 |
Sudarshan P. Bhat, Sumesh N. Dudani, M. D. Subhash Chandran and T. V. Ramachandra |
 |
SHILAPUSHPA - LICHENS: GENERAL CHARACTERISTICS
Shilapushpa or Lichens are simplest form of plants consisting of a very intimate association of a fungus (the mycobiont) with a photosynthetic partner (the photobiont), usually either a green algae or cyanobacterium. The intimate association of these two microorganisms results in the formation of a macro-organism, i.e. the lichen thallus whose morphology is quite different from the original organisms. The association between the algae and fungus is so intimate that the term symbiosis is often applied to it for its description. Lichens form easily distinguishable coloured patches on tree barks, rocks and soil and are universally distributed organisms occurring in varied climatic conditions ranging from the poles to the tropics in earth (Kumar, 2010). The word ‘Lichen’ (lie ken) was introduced into the Greek literature in about 300 BC by Theophrastus, to describe outgrowths from the bark of olive trees (Hawksworth & Hill, 1984). In spite of itsimportant roles in the ecosystem, the study of lichens remains quite neglected throughout the world, though they together with mosses form dominant organism in ecosystem covering over 10% of the earth terrestrial habitats, particularly at higher elevations (Nash & Egan 1988).
Lichens were the first examples of symbiosis in which the translocation process was demonstrated especially the movement of carbohydrates from algae to fungi. They have evolved with ability to absorb minute concentrations of water from air or dew and become metabolically active within a few minutes, whereas inversely in scorch sunny conditions, they lose water and become dry and crisp within an hour (Negi, 2003). The mycobiont part of the Lichen provides the necessary substratum and also aids in the assimilation of moisture, micro and macronutrients for the photosynthetic partner to grow and in turn receives the carbohydrates for their metabolic activity. This constant supply of carbohydrates enables the fungal partner to continuously grow and reproduce, unlike the free living fungi that appear only upon the availability of moisture and nutrients. Since the fungal constituent is unique in that symbiosis and usually dominates the association; lichens traditionally have been considered a type of fungus. Lichens have diversified extensively during the past 600 years, and occur over more than 10% of the terrestrial surface (Hawksworth & Hill, 1984). About 13,500 species are currently accepted and it is estimated that the actual world total will be in the range 17-20,000 (Galloway, 1992). They form an integral and important part of an ecosystem and serve as ecological indicators too. They are included in the lower groups of plants known as the cryptogams which reproduce by the means of spores and do not produce seeds.
It is believed that the algae, which belong to those families of Chlorophyceae and Myxophyceae that lived on dry land and had become aerial before their association with fungi to form lichens. The fungal hyphae have combined with a considerable number of different algae, so that, even as regards the algal symbiont, lichens are truly polyphyletic in origin (Smith, 1921). Two groups of fungi associated with lichens- Basidiomycetes found in only a few genera and Ascomycetes which form with the various algae and bulk of lichen families. Though lichens have no common origin, they are fitted for much longer existence than that of fungi and can persist through great climatic changes.
