Ramachandra and Rajinikanth, 2005). Temple ponds also referred as kunds/pushkarni/tirtha are created in enclosures of the temple premises to meet the water requirement for rituals in the temple. Efforts to maintain the sanctity of these water bodies were in practice by regular maintenance and also by restricting the use of water for anthropogenic purposes (such as community bathing, washing of cloth, etc.). These ponds in the temple vicinity serves myriads of benefits (groundwater recharge, providing uncontaminated water for rituals, etc.) while maintaining a good microclimate in the locality. These ponds not only act as perennial source of water for temple rituals but also maintains a good water balance in the region (Arya et al, 2011) and conserve the aesthetics of the locality. Sacred forests (with native flora) in the watershed of historic temple ponds ensures water availability during all seasons. Earlier studies in Uttara Kannada and Shimoga districts highlight that forests and water are intrinsically intertwined as forested watersheds have significantly helpful in infiltration of rainfall. The nature of vegetation in the catchment plays vital role in the ground water recharge, runoff and soil moisture conditions, soil erosion and soil quality (Ramachandra et al., 2012; Ray et al., 2014). Historically, the overall forest disturbance in the Western Ghats increased in spatial extent as well as in intensity, during the post World war era, with the emphasis on industrialization and economic development. Forest based industries coupled with large scale hydroelectric projects and conversions of forest land for agriculture have contributed significantly in the decline of primeval forests.
Land use and land cover changes with the unplanned developmental activities in the district coupled with growing demand of land for agriculture and horticulture in the district have further accelerated deforestation. Decline in native forest cover in the watersheds of rivers, lakes and ponds has resulted in the conversion of perennial to seasonal water bodies. This necessitates measures to safeguard the water resources within the auspices of holy places and temples (Mahananda et al, 2010). As the water from these water bodies are meant to be used for rituals, there are ample chances to revive the water quality through community’s active participation in rejuvenation as well as regular maintenance and management.
Gokarna in Uttara Kannada, being one of the pious destination (for pilgrims from various parts of the country) for performing rituals and pay homage to the departed soul/s. These rituals are performed (rituals of pithru karma - Pitrupurusha shraddh) throughout the year closer to the heritage temple pond - Kotiteertha. Subsequently, ritual offerings (comprising of rice balls, flowers, leafs, paddy, banana, linseed, etc.) are disposed in the temple pond. Sustained disposal of large quantity of organic constituents of the ritual offerings (by about 200-250 pilgrim families daily) has enriched the pond with nutrients (C and N). Increased pilgrims and consequent human activities have resulted in deteriorating water quality in the pond comparable to the earlier reports on temple ponds (Pal et al., 2012; Chaurasia and Pandey 2007; Gupta et al, 2011). Also, bathing (with soap) and washing (cloth with detergents) has further enriched the system with nutrients (N and P). Earlier studies in the pond ecosystem have also reported organic enrichment due to bathing (Chaturvedi and Kumar, 2011), and ritual debris (such as flowers, rice, fruits, leafs, coconut shells, saturated and unsaturated fats and occasionally cloth pieces, etc.). This is amplified with the unscientific waste disposal practices, sanitary liquid wastes (Raju et al., 2011) with the lifestyle changes of the residents and pilgrims (use of plastics, etc.) in the immediate vicinity.
Investigations of physico-chemical with biological parameters provide vital insights to the nutrient enrichment and also the trophic status (Ramachandra et al., 2001; 2003; 2005; 2007; 2009; 2015; Sharma et al, 2009; Mahapatra et al., 2011a,b,c; 2013). However, the influence of meteorological conditions and catchment integrity (Arya et al, 2011) also influences the water quality. Investigations of abundance and the distribution of biota aid in assessing the agents of water quality deterioration (Mahapatra et al., 2011). In this context, investigations were carried out by collecting water and biotic samples from the temple pond, to assess
- water quality (physical and chemical parameters) of the temple pond
- assessment of the nutrient status,
- the extent and distribution of aquatic macrophytes in the pond
- analyse the algal communities through sampling at select locations
- suggest appropriate measures to safeguard the water quality of the lake
- MATERIALS AND METHODS
2.1 Study area
Gokarna temple pond - Kotiteertha is located in Gokarna of Kumta taluk, in Uttara Kannada, Karnataka spans at 14ᵒ32’27.55’ to 14ᵒ32’31.62” N and 74ᵒ19’10.60” to 74ᵒ19’17.85” E (Figure 1). Figures 2.1 and 2.2 show the inlet and outlet of the temple pond. Many locations in the shoreline of the pond are earmarked for conducting rituals of pithru karma for paying homage to forefathers or pitrupurushas.
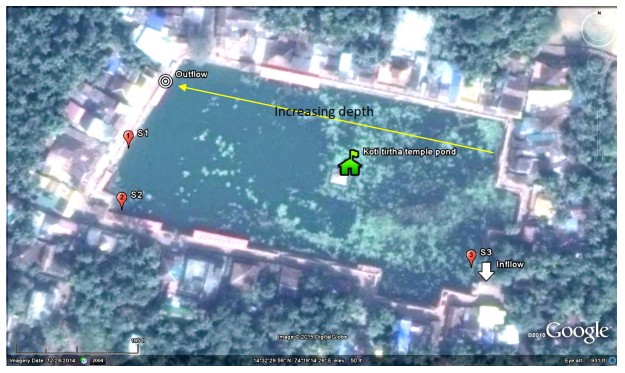
Figure 1. Kotiteertha pond

Figure 2.1) Inflow to the pond(east) and 2.2) Outflow from the pond

Figure 2.3 Temporal changes in surface cover of the temple pond from 2004-14
The pond (170m X 90m) has an area of ~1.53 hectares. The depth of the pond varies from ~5 m (at west) to 1.5 m (on eastern side). The estimated volume of the temple pond is >50,000 m3. The temple pond is surrounded by priest houses. The architecture of the temple pond highlight of historical design. Such deeper pond were constructed to store rainwater for meeting the water demand throughout the year. Several locations were earmarked on the bank of the pond, for performing rituals of pithru karma - Pitrupurusha shraddh, paying homage to the departed ancestor souls. During the field investigations on 27th September, it was observed (i) large number of devotees taking bath, ii) washing cloth with detergents, iii) washing utensils and iv) disposing organic matter (flowers, rice balls –pinda, grains, etc.) after rituals of pithru karma in the pond. Estimates indicate on an average about 200-250 families perform rituals of pithru karma every day. This means about 750 to 1000 gms of rice and other organic matters are disposed in the pond. These constitutes are rich in C (crabon) and N (nitrogen) and sustained disposal has enriched the pond with nutrients evident from the growth of filamentous algae and macrophytes. Figure 2.3 depicts further substantiates the temporal changes during 2004 to 2014, with the earlier events of siltation, algal blooms (2004, 2010 and 2011) and progressive increase in macrophytes cover (2013 onwards).
