1. Community Composition and Biomonitoring
Potential of Hill Stream Insects of the Western Ghats
2. Western Ghats Aquatic Biodiversity.
Community
Composition and Biomonitoring
Potential of Hill Stream
Insects of the Western Ghats
|
 |
K.A.Subramanian
Centre
for Ecological Sciences,
Indian Institute of Science, Bangalore-12 |
|
Current Address:
K.A.Subramanian,
National Centre for Biological Sciences,
GKVK Campus,
Bangalore-65
Email: subbu@ncbs.res.in
|
Introduction
Inland
waters comprise a small fraction of world’s water resource. Despite this, inland
aquatic habitats show far more variety in their physical and chemical characteristics
than marine habitats and contain a disproportionately high fraction of the world’s
biodiversity. Animal species are far more diverse and numerous in the inland
waters than plants. Apart from fishes, invertebrates form an important group.
As on land, insects (Phylum: Mandibulata) are the most diverse group of organisms
in inland waters (WCMC, 2000).
Aquatic
insects of running waters, comprising some well-known groups like mayflies,
dragonflies and caddisflies are important group of organisms in the stream
ecosystem function. Different functional group of aquatic insects such as shredders,
scrapers, filter feeders and predators are important links in nutrient recycling
in forest streams. Aquatic insects primarily process wood and leaf litter reaching
the stream from the surrounding vegetation. Nutrients processed by aquatic insects
of stream are further degraded into absorbable form by fungal and bacterial
action. Aquatic insects are also a primary source of food for fishes (Merritt
et al., 1984 & Wallace and Jackson,
1996).
In
addition to this significant ecosystem function, aquatic insects are very good
indicators of human impact on the fresh water ecosystem. Biological monitoring
methods using aquatic insects have been developed and reliably tested in both
temperate and tropical aquatic systems (Resh 1979, Armitage et al., 1983, Trivedi, 1991,
Sivaramakrishnan et al., 1996a).
The
Western Ghats of Peninsular India along with Srilanka is recognized as one of
the 25-biodiversity hotspots of the world (Myers et. al, 2000). The Western
Ghats is also well known for its high diversity of angiosperms, amphibians and
freshwater fishes. Invertebrates,
especially insects, which form the bulk of the diversity, are poorly known from
the region, except for butterflies (Daniels,
2002). Earlier studies on aquatic insects of the region were cursory
and restricted to very few localities. The present study conducted across the
entire length of the Western Ghats tries to address the distribution and diversity
of stream insect communities at different spatial scales.
The study also tries to evaluate and prioritize the riverine ecosystems
at various spatial scales for conservation using aquatic insects.
Objectives
- To
study the distribution and diversity patterns of stream insect communities
at the scale of (1) latitude (2) altitude (3) stream order (4) habitat and
(5) microhabitat.
- To
study the impact of catchment landuse on the composition and community structure
of stream insects.
- To
evolve a biomonitoring methodology using aquatic insects for riverine ecosystems
of the Western Ghats.
- To
identify and prioritize habitats and regions for conserving riverine biodiversity.
The above
questions were addressed by looking at the diversity and distribution of aquatic
insect communities at two scales in the Western Ghats. To address questions
at a regional scale (objectives i, iii and iv), aquatic insect samples collected
across 39 localities in the Western Ghats is used. Questions related to local
scale (objectives i & ii) are addressed by samples collected from the Swarna
and Bhadra drainage basin at Kudremukh National Park (Maps 1&2).
Methodology
Aquatic
insect communities were sampled from hill streams of 39 sites located between
8°N to 20°N of Western Ghats during August 1999 to February 2002.
The community was sampled from three major riverine habitats: runs, riffles
and pools (Plate-1). The insects were collected using kicknets, pond nets and
by all out search method. Collected specimens were preserved in 70% alcohol
in the field and brought back to lab for identification. In addition to biological
sampling, 16 environmental variables such as stream depth, width, pH, temperature,
substrate type, canopy cover etc. were also collected for each sample. Samples
were assigned to family and genus using keys for that particular group. Following
keys were used for identification: Ephemeroptera
(Dudgeon, 1999; Sivaramakrishan, unpublished); Odonata,
Plecoptera, Hemiptera, Megaloptera, Coleoptera, Diptera and Lepidoptera
(Fraser, 1933-36;Morse et al.,
1994; Dudgeon, 1999); Hemiptera (Thirumalai
1989,1999; Morse et al., 1994), Trichoptera (Wiggins, 1975, 1996). The distribution and diversity
of aquatic insects were looked at the spatial scales of: latitude, altitude,
stream order, habitat and microhabitat. In addition to this, the impact of riparian
landuse on the diversity and distribution of aquatic insects were also investigated.
Biomonitoring scores and conservation value for each genus was derived based
on the current knowledge on the distribution and diversity of that particular
taxon (Dudgeon, 1999).