HABITAT
The symbiotic relationship helps the lichens to live in variety of habitats and climatic conditions all over the world including extreme environments. Within a climatically uniform region each particular substrate tends to assume eventually a characteristic and often remarkably uniform lichen community (Yuan et.al, 2005). They grow in diverse climatic conditions and on diverse substrates. The ability to quickly absorb and retain water from many sources makes it possible for lichens to live in harsh environments like deserts and Polar Regions, and on exposed surfaces like bare rocks, walls, roofs, tree branches and manmade substrata like glass, metals etc. (Ahamadjian, 1995). They occur in virtually every pioneer terrestrial habitat from Arctic and Antarctic to tropical areas and in many desert areas where they are able to form long lived and stable communities (Hale, 1983). The different habitats of the lichens in nature can be summarized in the following chart (Fig.1):
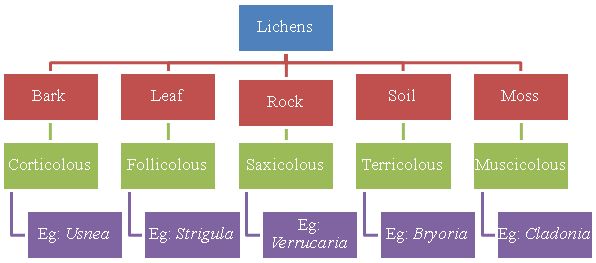
Fig.1 – Different habitats of Lichens
Based on the substrate of growing, the lichens can be broadly classified as – Corticolous (growing on the bark surface of trees), Follicolous (growing on the surface of leaves), Saxicolous (growing on rock surfaces), Terricolous (growing on soil) and Musicolous (growing on mosses). The various habitats of lichens are as described below:
Corticolous communities: These develop on bark and contain fruticose and foliose species (Fig.2). These include the species of Evernia, Parmelia and Usnea. Growth of lichens on tree bark depends on its stability, texture, pH and water retention ability. The rough barked trees encourage Parmelioid and Physioid lichens along with members of Buellia, Lecanoraceae, Lecideaceae and Pertusariaceae. The rough bark help lichens in trapping their spores or vegetative diaspores and retains moisture for longer duration.
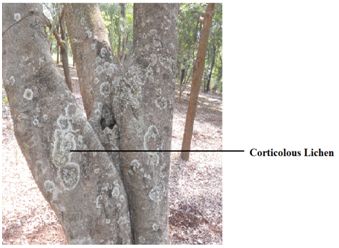
Fig.2 – Corticolous lichen
Follicolous communities: Species like Calicium, Cyphelium and Strigula occurring on leaf are called as follicolous lichens (Fig.3).The shiny, smooth evergreen leaves in outer canopy, shady understory, in light gaps and near water bodies provide suitable substratum for foliicolous lichens.
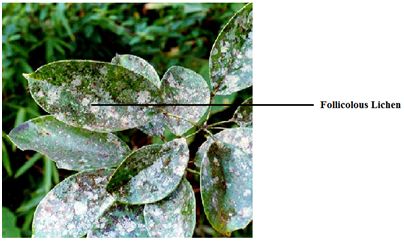
Fig.3 – Follicolous lichen
(Source - http://www.tnenvis.nic.in/Lichens/Field%20study.htm)
Saxicolous communities: Lichen communities developed on rocky substratum are called as Saxicolous and these vary according to rock type (Fig.4). The type of rock and pH are important factor responsible for colonization of the rock by lichen communities. The species like Caloplecta, Aspicilia grow on hard lime stones. Verrucaria species can be seen on well lit areas. Lepraria, Cystocoleus community grows on siliceous rocks.

Fig.4– Saxicolous lichen
Terricolous communities: The lichens of this community are growing on the ground or soil and often form a dominant component of the ground vegetation in the extreme environments (Fig.5).

Fig.5 –Terricolous lichen
(Source: www.waynesword.palomar.edu)
Muscicolous communities: These lichens grow on mosses (Fig.6). Some species like Cladonia, Peltigera grow along with mosses. They prefer the rough and bushy nature of the mosses which are efficient in trapping the lichen propagules. The hygroscopic nature of the mosses provides better water relation and microclimatic niche to the lichens growing on them (Nayaka et.al, 2007).

Fig.6 –Muscicolous lichen
(Source: www.google images/broad-horizon-photos.co.uk/lichens)
Different Growth forms of Lichens: The growth forms are usually conspicuous on the substrates, forming grey, green or even orange patches and are categorized primarily based on their morphology and size into three major types viz. crustose (crust like), foliose (leaf like) and fruticose (shrubby) (Negi, 2003). The lichens belonging to the first category are usually called microlichens and the latter two are referred to as macrolichens (Negi, 2003).