- 2.2 Site survey and discussions with the local community

Figure 2.4. Site visits and discussions with the temple priest communities
Field investigations were carried out on 27th September 2015 in response to the requests by the local community and the information of foul odour and the proliferation of macro algae and macrophytes. The filed investigations included (i) assessment of the present status of the pond (Figure 2.4 and 2.5), (ii) water sample collections from various locations (inlet and outlet) of the pond, (iii) collection of biotic components – macrophytes and algae, (iv) assessment of the spread and diversity of biotic components and (v) discussion with the local community to understand (a) causal factors of water quality changes and (b) the social perspective of the pond.
- 2.3 Water sampling and analysis
Water samples were collected from the pond at three representative locations based on depth and also extent of organic debris in the pond (Figure 2.5).
Hand held GPS was used for recording the geographical coordinates of sampling locations and also to map the boundary of the pond. Field investigations were carried out to find out the status of the pond and also the sources of contamination (if any). Water samples were collected in disinfected one litre sampling bottles. These bottles were thoroughly washed and rinsed with deionised water. Grab sampling was followed at all points. On-site estimation parameters include pH (pH probe), water temperature (temperature probe), Total Dissolved Solids (TDS) (TDS probe), salinity (salinity probe), conductivity (conductivity probe), dissolved oxygen (iodometry), Oxidation Reduction Potential (ORP) and transparency (visual observations). The samples were then carried to the lab and were analysed for other parameters according to Standard protocol (APHA AWWA WEF, 2000). Water samples were analysed for turbidity (turbidometer), total alkalinity (titrimetry), total hardness, Ca, Mg (complexometric titration), Na, K (flame photometer), chlorides (argentometric method), nitrates (phenol disulphonic acid method), phosphates (stannous chloride method), chemical oxygen demand (dichromate oxidation with open reflux) and BOD (5-d BOD).

Figure 2.5: Sampling locations a)-b) Site-01; c)-d) Site-02; e)-f) Site-03 and g)-h) On-site analysis of physic-chemical parameters at the sampling locations
- 2.4 Macrophyte sampling and analysis
Aquatic plants were collected from 11 different places apart from the three water sampling locations. These plants were identified based on morphological keys and published literature on flora (Cook, 1996). An aerial view of the pond using photographic camera at select elevated locations aided in assessing the extent of aquatic plant cover and their relative abundance. Samples were collected from the areas closer to the embankments (Figure 2.6.a) and specimen were transferred in the sealed polybags for further identification and analysis (Figure 2.6.b).

Figure 2.6 a) Macrophyte collection and b) Macrophyte collected in sealed polybags
- 2.5 Algal sampling and analysis: Macro algal samples were collected from the select locations and were placed gently in sealed polybags for further identification. The micro algal samples were concentrated from the water samples by centrifuging ~10 ml of the water samples. The pellet was observed under light microscope (40 X and 100 X) after mild dilution. The identification and enumeration was carried out using standard keys for identification of freshwater algae.
- Results and Discussions
- 3.1 Water Quality Analysis
- 3.1.1 Dissolved Oxygen: Dissolved oxygen (DO) is the most essential parameter in the aquatic ecosystems as it helps in the aquatic respiration as well as detoxification of complex organic and inorganic mater through oxidation. However, the sustained disposal of organic materials after rituals can impose a very high oxygen demand leading to oxygen depletion with severe impacts on the water ecosystem. The temple debris and the left out matter from the cultural practices can impart significant amount of oils, volatile organics, nutrients and solids in the pond. The DO of the analysed water samples varied between 2.7 (site 1) to 11.3 ppm (site 3). The higher variations of DO especially lower DO values are indicative of fast oxidising chemicals/bacterial activity in the ecosystem. Lower values of DO highlight the impact of disposing organic materials (such as rice balls, flower, etc.) in the pond. The shallower region (site-3) showed the highest DO levels indicating less abuse of the place compared to other sites.
- 3.1.2 Total Dissolved Solids (TDS): TDS affect the water quality in many ways impacting the water usage for domestic (drinking, cleaning, bathing, etc.). Total dissolved solids originate from organic sources such as leaves, silt, plankton, organic debris and also from sewage. Other sources come from runoff from nearby areas (APHA, 2000). Surface as well as groundwater with high dissolved solids are of inferior flavour and induce an unfavourable physiological reaction to the dependent population. TDS values in the samples analyzed, ranged from 38.5 to 41 ppm across all locations. It was higher at site 2, where disposal of organic materials is higher. TDS was comparatively high at locations with higher human activities such as washing of utensils, disposing rituals organic materials (rice balls, etc.).