Results
A
total of 15, 260 individuals belonging to 12 order, 59 families and 94 genera
were collected in 293 sampling sessions from 39 localities in the Western Ghats.
Of these 14,824 individuals were assigned to genus, 406 to families and remaining
30 to orders. The rarefied generic
and family richness decreased significantly with increasing latitude (Fig-1).
The aquatic insect family and generic richness decreased significantly with
increasing number of dry months (Fig-2) and it had no significant relation
with the average annual rainfall. Cluster analysis shows that there are two
distinct communities north and south of 15°N (Fig-3). Interestingly this
corresponds to two geological formations of the Western Ghats viz.
Deccan Trap and Precambrian. At the scale of altitude, the generic, family and
functional group richness decreases significantly with increasing altitude (Fig-4).
Aquatic insect faunal and functional group composition changes along the stream
order and habitat. Highest generic richness was observed in the second order
streams and in runs (Fig-5). The family and generic richness was significantly
positively correlated with microhabitat richness.
Of
the 94 genera, 60 (64%) were restricted to less than four microhabitats and
remaining were occupying four or more microhabitats (Fig-6). Maximum
generic richness was observed among cobbles for runs and riffles; bedrock harbored
maximum richness in pools. Riparian zone with natural vegetation had higher
family, generic and guild richness than human modified ones within the same
catchment (Fig-7). Family and generic composition changes with riparian
landuse and widespread taxa with pollution tolerance dominated streams with
human influenced catchments.
(Fig-8).
Sites north of 14°N had lowest and sites between 8°N-12°N had intermediate scores
and conservation values. The low altitude (1-200m) sites ranked high both in
terms of biomonitoring scores and conservation values than the high altitude
ones (1000-2400m). At the scale of stream order and habitat, the second order
streams and runs had highest biomonitoring scores and conservation values. Biomonitoring
scores and conservation values of streams reflected the riparian landuse (Table-1).
Streams with natural riparian cover had higher biomonitoring scores and conservation
value.
Discussion
Like
other taxa such as angiosperms and butterflies, aquatic insect generic and family
richness decreases towards northern latitude and shows a strong decreasing trend
with increasing number of dry months in the Western Ghats. Similar pattern is also observed for other aquatic organisms
such as amphibians (Daniels,
1992).
Similarly, low diversity of aquatic insects in high altitude streams is also
observed (Suren,
1994).
However, the high and low altitude streams harbour different sets of species.
The high altitude streams are characterized by Palaearctic genera such
as Rhyacophila and Polycentropus. The typical
Oriental genera such as Rheonathus
and Stenopsyche are restricted to
low altitude streams. Family and
generic composition changes with increasing stream order. The low order streams
are dominated by genera such as Glossosoma,
Teloganodes, Epeorus and Petersula, which
have morphological adaptations to cling to substratum in fast flowing streams.
On the other hand, burrowing families such as Libellulidae and Tabanidae
dominate the higher order streams. The generic and family richness is highest
in the second order streams. Similar patterns have also been reported from temperate
streams (Vannote
et al., 1980).
This poverty in higher order streams is probably due to decrease in available
substrate heterogeneity and canopy opening. The poor substrate richness allows
only few species to colonize in wider streams and streams with open canopy or
less riparian vegetation receive less organic inputs, which is a major source
of nutrition for the aquatic insects. At the scale of habitats, runs, riffles
and pools have different set of genera. For example, the aquatic bugs (Hemiptera)
and beetles with a mode of life on water surface dominate stream pools.
Genera such as Rhagovelia, Amemboa, Metrocoris,
Cylindrostethus and Dineutus
represent the fauna of a typical stream pool.
High
diversity of family and genera with increasing diversity of microhabitat is
very well known in temperate streams (Hynes, 1970; Rabeni and Minshall 1977;Allan,
1995). Similar pattern was also observed for all the three habitats viz.
runs, riffles and pools. Within a habitat, the high diversity was restricted
to microhabitats, which give maximum seclusion and access to food source. For
example, in runs and riffles highest diversity was among cobbles and trapped
litter. The influence of catchment landuse on the stream insect communities
was hitherto unknown from the tropics. The present study addresses this question,
which have important implications for developing a biomonitoring tool. High
family and generic richness was clearly confined to less disturbed areas in
the upper catchment. Families such as Leptophlebiidae,
Heptageniidae, Ephemereliidae, Perlidae, Calopterygidae, Euphidae, Naucoridae,
Helicopsychidae and Glossosomatidae
dominate the natural stream community.
The streams flowing through human impacted areas such as paddy fields
and mines were poor in family and genera. The dominant families of the community
are Baetidae, Libellulidae, Psephinidae,
Dytiscidae, Hydropsychidae and Simulidae.