Crustose lichens: These types of lichens lack an organized thallus and are closely attached to the substratum (Fig.7). They consist of an indeterminate hyphal mat which entraps and encloses algal colonies. Such rudimentary thalli occur in the lower species of Calicium, Pyrenula, Trypethelium, Xylographa and Arthonia. Majority of crustose lichens like species of Lecanora and Lecidea grow on the surface of rocks and trees with distinct thallus. The surface is often warty or the entire thallus is marked off into many-sided areas or areoles and is therefore spoken of as areolate (Schneider, 1904). The highest stage in development of crustose lichens is squamulose thallus. In this type the individual lobes still lacking a lower cortex become partially free of the substrate (Hale, 1983). Soil lichens like Cladonia, Catapyrenium, Psora contains this type of thallus.

Fig.7 – Crustose lichen
Foliose lichens: They are also called as leafy lichens. The thallus in this case is loosely attached to the substratum by rhizines with distinct upper and lower surfaces (Fig.8). The thallus is typically divided into branching lobes as in Heterodermia, Physcia, Xanthoria,Cetraria and Parmelias. The foliose type of lichens merges into the fruticose type in the ascending series and into the crustose type in the descending series (Schneider, 1904).

Fig.8 –Foliose Lichen
Fruticose lichens (shrubby): These are hair like,shrubby,finger like or strap shaped (Fig.9). Here the lichen thallus is attached to the substratum at one point and remaining major portion is either growing erect or hanging. These vary in size from minute cladonias only 1-2 mm high to strands of Usnea upto 5 m long. The internal structure is radial with a dense outer cortex, a thin algal layer, a medulla and more or less hollow centre or a dense central cord. The thallus is round or flattened and richly branched. It is attached at the central or basal point known as the umbiculus, which consists of a hyphal tissue holding the plant firmly attached to the substratum and taking there from moisture and soluble food-substances (Schneider, 1904).

Fig.9 – Fruticose lichen
MORPHOLOGY OF LICHENS The main plant body of the lichen is a vegetative portion known as thallus which is comparable to the vegetative portions of other cryptogams such as mosses and liverworts. The fungal component (mycobiont) is an Ascomycete or Basidiomycete which forms a symbiotic relation with green algae or blue green algae (phycobiont). After this association, both the phycobiont and mycobiont components lose their individuality and the lichen behaves as a single organism, both morphologically and physiologically (Nayaka & Upreti, 2006). In lichen thallus (body) the mycobiont predominates with 90% of the thallus volume and provides shape, structure and colour to the lichen with partial contribution from algae and hence, the lichens are placed in the kingdom – Mycota (Nayaka & Upreti, 2006). Lichens have highly organized thallus than corresponding non-lichenized Ascomycetes and also produce vegetative structures not known in other fungi (Hale, 1983).
Vegetative structures
Lichens are characterized by a variety of vegetative structures on the upper and lower surfaces of the thallus. The colour of the thallus, texture (smooth, rough, warty), presence of finger like projections (isidia), granular powder in groups (soredia), fine powder (pruina), black dots (pycnidia) and whitish decorticated areas (pseudocyphellae) are to be noted on the upper surface of crustose and foliose lichens, whereas the colour of lower surface, presence of any pores, presence or absence of rihizines (root like structures), their colour, distribution, branching, abundance is to be noted on the lower surfaces of foliose lichens (Nayaka & Upreti, 2006).
Soredia: These are clumps of algal cells closely enveloped by hyphae (Fig.10). They originate in the medulla and algal layer and erupt through pores or cracks in the cortex. They are classified as marginal and laminal according to their position and farinose and granular according to their fineness (Hale, 1983). Delimited masses of erupted soredia called soralia are common in foliose and fruticose lichens but rare in crustose groups.

Fig.10 – A microscopic view showing soredia
Isidia: They are cylindrical finger like protuberances of the upper cortex in which algal and fungal tissues are incorporated (Fig.11). Though they are often fragile and can be easily broken, they form an integral and important part of the thallus and are produced uniformly over the upper surface without any unique patterns of orientation (Hale, 1983). They primarily act as vegetative propagules for the lichens; however, it is also presumed that they increase the surface area and assimilative capacity of the thallus. Upto 25-30% of the species in foliose and fruticose genera have been found to produce isidia whereas this is a much rarer in the case of crustose groups (Hale, 1983).