- 3.1.3 pH: pH is a numerical expression that indicates the degree to which water is acidic or alkaline, with the lower pH value tends to make water corrosive and higher pH has negative impact on skin and eyes. pH value of water samples of the pond are acidic and ranged from 5.8 – 6.1. A slightly lower pH value can be attributed to either the humic and fulvic acids (high detrital matter) or due to the volatile organic acids emanating from the decomposing and semi decomposed organic materials (of rituals). Also, aquatic macrophytes that were cut were left in the shallow region of the pond and with decomposition it is emanating low molecular weight organic acids.
- 3.1.4 Turbidity: Turbidity indicates the amount of suspended matter that are of a colloidal dimension. All water samples showed a very low turbidity indicating no suspended micro algae. The values ranged from 1.69 (site 3) to 4.81 NTU (site 2). Slightly higher values at site 2 is due to the disposal of organic materials after ritual.
- 3.1.5 Chlorides: Chlorides are essentially potential anionic radical and excess of chlorides leads to the formation of potentially carcinogenic and chloro-organic compounds. Chloride values in samples ranged from a mere 22.72 – 24.14 ppm. Relatively higher values were observed in sites 2 and 3. It may be due to washing (cloth and utensils) with bleaching powder or chloride detergents.
- 3.1.6 Sodium: Sodium (Na) is one of the essential cations that stimulate various physiological processes and functioning of nervous system, excretory system and membrane transport in animals and humans. Increase of sodium ions has a negative impact on blood circulation, nervous coordination, hence affecting the hygiene and health of the nearby localities. The concentration of sodium in the pond water samples were low and ranged from 21.6 (site 2 and 3) to 22.8 ppm (at the deeper reaches, site 1). Sodium values were significantly correlated with chlorides (r= 0.99; p<0.0001) and Ca Hardness ((r= 0.99; p<0.0001).
- 3.1.7 Potassium: Potassium (K) is an essential element for both plant and animal nutrition, and occurs in ground waters as a result of mineral dissolution, decomposing of plant materials and also from agricultural runoff. Potassium ions in the plant root systems helps in the cation exchange capacity to transfer essential cations like Ca and Mg from the soil systems into the vascular systems in the plants in replacement with the potassium ions (Mahapatra et al., 2011a). Incidence of higher potassium levels in soil system affects the solute transfer (active and passive) through the vascular conducting elements to the different parts of the plants. The potassium content in the water samples of Gokarna tank ranges between 3.2-4.8 ppm. Higher potassium values were at disturbed regions (site 2) due to the sustained disposal of organic materials (of rituals).
- 3.1.8 Alkalinity: Alkalinity is a measure of the buffering capacity of water contributed by the dynamic equilibrium between carbonic acid, bicarbonates and carbonates in the water. Sometimes excess of hydroxyl ions, phosphate, and organic acids in water causes alkalinity. High alkalinity imparts bitter taste. The acceptable limit of alkalinity is 200 ppm. The alkalinity of the samples ranges from 64 – 76 ppm. A comparatively higher value was observed in site 2 owing to the disposal of organic materials (rice balls, flowers, etc.) after performing rituals.
- 3.1.9 Total hardness: Hardness is the measure of dissolved minerals that decides the utility of water for domestic purposes. Hardness is mainly due to the presence of carbonates and bicarbonates. It is also caused by variety of dissolved polyvalent metallic ions predominantly calcium and magnesium cation although, other cations like barium, iron, manganese, strontium and zinc also contribute. In the present study, the total hardness ranged between 22 to 24 ppm. It was relatively high at site 2. Hardness is significantly correlated with the EC values (r= 0.9; p<0.05).
- 3.1.10 Calcium hardness: Calcium (Ca) is one of the major macro nutrients which are needed for the growth, development and reproduction of both plants and animals. The presence of Ca in water is mainly due to its passage through deposits of limestone, dolomite, gypsum and other gypsiferous materials (APHA, 2000). It contributes to the total hardness of the water. Ca hardness concentration in the samples analysed varied from 3.74 to 4.81 ppm.
- 3.1.11 Magnesium hardness: Magnesium (Mg) in one of the most essential macro nutrients that helps as a co-factor in the enzyme systems and in the central metal ions that constitutes the chlorophyll molecule essential for plant photosynthesis. According to WHO guidelines the maximum admissible limit is 50 ppm. In this study the concentration of magnesium hardness ranged from 4.18-4.66 ppm. Mg hardness was significantly correlated with turbidity (r= -0.9; p<0.05).
- 3.1.12 Nutrients (nitrates and phosphates): Nutrients essentially comprise of various forms of N and P which readily dissolve in solutions that are uptaken in the form of inorganic mineral ions by plant root systems through microbes. Accumulation of N (as nitrates) and P (as inorganic P) in aquatic ecosystems leads to higher net productivity causes with significant water quality problems. Sustained input of organic materials (of rituals consisting of large quantity of rice balls, banana, flower, etc.) beyond the supportive and assimilative capacities of the pond has resulted in nutrient enrichment and profuse growth of invasive floating plant species. Together with phosphorus, nitrates in excess amounts in streams and other surface waters can accelerate aquatic plant growth causing rapid oxygen depletion or eutrophication in the water. Nitrates at high concentrations (10 mg/l or higher) in surface and groundwater used for human consumption are particularly toxic to young children affecting the oxygen carrying capacity of blood cells ( RBC) causing cyanosis (methemoglobinemia). In the present study, nitrate values ranged from 0.15 to 0.23 ppm. Nitrate values were significantly correlated with TDS (r= -0.9; p<0.05). The phosphate values ranged between 0.2 to 0.4 ppm. Ponds with phosphate values >0.02 ppm have been reported as eutrophic in nature.