These families are tolerant to aquatic pollution. Absence of pollution-sensitive
families of aquatic insects and low biomonitoring scores indicate the poor quality
of the water flowing through human impacted area.
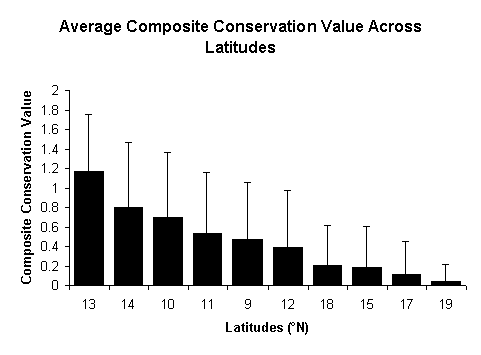
Composite
Conservation Value (CCV) developed for Indian birds were adopted to develop
CCV for aquatic insect genera. Using this, riverine ecosystems were assessed
for conservation at various spatial scales. It is interesting to note that rivers
flowing through the central Western Ghats have (13°N-15°N) highest conservation
value. This may be due to the presence of more rivers without impoundment than
in northern or southern Western Ghats. However, this pattern needs to be corroborated
with other taxa such as freshwater fishes.
Summary
and Synthesis
Of
the factors examined, the length of the dry season, altitude, stream order,
microhabitat richness and the riparian landuse influenced stream insect diversity.
The species richness at a habitat is a result of interplay between factors
acting locally and regionally. The biogeographic evolution, historical immigration
and climatic factors determine the regional taxon pool. Within a region, the
factors such as altitude, microhabitat richness and riparian landuse play an
important role in influencing taxa composition and richness. It is evident from
the study that, the stream insects are very good indicators of the health of
the riverine ecosystem and could be incorporated into the environmental impact
assessment protocols. However,
some basic questions need to be immediately addressed to fully understand and
appreciate the role of stream insects in the ecosystem functioning. First and
the foremost, the taxonomy of the stream insects of the Western Ghats is poorly
known. A concentrated effort is required to fill this gap and need to update
with the recent international taxonomic revisions. The process oriented experimental
studies needs to be given immediate attention to understand the role of the
stream insects in the nutrient dynamics.
Moreover, the importance of other macroinvertebrates in the stream ecosystem
function is little known from the region that is essential for understanding
the nutrient dynamics. Future investigations
should focus on the whole macroinvertebrate communities of the streams and studies
should extend to full river stretches. This will create baseline information
for identifying and conserving riverine ecosystems of the region.
Subramanian in Field:

Acknowledgements
This work
was carried out during author’s tenure at CES, IISc between 1999-2003. Students
and teachers of the Western Ghats Biodiversity Network were the inspiring force
behind this study and assisted me in the field. The forest departments of Maharastra,
Goa, Karnataka, Kerala and Tamil Nadu were cooperative and helpful. I also thank
my friends and former colleagues at CES for valuable suggestions and constant
encouragement during the study.
Reference
-
Allan,
D.J.(1995). Stream Ecology: Structure and function of running waters. Chapman
& Hall, Madras.388pp.
-
Armitage,
P.D., D. Moss, J.F. Wright and M.T. Furse. (1983). The Performance of A
New Biological Water Quality System Based On Macroinvertebrates Over a Wide
Range Of Unpolluted Running Water Sites. Water
Research.17 (3): 333-347.
-
Daniels,R.J.R
.(2002). A Report on the National Biodiversity Strategy and Action Plan-the
Western Ghats Ecoregion: Submitted to the Ministry of Environment and Forests,
India. URL.http://sdnp.delhi.nic.in/nbsap/index1.html
-
Daniels,R.J.R.(1992).
Geographical distribution patterns of amphibians in the Western Ghats, India.
J.Biogeography. Vol.19. 521-529.
-
Dudgeon,
D. (1999). Tropical Asian Streams-Zoobenthos, Ecology and Conservation.
Hongkong University Press. Hongkong. 828pp.
-
Fraser,
F.C. (1933-36). The fauna of British India, including Ceylon and Burma,
Odonata, Vols.I-III. Taylor &
Francis Ltd., London.
-
Hynes,
H.B.N. (1970). The ecology of running waters. Liverpool
University Press. 555pp.
-
Merrit,
W.R, Cummins, W.K and Burtorn, M.T. (1984). The role of aquatic insects
in the processing and cycling of nutrients. In: The Ecology of Aquatic Insects.(eds.Resh,
H.V and Rosenberg ) M.D. Praeger Publishers. 625 pp.
-
Morse
C.J, Yang Lianfang and Tian Lixin (ed.) (1994). Aquatic Insects Of China
Useful For Monitoring Water Quality. Hohai
University Press, Nanjiing People’s Republic Of China pp 569.
-
Myers
Norman, Mittermeier, R.A., Mittermeier, C.G.,da Fonseca, Gustavo A.B., Kent,J.