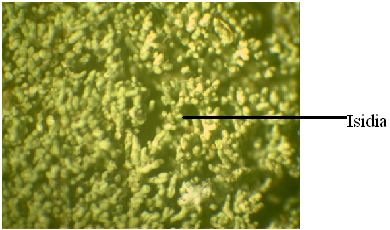
Fig.11 – A microscopic view of a lichen showing isidia
Rhizines: They are compacted strands of colourless or blackened hyphae that originate largely from the lower cortex and anchor the thallus to the substrate (Fig.12) (Hale, 1983). The simplest rhizines are unbranched as in the case of Cetraria, Physcia and many Parmelias; while the branching rhizines are of two kinds – squarrose as in the case of Anaptychia and dichotomous as in the case of Hypotrachyma species (Hale, 1983).
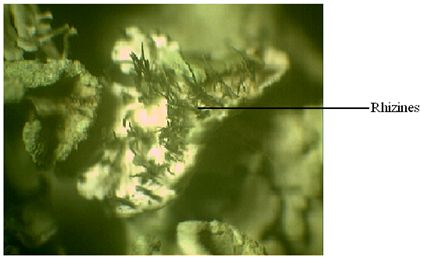
Fig.12 – A microscopic view of lichen showing rhizines
Cilia: They are hair like thalline appendages, decolourized or carbonized strands of hyphae that originate along the lobe margins or on the exciples of lecanorine apothecium (Fig. 13) as in Usnea (Hale, 1983). The cilia appear to be related to the rhizines and are restricted to more or less structurally advanced foliose genera such as Parmotrema and Physcia.

Fig.13 – A microscopic view of lichen showing cilia
Pores: Foliose lichens produce pores on the lower surface of the thallus which help in gas exchange similar to that of stomata on the leaves of higher plants. The most highly specialized pores are called Cyphellae (Fig.14) can be seen lower surface of Sticta. Simple undifferentiated pores in upper and lower cortex of foliose lichens such as Lobaria, Parmelia, Cetralia and in fruticose Ramalina, Bryoria, Alectoria are called as pseudocyphellae (Fig.15).

Fig.14 – A microscopic view of lichen showing cyphellae
Source: http://www.sharnoffphotos.com/lichensNH/anm_img/cyphellae_s_weigelii
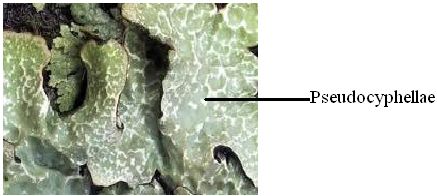
Fig.15 – A view of lichen showing pseudocyphellae
Source: http://www.lichens.ie/wp-content/themes/lichens/thumb
ANATOMY OF LICHENS
A sharp blade is used for procuring thin and clearly differentiable sections for analyzing the anatomical features of the thallus and fruiting bodies. The anatomy gives important details such as thickness of various layers (upper cortex, algal layer, medulla, lower cortex), type of algae and their distribution (stratified – heteromerous or uniform – homeomerous) and the arrangement of fungal hyphae (vertical or horizontal) within the thallus (Nayaka & Upreti, 2006). The sections are observed under a good compound microscope with a magnification of 40x. The vertical section of the vegetative thallus usually shows three distinct layers – uppermost layer is the cortical layer, bottom most is the medulla and in between these two, the algal layer is embedded (Fig.16). The anatomical characters of the fruiting bodies are also essential as identification aids and for noting down the features such as type of spores, their shape, size, and number of spores per spore-sac, colour of ascocarp wall, presence or absence of crystals, algal cells in the wall and colour of different layers of ascocarps (Nayaka & Upreti, 2006).