- 3.1.13 BOD and COD: BOD and COD are important parameters that indicate contamination with organic wastes. Biochemical oxygen demand (BOD) is the amount of oxygen required by bacteria while stabilizing decomposable organic matter under aerobic. These parameters help to assess the extent of pollution of surface and ground water where contamination occurred due to the organic inputs (of rituals) to the pond. Figure 3.1 indicates the type of activities around the pond.

Figure 3.1 a) organic materials (rice balls, etc.); b) washing of utensils in the pond
Chemical oxygen demand (COD) determines the oxygen required for chemical oxidation of most organic matter and oxidisable inorganic substances with the help of strong chemical oxidant. In conjunction with the BOD, the COD test is helpful in indicating toxic conditions and the presence of biologically resistant organic substances (Sawyer and McCarty 1978). In this study the BOD values ranged from 4.07-8.13 ppm. BOD values were negatively correlated with EC values (r= -0.9; p<0.05). The COD values ranged from 12 to 24 ppm. The COD were relatively high at site 2. The ORP values indicate highly oxidising conditions in the temple pond. The physicochemical analysis of the select sampling sites is elucidated in Table 3.1.
Table 3.1 Physicochemical parameters of water samples from select sampling locations
Parameters \ Sampling Locations |
Site-01 |
Site-02 |
Site-03 |
Sam-Label
|
867 |
868 |
869 |
Time
|
11:00 |
11:40 |
12:30 |
Latitude (ᵒN)
|
14.54156 |
14.54139 |
14.54119 |
Longitude (ᵒE)
|
74.31965 |
74.31973 |
74.32137 |
Altitude (meter above msl)
|
7 |
6 |
1 |
pH
|
5.9 |
5.8 |
6.1 |
Temp (ᵒC)
|
33 |
35.6 |
31.3 |
TDS (ppm)
|
38.5 |
41 |
38.8 |
Sal (ppm) |
27.8 |
30.7 |
28.7 |
EC (mic.S/cm) |
55.6 |
58 |
55.5 |
ORP (mV)
|
158 |
164 |
166 |
Turbidity (NTU)
|
3.45 |
4.81 |
1.69 |
DO (ppm)
|
2.70 |
5.00 |
11.3 |
BOD (mg/l)
|
8.13 |
4.07 |
8.13 |
COD (mg/l)
|
16 |
24 |
12 |
Alkalinity (mg/l)
|
68 |
76 |
64 |
Chloride (mg/l)
|
22.72 |
24.14 |
24.14 |
Total Hardness (mg/l)
|
22 |
24 |
22 |
Ca Hardness (mg/l)
|
3.74 |
4.81 |
4.81 |
Mg Hardness (mg/l)
|
4.44 |
4.66 |
4.18 |
Phosphate (mg/l)
|
0.211 |
0.243 |
0.41 |
Nitrate (mg/l)
|
0.23 |
0.146 |
0.217 |
Sodium (mg/l)
|
21.6 |
22.8 |
22.8 |
Potassium (mg/l)
|
3.2 |
4.8 |
4 |
- 3.2 Macrophyte diversity and abundance
During the site visit it was observed that ~70 % of the temple pond surface was covered by macrophytes and to minor extent filamentous algae. The extent and spread of macrophytes was higher in the shallower regions of the pond. Eleven macrophytes species (Table3.2) were recorded during the field investigation.
Table 3.2: Macrophytes of Gokarna temple pond
No. |
Macrophytes |
|
1 |
Valisneria spiralis |
|
2 |
Nymphae nouchali |
|
3 |
Nymphae pubescens |
|
4 |
Ceratophylum demersum |
|
5 |
Limnophylla heterophylla |
|
6 |
Limnophylla aromatica |
|
7 |
Rotala macranadra |
|
8 |
Ceraptoteris thalictroides |
|
9 |
Utricularia gibba |
|
10 |
Blyxa aubertii |
|
11 |
Nymphoides sp. |
|
Macrophytes observed in the pond are i) submerged floating leaf type e.g. water lily etc., and ii) submerged e.g. Ceratophyllum, Blyxa, Valisneria. Macrophytes specimen were collected through opportunistic sampling from accessible parts of the pond. Most of the samples were collected either by hand or with the help of a wooden pole. Macrophytes near the shallow regions were trapped between filamentous cyanophycean members as Oscillatoria and Pseudoanabaena.
The proliferation of macrophytes in the ponds highlights of nutrient enrichment and internal recirculation of nutrients especially P from the dead and decaying plant matter, organic inputs of rituals and nutrient laden sediments. Deposition and decay of organic matter has contributed to the foul odour. Higher macrophyte density also indicates of increased primary productivity due to nutrient enrichment both from autochthonous and allochthonous sources, which eventually leads to conversion of ponds into swamps and marshes. No emergent macrophytes were recorded during the site inspection. Macrophytes recorded at sampling locations are
- Site 1, corresponding to deeper part of the pond comprised of Nymphae and Limnophylla species.
- Site 2, mainly comprised of Lymnophylla, Nymphae and Nymphoides species and
- Site 3 with the higher macrophyte diversity (Figure 3.2c and d). Water lily is the dominant macrophytes in the deeper reaches where as plants like Valisneria and Ceratophyllum along with that Nymphae, Limnophylla and Blyxa species were dominant in the shallower reaches.
- ALGAL ANALYSIS
- Site 1 recorded the highest diversity (>16 species) and majority were green algae (10). In all the three sites the chlorophycean members dominated over the members of other classes. These include Cosmarium spp., Kirchneriella spp., and Staurastrum spp.
- Site 2 has higher diversity of the euglenophycean members and the bacillariophycean members, attributable to the presence of higher organic matters that correlates with the higher COD and TDS values. Abundance of eugleophytes is an indication of the nutrient enrichment with the input of higher organic matter. These include chrolophycean members the Staurastrum spp.