(2000).Biodiversity hotspots for conservation priorities. Nature
Vol.403.853-858.
-
Rabeni,
C.F. and Minshall, G.W.(1977). Factors affecting micro-distribution of stream
benthos insects. Oikos. 29:33-43.
-
Resh.V.H.(1979).
Biomonitoring, species diversity indices and taxonomy. Pages 241-253 In:
Ecological diversity in theory and practice (eds.J.F. Grassle, G.P.Patil,W.K.Smith
and C.Taillie). International Cooperative
Publishing House, Fairland, M.D.365 pp.
-
Sivaramakrishnan,
K.G, Hannaford, J Morgan and Resh H Vincent. (1996a). Biological Assessment
of the Kaveri River Catchment, South India, and Using Benthic Macroinvertebrates:
Applicability of Water Quality Monitoring Approaches Developed in Other
Countries. Int. J. Eco.and Env.Sci.32: 113-132.
-
Suren,
A.M. (1994). Macroinvertebrate communities of streams in western Nepal:
effects of altitude and land use. Freshw.Biol.32:
323-336. Thirumalai, G (1999). Aquatic and semi-aquatic
Heteroptera of India. Indian Association
of Aquatic Biologists. Publication No.7. Hyderabad. pp 74.
Thirumalai, G. (1989). Aquatic and semi aquatic Hemiptera (Insecta)
of Javadi Hills, Tamil Nadu. Occasional paper no.118. Zoological
Survey of India, Culcutta.
-
Trivedi,R.C.
(1991). Biomonitoring A Case Study On Yamuna River. On The Implementation
of a Biomonitoring yardstick for Water Quality Management in Indian Rivers.
Proceedings of Indo-Dutch Workshop New Delhi-29th -31ST
Oct, 1991.
-
Vannote,
R.L., Minshall, G.W., Cummins, K.W., Sedell, J.R. & Cushing, C.E. (1980).
The River Continuum Concept. Can.J. Fish. Aquat. Sci. 37: 130-137.
-
Wallace,
B. J. and Jackson, R. W. (1996). The role of macroinvertebrates in stream
ecosystem function. Annu.Rev.Entomol.1996
41:115-39.
-
Wiggins.B.
(1975). Larvae Of The North American Caddisfly Genera (Trichoptera). University
Of Toronto Press, Tronto. pp401.
-
Wiggins.B.
(1996). Larvae Of The North American Caddisfly Genera (Trichoptera). 2nd
edition. University Of Toronto Press, Tronto. pp457.
-
World
Conservation Monitoring Centre (2000). Global Biodiversity: Earth’s living
resources in the 21st century.By:Groombridge,B.and Jenkins,M.D.World
Conservation Press,Cambridge,UK.
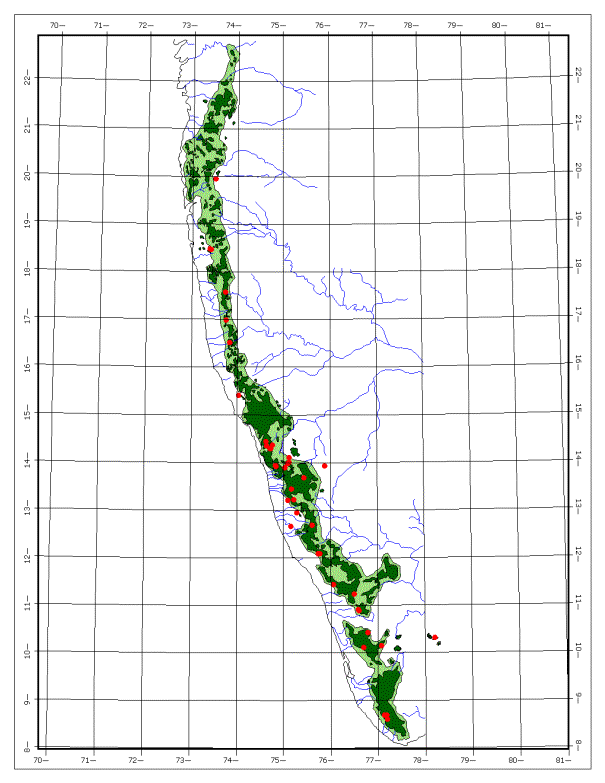
Map-1:
The map of the Western Ghats. The
study sites are shown as red spots.