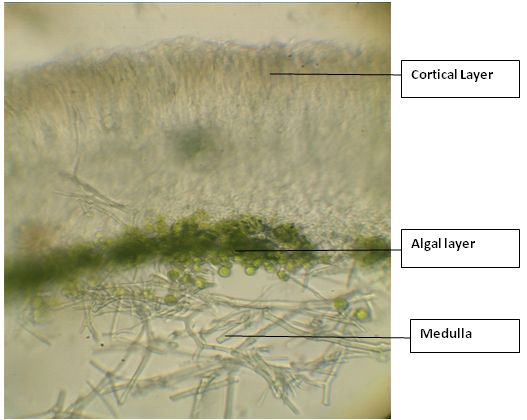
Fig.16 – A vertical section of lichen thallus showing anatomical features
Cortical layer
The cortex serves as protective cover over the thallus surface and consists of upper cortex and lower cortex layers, comparable to the upper and lower epidermis of the leaves of higher plants. The commonest type of cortex consists of dense heavily gelatinized paraplectenchymatous layer 10-40µm thick with several layers of cells (Hale, 1983). The upper cortical layer acts as a protective layer for the tissues beneath and also supplies mechanical support to prevent the breaking or tearing of thallus due to winds or other external forces (Schneider, 1904). In some genera the lower cortex also occurs which resembles the upper cortex. However, in general from the lower surface of the thallus, rhizoids occur which are parts of the fungal hyphae which extend vertically downward into the substratum and are usually black in colour.
Algal component (Phycobiont)
The algal component in the lichen thallus is completely surrounded by the fungal tissues on all sides and hence, in the cross section, the algal layer can be seen compactly arranged with a thickness of 10-30 µm thick between the upper cortex and medulla. This layer is responsible for the assimilation of carbon through the process of photosynthesis. The phycobiont component of algae, usually Nostoc, is commingled with loosely interwoven hyphae and in such cases, the algae makes a greater contribution to thallus colour, shape and constituency than the fungus (Hale, 1983). Certain hyphal branches of the fungal component of lichen thallus enclose and penetrate the algae and, and take from them the assimilated food-substances required by the fungal symbiont, while the algae in turn receives water and certain soluble food-substances from the fungal symbiont (Schneider, 1904).
Medulla
The bulk of the lichen thallus consists of medullary tissue which may be as much as 500 µm thick and in this region hyphae are weakly gelatinized in comparison with the cortical hyphae and have relatively large lumina (Hale, 1983). The medullary layer is present just below the algal layer with a network of loosely interwoven hyphae and has a large water holding capacity along with being the storage site of most of lichen substances responsible for encrusting the hyphae. The medulla in the crustose lichens penetrates between the rock crystals or bark periderm firmly anchoring the thallus to the substrate (Hale, 1983).
REPRODUCTION IN LICHENS
The method of reproduction in lichens is totally different from that of fungi as well as algae and even though the spores of lichens have a close morphological resemblance to the spores of certain fungi, they differ widely in their functions (Schneider, 1904). In natural conditions, the spores of fungi can germinate and develop into new spore producing individuals, but the spores of lichens cannot produce new lichens unless they are associated with the essential algae. Thus, this indicates that the fungal component of lichen can no longer mature independently and requires the presence of specific algal components to fulfill the symbiotic association. The vegetative reproduction in lichens is achieved by the development of special propagules which are unique to lichens and the sexual reproduction in the lichens commences with the production of fruiting bodies by the fungal component.