- Site 3 with higher DO values has more than 12 algal sp. and were dominated by Xanthidium sp. (which are absent in the other sampling sites).
- RECOMMENDATIONS
- Regular partial removal of water plants (kalé) twice a year: at least before (a) Ganesha / vinayaka Chaturthi (b) Shivaratri – water plants to be removed by uprooting (about 60% - not complete removal). A community program for regular harvesting of pond macrophytes mostly the floating macrophytes will ensure adequate sunlight penetration that is helpful for the growth of phytoplankton’s and submerged macrophytes and also helps in decontamination through destruction of pathogens i.e. harmful bacteria. Moreover free pond surface is highly required for gaseous exchange and diffusion of oxygen into the pond systems that is essential for the survival of pond biota like fishes.
- De-silting and sludge removal of the pond once in 5 years during the dry periods will help in checking the sedimentary P deposition and P flux that buffers the phosphate levels in the overlaying water.
- Regular water quality monitoring by involving school and college students (co-ordinated by the regional centre of the Karnataka State Pollution Control Board)
- Setting up eco-clubs in the education institutions (as per the provisions available with the Karnataka State Pollution Control Board) in the local education institutions (schools and colleges)
- Regular maintenance of the pond (through cost effective ecological methods) involving local NGO’s, NSS volunteers, students from schools and colleges - Mandatory Shramdhan for removal of silt as well as partial removal of water plants (kalé)
- Safeguard cultural heritage through the community awareness programmes (street play, painting competition among school kids, essay and debate competition among college students, cultural programmes, etc.)
- ‘HERITAGE TOWN’ status to GOKARNA considering the cultural heritage of the region. This entails environment management focussing on waste management, prevention of littering on roads, construction of public toilets (pay and use), construction of community bathrooms, arrangements for disposal of organic fraction (flowers, rice balls–pinda, grains, banana, etc.) of pithru karma rituals. Alternate arrangements for disposal of these organic wastes (flower, rice balls, etc.) and an arrangements to transfer these organic constituents to goshala (cattleshed). This could provide nourishment and also serving to milking cattle could be the best option of offering to the lord.
- Management of watershed – arrest deforestation -Planting of natives species in the catchment /watershed. This could be implemented by introducing the mandatory plant native sapling by the devotees performing rituals (with a nominal fee to cover the sapling cost).
- Celebration of the World Environment Day on June 5th, every year through large scale afforestation programme involving NSS volunteers, devotees visiting the temple (on June 5th), students (from nearby schools and gurukula) and local NGO’s.
- Provision of wetlands at the entry of the pond (which will remove the contaminants entering the pond) during run-off
- Implement bio-manipulation (Labeo rohita and Catla catla– surface phytoplankton feeders, Rohu – Column zooplankton feeder, Gambusia and Guppies – larvivorous fishes for mosquito control, bottom dwellers – common carp, scavenging fish – Labeo fimbriatus).
- Restrictions on the disposal of organic matter (flowers, rice balls –pinda, grains, etc.).
- Introduce ducks (at least four pairs to begin with), which will aid in aeration and control of water plants, algae, etc. Introduction of water fouls (such as duck and coots) will also help in regulating the macrophytes to lower extent and at the same time providing surface aeration. This will activate the food web in the pond and will ensure recycle of the nutrients across the food chain.
- Plug cracks and other defects in the pond embankments. Timely management of the cracks and the fractures of the embankments will help in stopping waste water entering the pond.
- Restrictions on the disposal of organic matter (flowers, rice balls –pinda, grains, etc.) after rituals of pithru karma.
- Construction of ‘PINDA KALYANI’ - Alternate arrangements for disposal of organic wastes (flower, rice balls) and an arrangements to transfer to goshala (cattle shed). This could provide nourishment and also serving to milking cattle could be the best option of offering to the lord.
- Awareness among priests about the need for environment friendly option of disposal of organic materials of the ritual (as contaminating the pond water would also contaminate the nearby groundwater sources – bore well, open wells, etc.).
- As this pond is also used in the temple, restrictions on the use of pond for bathing by the community visiting the temple. Appropriate signage to this effect, would help in controlling the pollutants entering the pond.
- Only the head priest performing pooja to the deities at Gokarna be allowed to take bath (without synthetic soap).
- Ban on washing of cloth and utensils in the pond.
- Deployment of NSS volunteers and Local NGO’s in raising the awareness among the public.
- Environment friendly Ganesha festival celebration in the temple or use of silver Ganesha / Pancha loha idols and worship at home.
- Complete ban on immersion of idols (painted, plaster of paris idols, etc.) in the pond. This will help in avoiding the influx of heavy metals and other contaminants getting into the temple pond.
- Environmental awareness among the local residents about the need to protect water bodies (at least keeping the next generation in mind).
Decomposed plant debris (uprooted and dead Valisneria species) and organic inputs (of ritual) have contributed to foul odour as well as lowering of DO that might eventually harm the entire aquatic biota. The large floating leafs of lily provides aesthetic looks to the pond but at the same time their over growth acts as an obstacle in light penetration, that is detrimental to the submerged algae or macrophyte community and this ultimately results in reduction of the overall dissolved oxygen creating partial anoxia in deeper zones.
During the field investigations, it was observed that Valisneria spiralis domination were mainly confined to the littoral zone (1.5–2.0 m), which had higher transparency and also observed at higher depth (>3 m) locations. This indicates its potential in stabilising the suspended particles and making the water clear. Such type of tropical pond systems with submerged plants like Valisneria spiralis have the potential to maintain clarity of the water through out the year. These submerged species restricted suspended algal growth, even though the nutrients levels and the organic loads were slightly high. Higher phosphates (0.2 – 0.4 mg/l; i.e. > 0.02 mg/l) value indicates an eutrophic status of the lake. Figure 3.2 depicts the extent and spread of macrophytes communities in the temple pond. Figure 3.3 elucidates sampling location wise, the percentage composition/distribution of macrophytes in the lake. Macrophyte specimen collected are depicted in figure 3.4.