Map-2:
Sampling localities at Kudremukh National Park
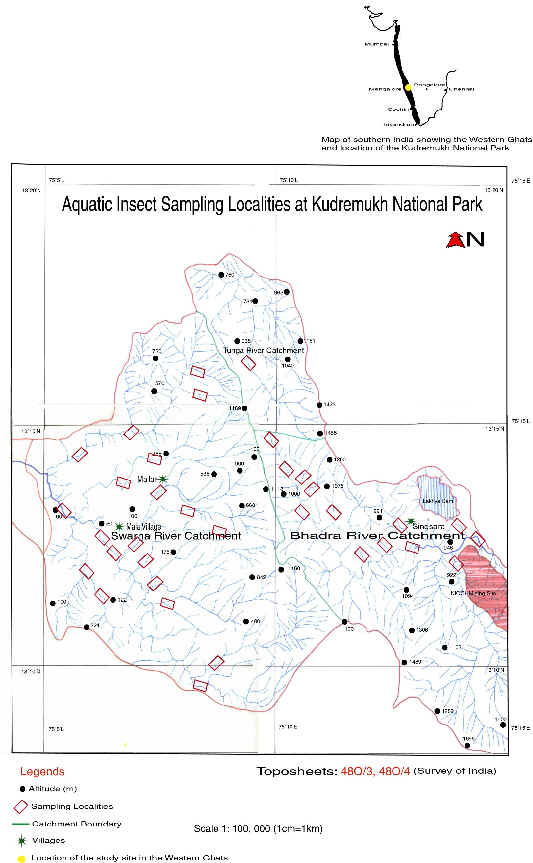

Fig.1.
Rarefied generic richness across latitude
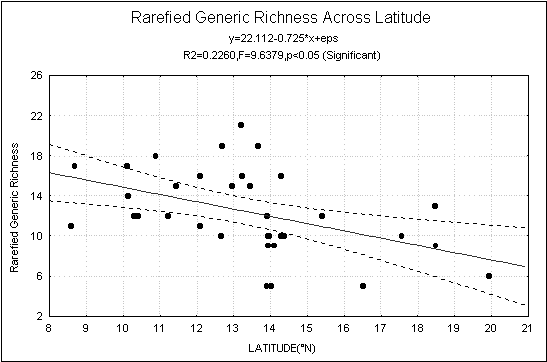
Fig.2.
Generic Richness and number of dry months
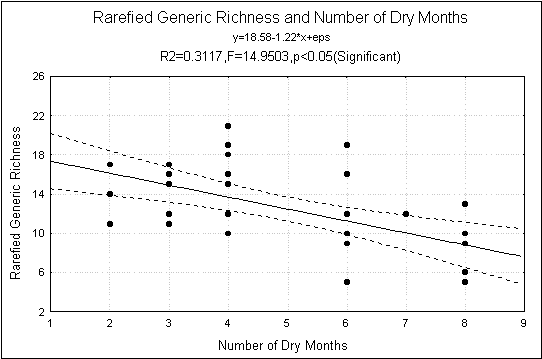
Fig:3.
Dendrogram showing similarity in Ephemeroptera genera composition across latitude
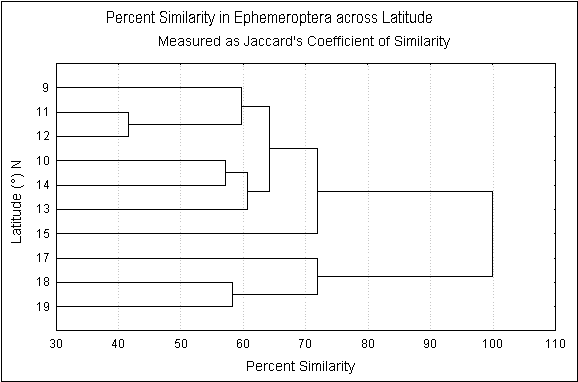
Fig.4.