Asexual reproduction (Vegetative reproduction) in Lichens -
Vegetative reproduction occurs by the production of vegetative propagules (diaspores) which are lichenized structures in which algal and fungal components together act as separable autonomous subunits of the thallus (Hale, 1983). In lichens these diaspores are Soredia, Isidia, rare harmocysts, and various thalline structures such as squamules, lobules, phyllidia, blastidia, fragments or even the whole thallus (Pyatt, 1974). The commonest among these are soredia and isidia. Soredium contains clumps of algal cells closely enveloped by hyphae, which arise over the whole surface of thallus. Isidia are finger like outgrowth of upper cortex which contain algal and fungal tissues. They are easily broken off and are produced uniformly over the upper surface. Lobules are in general any adventives growths from the thallus often originating along the margins of the lobes. More rarely occurring types of propagules include Phyllidia, abstracted leaf or scale like dorsiventral portions of the whole thallus (eg: Collema flaccidum, Peltigera praetextata); blastidia-segmenting yeast like thaline propagules in Physcia opuntiella and harmocysts of several Lempholemma species where photobiont filaments and hyphae grow together in a chain like manner which break into clumps (Hawksworth & Hill, 1984). Most often vegetative reproduction in fruticose lichens like Cladonia and Bryoria is by thallus fragmentation.
Sexual Reproduction in Lichens -
The fungal partner in lichens reproduces sexually. Sexual reproduction introduces the possibility of variation into a population, and this is why most fungi have a sexual phase. To achieve sexual reproduction it is necessary to have two mating type haploid nuclei (n + n), or a diploid (2n) nucleus. In the case of the two haploid nuclei they must fuse to form a diploid first, but once fused the nuclei undergo meiosis, which is the reduction division that potentially brings about variation in the progeny. These events are followed by the formation of spores (ascospores), which in most cases are resting spores that can withstand adverse conditions. A lichen thallus is reconstituted by the combining of germinated ascospores and a suitable alga.
Morphology of reproductive structures
Majority of lichens are Ascomycetes which produce spores in sac like structures called as Asci. Asci are formed in structures called as fruiting bodies (Ascocarps) like apothecia, perithecia or pseudothecia same as that of non-lichenized fungi. These are characterized by ascocarps (ascomata) containing a fruiting layer (hymenium) of spore containing asci and interascal sterile thread like paraphyses. Basidiomycetes contain basidiocarp.
Apothecia: Apothecia are typically open disc or cup-shaped structures. The hymenium consisting of apically free paraphyses and asci forms a thin layer lining the inner surface of the disc. The disc in most crustose species is 0.5-3 mm in diameter. In larger foliose lichens it exceeds 10-20 mm. Apothecia may be weakly delimited patches as in Cryptothecia. Elongate apothecia exposing the disc by a narrow or broad slit, called as lirellae (Fig.17) can be seen in Graphidaceae and Opegraphaceae. The tissue supporting the margins of the discs in apothecia (excipulum) may lack photobiont cells (true exciple) when its color is similar to disc or stalk. These types of apothecia called as lecideine are characteristic of species in Cladoniaceae, Lecideaceae, Stereocaulaceae etc. The exciple may be combined with an outer photobiont containing tissue (thalline exciple) and same colour as the thallus. This type of apothecium with thalline exciple is called as Lecanorine (Fig.17) and can be seen in Lecanoraceae, Parmeliaceae, Lobariaceae (Hawksworth and Hill, 1984).
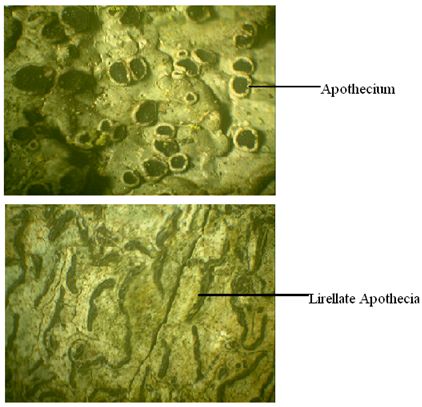
Fig.17 – Lecanorine apothecia (above) and Lirellate apothecia (below)
Perithecia: Perithecia are flask shaped structures 0.5-2 mm in diameter and immersed in thallus (Fig.18). The spores are released through the pore which is in upper surface of thallus called as ostiole. The perithecial wall is derived from thalline tissue and may be carbonized or made up of several layers. In some genera a separate carbonized shield like layer (involucrellum) extends outwards from the ostiole as in Verrucaria species.