Figure 3.2 a)-d): distribution and types of macrophytes in various sampling lications and
e)-f): the extent of macrophyte proliferation in the temple pond
g) Nemachilichthys ruppelli and h) Common carp – fishes found in temple pond
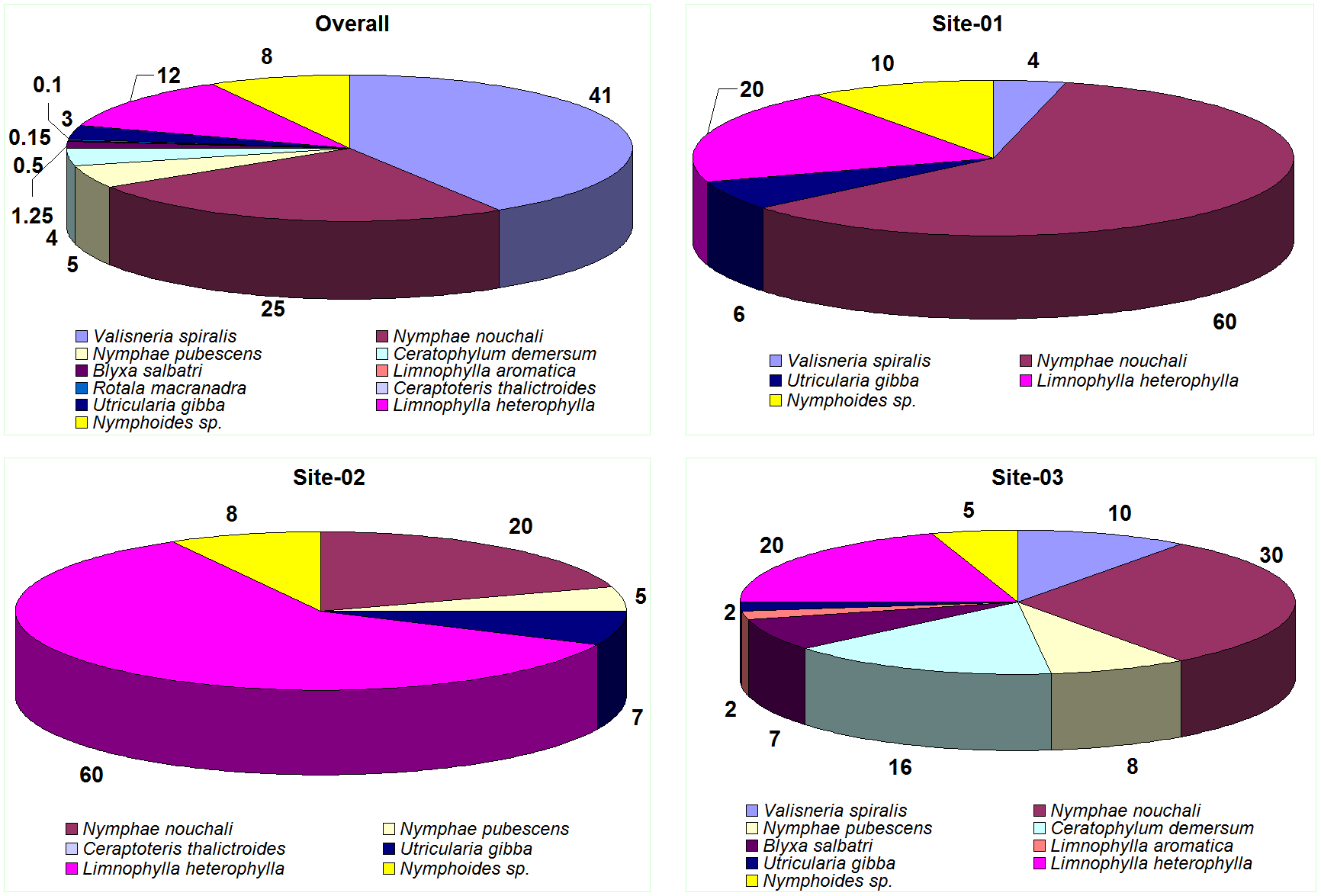
Figure 3.3 a) percentage cover of macrophyte species in pond
b)-d) percentage composition of macrophytes at sampling locations (Sites 1-3)

Figure 3.4: Macrophyte specimen - a) Ceratophylum demersum b) Ceraptoteris thalictroides c) Valisneria spiralis d) Rotala macranadra e) Limnophylla heterophylla f) Limnophylla aromatica g) Nymphoides sp. h) Nymphae nouchali
Analysis of macrophytes samples with the help of standard morphological keys indicate the presence of 24 algal species (Table 3.3) belonging to four taxonomic divisions, Cyanobacteria (1), Bacillariophyta (6), Chlorophyta (14), and Euglenophyta (3). Sampling location wise, algal species are:
Apart from these macro algal species were also identified and analysed (figure 3.5-3.7). Figure 3.8 elucidates the composition - green algae (~58 %) diatoms (25 %) > euglenophytes (13 %) > blue green algae (4 %) during the study. Figure 3.9 provides select algal microscopic illustrations.
Filamentous macro algae in the peripheries of the pond were Oscillatoria sp. This species were mostly found along with organic debris and sludge masses floating on the top trapping air bubbles and are the most common bloom forming algae in the nutrient enriched waters. Most interestingly these species were also observed entangling the aquatic hydrophytes like Vallisneria spiralis, due to which the long leafs of the plant were bundled together, obstructing sunlight that inhibit photosynthesis. Further more the submerged aquatic plants also uptake nutrients through leafs, and the presence of dominant cyanophycean members such as Oscillatoria hampers the growth and productivity. Pseudoanaebaena species were also observed associated with some of the Oscillatoria clumps near the shore lines.