Generic Richness Across altitude at Kudremukh
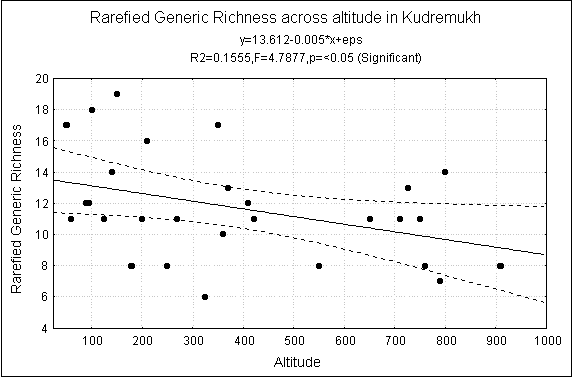
Fig.5:
Generic richness of across stream orders
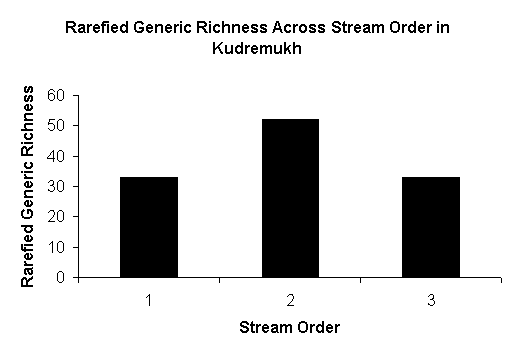
Fig.6:
Frequency distribution of genera across microhabitat
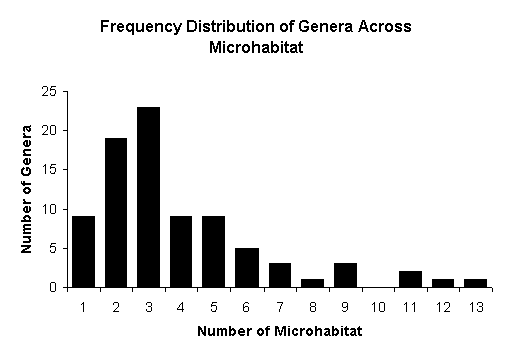
Fig.7:
Generic richness across landscape elements types
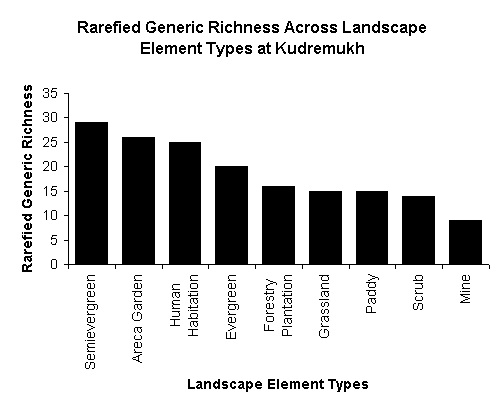
Fig.8:
Composite Conservation Values across latitudes
|
SLNo.
|
LSE Types
|
BMWP
|
ASPT
|
|
1
|
EVG
|
365
|
7.448979592
|
|
2
|
MDF
|
284
|
7.282051282
|
|
3
|
SEVG
|
280
|
7.368421053
|
|
4
|
HAB
|
268
|
7.657142857
|
|
5
|
ARE
|
255
|
7.5
|
|
6
|
SCR
|
234
|
7.3125
|
|
7
|
FOR
|
180
|
7.5
|
|
8
|
PAD
|
171
|
7.434782609
|
|
9
|
GSL
|
157
|
7.85
|
|
10
|
SHOL
|
123
|
7.6875
|
|
11
|
DDF
|
92
|
7.666666667
|
|
12
|
MINE
|
64
|
8
|
Legend: EVG-Evergreen;
MDF- Moist Deciduous Forests; SEVG-Semievergreen;
HAB- Habitation; ARE-Arecanut garden; SCR-Scrub; FOR-Forestry Plantations; PAD-Paddy;
GSL- Grasslands; SHOL-Shola; DDF:
Dry deciduous forests;MINE- Mine
BMWP: Biomonitoring working
party score system.
ASPT: Average score per
taxon
Top
Western
Ghats Aquatic Biodiversity
|
Ahalya
N. and Ramachandra T.V.
|
|
Western Ghats is a mountain chain running north-south and parallel to the west
coast of India. The hill chain is 1600 km long and between 5 and 150 km wide,
separated from the coast by a low land strip usually 40 - 60 Km wide. The Western
Ghats traverse 6 south Indian states viz., Gujarat, Maharashtra, Goa, Karnataka,
Tamilnadu and Kerala. The area experiences an average annual rainfall of 2500
mm. Depending upon the geographical location and topography, rainfall is locally
much higher crossing 10,000 mm a year. Forests cover nearly one - fourth of
the total area in Western Ghats and give rise to east flowing rivers that form
the water source for the entire peninsular India. The Western Ghats is the catchment
of 3 large, 13 medium and 17 minor rivers of the peninsular India.
The Western Ghats have an ancient history. The past 12,000-5,000
years have seen a lot of changes in terms of the magnitude and distribution
of biodiversity due to settled agriculture and extensive transformation of habitats
in and around the Western Ghats (Subash Chandran, 1997). What is present today
is the testimony to the human interference in the area. It is estimated that
12, 450 km2 of natural forest cover exist in the Western Ghats, which is about
6.8 % of the original forest cover. Irrigation and hydroelectric power projects,
timber operations and agriculture expansion are the major reasons for the decline
in forest cover.
The once continuous tropical rainforests in the Western Ghats have been modified
into heterogeneous mosaic of evergreen, semi-evergreen and moist deciduous formations.
Though the Western Ghats have undergone changes, they still harbor an exceptionally
high magnitude of biodiversity and provide immense ecosystem services.
The estimated number of species of microorganisms, plants and animals in the
Western Ghats is in the range of 10,000-15,000. Roughly 40% of these could be
endemic (http://sdnp.delhi.nic.in/nbsap/dactionp/ecoregion/wghatsd.html). The
magnitude of diversity coupled with the anthropogenic threats has made Western
Ghats one of the biodiversity hotspots.