Fig.18 – Perithecia
Ascus: These are sac like structures in which ascospores are formed. The ascus contains two layers of wall- an outer inextensible and inner extensible one. The outer ascal wall (exotunica) dehisces apically by means of a cap, and inner wall (endotunica) glides out and spores are released through an elastic pore. This type of ascus is called as functionally bitunicate or fissitunicate (Hale, 1983).
Ascospores: Ascospores in lichen vary in size, shape, structure and septation and may be colourless, greenish or brown (Fig.19). The septation is most useful feature separating spores. The different types of spores are:
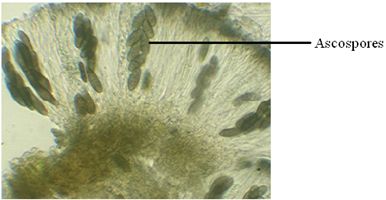
Fig.19 – A microscopic view of Ascospores
Simple spores: Unicellular and non-septate, small (10-30 µm) and thin walled as in Lecanora, Parmelia, Usnea. Rarely (Pertusaria) it is large and thick walled.
Transversely septate spores: Elongate and multicellular with 1-40 transverse cross walls. (Graphis, Pyrenula, Bacidia)
Muriform spores: Multicellular with both transverse and longitudinal walls, large in size (Phaeographina, Umbilicaria, and Lopadium)
Polarilocular spores: The septum is thick and penetrated by a thin canal (Caloplaca, Xanthoria). The number of spores is commonly eight but it can vary from one in certain Mycoblastus species to several hundred in Acarospora.
Pycnidia
Another group of structures that are important in sexual reproduction of lichen are the pycnidia. Pycnidia occur in all lichen groups. They are flask shaped structures embedded in the thallus of the lichen (similar in shape and size to perithecia) that produce hundreds of little flecks of fungal hyphae called conidia. Conidia can function as male spermatia, fusing with the “female” nucleus in the asci. However, conidia can also function as asexual fungal spores by falling onto the substratum, germinating, encountering a suitable alga and forming lichen.
Life Cycle of Lichens
Life cycle of lichens consists of two phases - Sexual phase and asexual phase (Figure 20). The fungal part of the lichen carries its normal sexual cycle. It leads to the formation of fruiting bodies like apothecia, perithecia, pycnidia which produce the spores. These spores germinate and produce new mycelium, which combine with suitable algae and produces new lichen thallus. The alga of lichen reproduces by cell division. Most of the lichens reproduce asexually. Asexual phase (Vegetative dispersal) is with the help of special propagules unique to lichens. They produce vegetative propagules like Soredia, Isidia, lobules and fragments. After growth these detach from lichen body and develop directly into a new thallus.
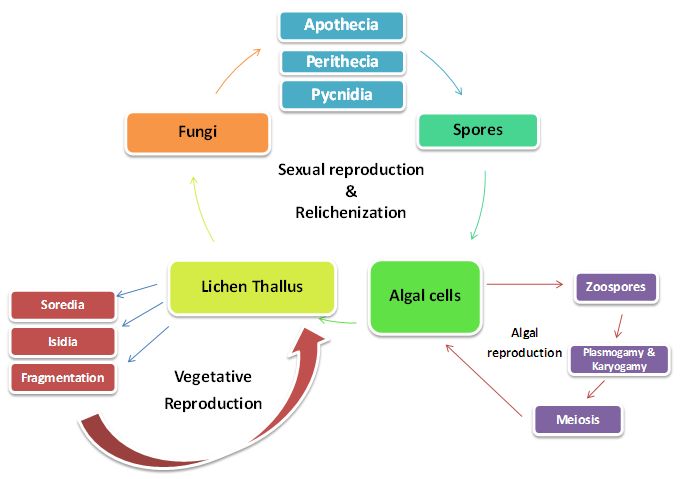
Fig.20 – Life cycle of a Lichen
| Back | Next |