Figure 3.5: Filamentous Cyanophyceae attachments with Valisneria sp.
The surface of the pond in the deeper regions were mostly occupied by a composite of green algae Spirogyra sp. (known as pond silk), water lily with intermittent Limnophylla sp. This forms a network like structure floating on the top of the pond over which small Utricularia sp. flowers were observed. This mesh restricts the penetration of sunlight and might also limit air diffusion creating problem to the fish and aquatic biota (Figure 3.7).

Figure 3.6: Filamentous algae in the temple pond

Figure 3.7: Network of filamentous algae with macrophytes trapping organics
High percentage composition of Cyanophyceae is indicative of eutrophic water (Lund 1965). Percentage contribution of Cyanophyceae in Kotiteertha pond shows that the nutrient levels are low and this could be an oligotrophic state. Dominance of algal genera like Euglena, Navicula, Nitzschia, Microcystis, Oscillatoria and Scenedesmus are indicative of organically pollution in waters, supported (Palmer, 1969). However the present study recorded abundant Oscillatoria members indicative of organic pollution. The epiphytic and epilithic algae may form excellent indicators of water pollution (Vyas and Kumar, 1968). In the present study the occurrence of spirogyra (mostly epilithic) and Oscillatoria (mostly epiphytic) were observed. Ramachandra et al., 2015 reported that Microcystis aeruginosa can be used as the best single indicator of pollution. However in the present study Microcystis sp. were not observed.
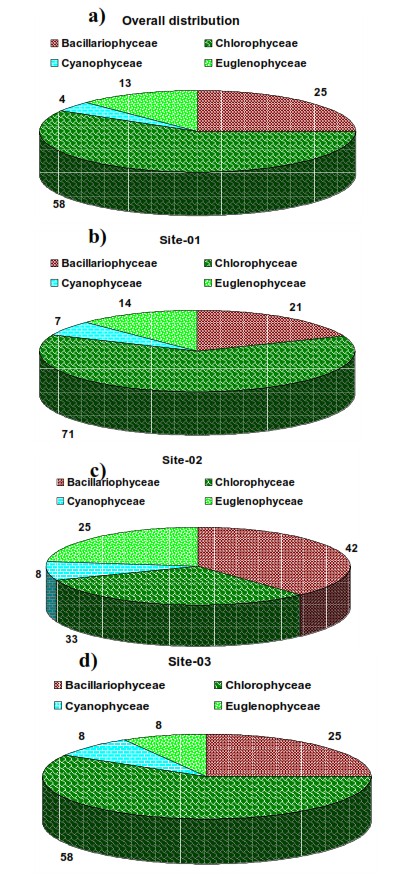
Figure 3.8: Algal composition a) Overall, b) Site 1, c) Site 2 and d) Site 3
Figure 3.9: Microscopic images of some selected algae found in various sites of the temple pond
Table 3.3: Algal species of Gokarna temple pond, Kotiteertha
Sl.no. |
Phytoplankton |
Sites |
||
I |
Chlorophyceae |
S1 |
S2 |
S3 |
1 |
Ankistrodesmus sp.
|
+ |
- |
+ |
2 |
Coelastrum sp.
|
- |
- |
+ |
3 |
Cosmarium spp.
|
++ |
++ |
+ |
4 |
Dimorphococcus sp.
|
+ |
- |
- |
5 |
Gloeocystis sp.
|
+ |
- |
- |
6 |
Kirchneriella spp.
|
++ |
- |
+ |
7 |
Nephrocytium sp.
|
+ |
- |
- |
8 |
Pleurotaenium sp.
|
- |
- |
+ |
9 |
Scenedesmus sp.
|
- |
+ |
- |
10 |
Selenastrum sp.
|
+ |
- |
- |
11 |
Staurastrum spp.
|
++ |
++ |
+ |
12 |
Staurodesmus sp.
|
+ |
- |
- |
13 |
Westella sp.
|
+ |
+ |
- |
14 |
Xanthidium spp.
|
- |
- |
++ |
II |
Cyanophyceae |
|||
1 |
Oscillatoria sp.
|
+ |
+ |
+ |
III |
Euglenophyceae |
|||
1 |
Euglena sp.
|
+ |
+ |
- |
2 |
Phacus sp.
|
+ |
+ |
- |
3 |
Trachelomonas sp.
|
- |
+ |
+ |
IV |
Bacillariophyceae |
|||
1 |
Fragilaria sp. |
- |
+ |
- |
2 |
Gomphonema sp. |
+ |
+ |
+ |
3 |
Melosira sp.
|
+ |
+ |
- |
4 |
Navicula sp.
|
- |
- |
+ |
5 |
Nitzschia sp.
|
+ |
+ |
- |
6 |
Pinnularia spp.
|
- |
+ |
+ |
Field investigation, water and biological sample collection and analyses reveal of higher nutrient levels, signifying more cultural pressure due to (i) disposal of organic materials (flowers, rice balls, linseed, banana, curd, ghee, etc.) after performing rituals, (ii) bathing of large number of devotees, (iii) washing of cloth with detergents, (iv) disposal of solid waste and (iv) washing of utensils, etc. The algal diversity analyses show the proliferation of filamentous cyanophycean members and minimal suspended algae. Higher biomass growth, productivity and spread of aquatic plants Vallisneria spiralis, Nymphae nouchali, followed by Certophyllum demersum and Blyxa species highlight of nutrient enrichment. The overgrowth of such species triggers internal nutrient loading leading to further enrichment and subsequent deterioration of water quality. Therefore following action plans are recommended to regain the sanctity of the pond and to ensure pristine water in the surrounding wells.