(Biodiversity is the variety and differences among living organisms from all
sources, including terrestrial, marine, and other aquatic ecosystems and the
ecological complexes of which they are a part.
India is one of the mega biodiversity centres in the world and has two of the
world's 25'biodiversity hotspots' located in the Western Ghats and in the Eastern
Himalayas)
Forest clearing and human encroachment remain the biggest threats to this regions
natural habitat and biodiversity. Large areas have already been converted into
rubber, tea, and coffee plantations. Because of extensive forest fragmentation,
it may not be possible to create additional large protected areas without extensive
restoration.
The dense human population led to a change in conservation status from endangered
to critical. This physical alteration, habitat destruction and creation of new
dams and reservoir has caused a decline in the aquatic biodiversity in the Western
Ghats. Apart from the decline in invertebrate and fish population in the streams
of the Western Ghats, other vertebrates like amphibians, reptiles, birds and
mammals are also affected by deterioration of freshwater systems (Subramanian,
2003).
There are around 218 species of primary and secondary freshwater fishes in the
Western Ghats. 53% of all fish species (116 species in 51 genera) in the Western
Ghats are endemic (Talwar and Jhingran, 1991; Jayaram, 1999; Menon, 1999; Daniels,
2001a.).
Freshwater fishes of the Western Ghats have economic value as food and ornamental
fish. Gopalakrishnan and Ponnaiah have listed at least 100 species, many of
which are endemic, as having potential economic value. Such species belong to
the genera Tor, Neolissochilus, Gonoproktopterus, Hypselobarbus, Labeo, Barbodes,
Osteocheilus, Horabagrus, Mystus, Ompok, Silurus, Wallago, Clarias, Channa,
all of which are considered as food and sport fishes and in the genera Puntius,
Danio, Rasbora, Barilius, Chela, Bhavania, Homaloptera, Travancoria, Balitora,
Nemacheilus, Garra, Glyptothorax, Pristolepis, Aplocheilus, Tetradon, Macropodus,
Etroplus, etc., are of potential ornamental value (Daniels and Ouseph, unpublished;
Gopalkrishnan and Ponniah, unpublished).(http://sdnp.delhi.nic.in/nbsap/dactionp/ecoregion/wghatsd.html)
One hundred and twenty one species of amphibians are known from the Western
Ghats (Daniels, 1992). Of these, 94 species are endemic (Daniels, 1992, 1993
& 1997c; Dutta 1997). Few studies have specifically focused on habitat use by
amphibians. In the early 1990s, Daniels (1991) highlighted the role of habitat
destruction in the loss of amphibians in the Western Ghats. At very local scales
it has been observed that amphibian species richness is determined by the proximity
to water - most species tending to aggregate closer to a source of water (Vasudevan
et al, 2001). 157 species of reptiles including a species of crocodile Crocodylus
palustris is known from the Western Ghats, majority being snakes. 97 species,
representing 36 genera (2 genera of turtles/tortoises, 14 lizard genera and
20 genera of snakes) of all reptiles in the Western Ghats are endemic (Table
1). Endemism is highest amongst snakes, especially with the family Uropeltidae
alone contributing 33 species. Amongst lizards, dwarf geckoes (Cnemaspis spp)
and skinks (Ristella, Lygosoma, Mabuya and Scincella) have the maximum number
of endemic species.
Table 1: Taxonomic breakup of reptilian
diversity in the Western Ghats
|
Group
|
No.
of species
|
Endemic
species
|
|
Turtles/tortoises
|
6
|
2
|
|
Crocodiles
|
1
|
0
|
|
Lizards
|
63
|
34
|
|
Snakes
|
87
|
61
|
|
Total
|
157
|
97
|
Source: Whitaker (1978); Das (1985 & 1997); Murthy (1985 & 1990).
http://sdnp.delhi.nic.in/nbsap/dactionp/ecoregion/wghatsd.html
With regard to freshwater
invertebrates in the Western Ghats, the species level inventories have been
lacking. But an exception to this
is Odonata (Dragonflies and Damselflies). Out of the 176 species of Odonates
in Western Ghats, 67 i.e., 38% are endemic (Subramanian K.A). Stream insects
belonging to 13 orders, 53 families and 80 genera have been collected. Families
such as isonychidae (Ephemeoptera: Mayflies) and blephariceridae (Diptera: Netwinged
midges) known to be present in the cold streams of the Himalayas were collected
for the first time in the Western Ghats. A preliminary study by Madhyastha has
suggested that Silent Valley and many other well preserved parts of the Western
Ghats may well support over a 100 species of molluscs locally (http://sdnp.delhi.nic.in/nbsap/dactionp/ecoregion/wghatsd.html).
The principal causes
for the loss in biodiversity is anthropogenic. The direct impacts are due to
collection, harvest and poaching and indirectly through habitat destruction.