REGULAR MAINTENANCE:
REGULAR MONITORING
SUSTAINABLE MANAGEMENT:
6.0 REFERENCES
Arya S, Kumar V, Raikwa M, Dhaka A and Minakshi. (2011), Physico-chemical Analysis of Selected Surface Water Samples of Laxmi Tal (Pond) inJhansi City, UP, Bundelkhand Region, Central India, J. Exp. Sci. 2,8: 1-06.
APHA. (2000), Standard methods for the Examination of water and waste water, 10th Edition, American Public Health Association, Washington DC.
C.D.K. Cook, Aquatic and Wetland Plants in India(Oxford University Press, Oxford, 1996).
Chaturvedi V and Kumar A. (2011), Diversity of culturable sodium dodecyl sulfate (SDS) degrading bacteria isolated from detergent contaminated ponds situated in Varanasi city, India, Intel. Biodeterioration Biodegradation, 65: 961-971.
Chaurashiya M and Pandey GC. (2007), Study of Physico-chemical Characteristic of Some Water Ponds of Ayodhya- Faizabad, Indian J. Environ. Prot. 27, 11:1019-1029.
Gupta AK, Mishra K, Kumar P, Singh C and Srivastava S. (2011). Impact of religious activities on the water characteristics of prominent ponds at Varanasi (UP.), India Plant Arch., 11,1: 297-300.
Lund JWG (1965) The ecology of fresh water phytoplanktons. Biol Rev 40:231–293
Mahananda MR, Mohanty BP and Behera NR. (2010), Physico-chemical analysis of surface and ground water of Bargarh District, Orissa, India, Intel. J. Res. Rev. Appl.Sci., 2, 3.
Mahapatra DM, Chanakya HN and Ramachandra TV. (2011a). Assessment of Treatment capabilities of Varthur Lake, Bangalore. International Journal for Environment, Technology and Management. UNESCO. 14, 1-4:84-102.
Mahapatra DM, Chanakya HN and Ramachandra TV. (2011b). C:N ratio of Sediments in a sewage fed Urban Lake. International Journal of Geology. 5,3:86-92.
Mahapatra DM, Chanakya HN and Ramachandra TV. (2011c). Role of Macrophytes in urban sewage fed lakes. Institute of Integrative Omics and Applied Biotechnology. 2,7: 1-9.
Mahapatra DM, Chanakya HN, Ramachandra TV (2013). Treatment efficacy of Algae based sewage treatment plants. Environmental Monitoring and Assessment
Mehta P. (2013), Alteration in water quality parameters and consequential impacts due to festival waste in jodhpur, Intl. J. Sci. Tech. 17,1: 1166-1176.
Pal A, Sinha DC, and Rastogi N. (2012), Gerris spinolaeLethierry and Severin (Hemiptera: Gerridae) and Brachydeutera longipesHendel (Diptera: Ephydridae): Two Effective Insect Bioindicators to Monitor Pollution in some Tropical Freshwater Ponds under Anthropogenic Stress, Hindawi publishing corporation psyche, Res Article, 818490, pp. 10
Palmer CM (1969) A composite rating of algae tolerating organic pollution. J Phycol 5:78–82
Raju NJ , Shukla UK and Ram P. (2011), Hydrogeochemistry for the assessment of groundwater quality in Varanasi: a fast-urbanizing center in Uttar Pradesh, India, Environ. Monit. Assess. 173, 279–300.
Rajashhri Ray, M D Subhash Chandran, Ramachandra. T.V, Hydrological importance of sacred forest fragments in Central Western Ghats of India, Tropical Ecology, Volume 56(1) 2014, 87-99
Ramachandra TV and Ahalya N. (2001). Essentials of Limnology and Geographical Information System (GIS). Energy and Wetlands Research Group, Center for Ecological Sciences, Indian Institute of Science, Bangalore.
Ramachandra TV and Solanki M (2007), Ecological assessment of lentic water bodies of Bangalore. Technical Report 25, CES, Bangalore.
Ramachandra TV, Ahalya N, and Payne M, (2003).Status of Varthur lake: Opportunities for restoration and sustainable management. Technical report 102. Centre for Ecological Sciences, Indian Institute of Science, Bangalore,
Ramachandra TV, Asulabha K S, Sincy V, Vinay S, Sudarshan P. Bhat and Bharath H Aithal, (2015). Sankey Lake: Waiting for an immediate sensible action, ENVIS Technical Report 74, CES, Indian Institute of Science, Bangalore 560012.
Ramachandra TV. (2005) Conservation, restoration and management of aquatic ecosystems, In Aquatic Ecosystems - Conservation, Restoration and Management, Ramachandra, Ahalya N. and Rajasekara Murthy (ed.), Capital Publishing Company, New Delhi.
Ramachandra, T. V., 2001, Restoration and management strategies of wetlands in developing countries. Electronic Green Journal, 1(15).
Ramachandra, T. V., and Rajinikanth, R., (2005), Economic valuation of wetlands. Journal of Environmental Biology, 26, 3: 439-447.
Ramachandra T V, Subash Chandran M D, Ananth Ashisar, Rao G R, Bharath Settur, Bharath H Aithal, Sreekanth Naik, Prakash Mesta, 2012, Tragedy of the Kan Sacred Forests of Shimoga District: Need for Urgent Policy Interventions for Conservation, CES Technical Report 128, Centre for Ecological Sciences, Indian Institute of Science, Bangalore
Sharma KK, Shvetambri PVerma and Sharma SP . (2009), Physico-chemical assessment of three freshwater ponds of Jammu (J&K), Curr. World Environ., 4, 2: 367-373.
Vyas LN, Kumar HD (1968) Studies on phytoplankton and other algal of India Sagar Tank, Udaipur, India. Hydrobiologia 31:421–434