The loss of aquatic biodiversity would be attributed to change of flow, depth
and turbidity in aquatic habitats, opening of canopy (often due to selective
logging) in the catchment area, overuse of pesticides ands fertilisers, etc.
References:
-
Daniels,
R J R (2001a) Endemic fishes of the Western Ghats and the Satpura hypothesis.
Current Science 81(3): 101-105.
-
Daniels,
R J R (2001b) Amphibians and reptiles of the Tamil Nadu Eastern and Western
Ghats. Cobra 43: 1-8.
-
Daniels,
R J R and Ouseph, A (unpublished) Diversity of tropical aquarium fishes
in the Western Ghats. National Bureau of Fish Genetic Resources - Workshop
on Germplasm Inventorisation and Gene-banking of Freshwater Fishes, 1998,
CMFRI, Cochin.
-
Das,
I (1985) Indian Turtles: A field guide.
WWF-India (Eastern Region), Calcutta, pp119.
-
Das,
I (1997) Checklist of reptiles of India with English common names. Hamadryad
22: 32-45.
-
Das,
I and Dutta, S K (1998) Checklist of amphibians of India, with English common
names. Hamadrayad 23 (1): 63-68.
-
Dutta,
S K (1992) Amphibians of India: updated species list with distribution record.
Hamadryad 17: 1-13.
-
Dutta,
S K (1997) Amphibians of India and
Sri Lanka (Checklist and Bibliography), Bhubaneshwar, Odyssey Publishing
House, pp342.
-
Easa,
P S (1998) Survey of reptiles and
amphibians in Kerala part of Nilgiri Biosphere Reserve. KFRI Research
Report no. 148, pp40.
-
Easa,
P S (1998) Survey of reptiles and
amphibians in Kerala part of Nilgiri Biosphere Reserve. KFRI Research
Report no. 148, pp40.
-
Easa,
P S and Shaji, C P (1997) Freshwater fish biodiversity in Kerala part of
the Nilgiri Biosphere Reserve. Current
Science 73(2): 180-182.
-
Gopalakrishnan,
A and Ponniah, A G (unpublished) Economically important cultivable/sport
fishes endemic to the peninsular India with special reference to Western
Ghats & Potential ornamental freshwater fishes endemic to peninsular
India with special reference to Western Ghats. National Bureau of Fish Genetic
Resources - Workshop on Germplasm Inventorisation and Gene-banking in Freshwater
Fishes, 1998, CMFRI, Cochin.
-
Hussain,
S A and Achar, K P (1999) (eds) Biodiversity
of the Western Ghats complex of Karnataka.
Mangalore, Bidiversity Initiative Trust. pp262.
-
Inger,
R F (1996) Commentary on a proposed classification of the family ranidae.
Herpetologica, 52(2): 241-246.
-
Inger,
R F and Dutta, S K (1986) An overview of the amphibian fauan of India. J
Bombay Nat Hist Soc 83(suppl):135-146.
-
Inger,
R F, Shaffer, H B, Koshy, M. and Badke, R. (1984) A report on the collection
of amphibians and reptiles from the Ponmudi, Kerala, South India. J
Bombay Nat Hist Soc. 81: 406-427; 551-570.
-
Inger,
R F, Shaffer, H. B, Koshy, M. and Badke, R. (1987) Ecological structure
of a herpetological assemblage in south India. Amphibia-Reptilia,
8: 189-202.
-
Jayaram.
K C (1999) The freshwater fishes of
the Indian region. New Delhi: Narendra Publishing House, pp 551.
-
Johnsingh,
A J T (2001) The Kalakad-Mundanthurai Tiger reserve: a global heritage of
biological diversity. Current Science
80(3):378-388.
-
Menon,
A G K (1999) Checklist – Freshwater
Fishes of India. Zoological Survey of India. Occassional
Paper No.175, pp 366.
-
Pillai,
R S (1986) Amphibian fauna of Silent Valley, Kerala, S. India. Rec.
Zool. Surv. India 84(1-4): 229-242.
-
Subash
Chandran, M D (1997) On the ecological history of the Western Ghats. Current
Science 73(2): 146-155
-
Vasudevan,
K. (1996) Effect of rainforest fragmentation on WG amphibians. Frog
Leg 1(2): 1.
-
Vasudevan,
K, Kumar, A and Chellam, R (2001) Structure and composition of rainforest
floor amphibian communities in Kalakad-Mundanthurai Tiger Reserve. Current
Science 80(3):406-412.
http://sdnp.delhi.nic.in/nbsap/dactionp/ecoregion/wghatsd.html
Subramanian K.A, (2003).
Strean Insect Communities of Western Ghats and their Bioindicator Potential.
(Thesis submitted to Madurai Kmaraj University)
Top












