|
ABSTRACT
11 species of algae of 10 genera were collected, of which 6 genera and 5 species belong to chlorophyta, 6 genera of 7 species belong to Rhodophyta,1 genus of 1 species belongs to Phaeophyta. Out of the 11 seaweeds recorded in this estuary Chlorophyta Division dominated during the entire period of study across all stations. Utilization of estuarine algae- great importance as the only natural source of phycocolloids (Agar, Algin and Carrageenan), consumed as staple food in Southern Asian countries also supplements for protein and mineral deficient food items and animal/ poultry feeds. With increasing fuel demand around the world leading to dearth of inventing alternative and sustainable fuel - Seaweed serves as a promising feedstock for biofuel production.
Keywords__ Seaweeds, Estuary, Phycocolloids, Biofuel, Feedstock, Sustainable fuel
INTRODUCTION
The Indian west coast is characterised by numerous rivers which merge with the Arabian Sea forming estuarine areas towards their confluences. Estuaries are highly productive ecosystems, supporting rich biodiversity adapted to very fluctuating and dynamic environment, subjected to, notably, great variations in salinity and water flow regimes, depending on the quantities of fresh water mingling with marine water and variations in rising and receding tides. The macro-algae or large sized algae inhabiting the estuaries are normally also marine inhabitants. They are also included in the general category of ‘seaweeds’. The Indian coastline harbours diverse kind of such seaweeds (Oza and Zaidi 2001). These seaweeds of the shallow coastal waters are very important primary producers, having special affinities towards rocky substratum and coral formations, where they are attached, at least in their early stages, and many throughout their lifespans.
Some of the estuarine seaweeds, especially of green algae of Chlorophyta may even tolerate inundation with fresh water, especially during the rainy season. Others of the Rhodophyta and Phaeophyta are found in more saline areas only. The growth and accumulation of seaweeds in estuaries are common sight throughout the world (Boyer and Fong, 2005). The products of many of the seaweeds are unique nature, which can hardly be obtained from land or fresh water plants for e.g. agar, carrageenan and alginates (Rao et. al, 2006). For many countries of the East and South East like Japan, China, Philippines, Indonesia, seaweeds find numerous uses as food, fodder, manure, medicine etc.
Apart from these classical utilizations of seaweeds, attempts are currently on way for screening estuarine algae as a potential feedstock for biofuel production, which gains significance in these times of energy crisis due to increasing population, urbanization and industrialization (Sharma & Singh, 2017). The inexorable usage of energy primarily supplied from fossil fuel over past few decades has increased the rate of emission of greenhouse gases (GHG) dramatically. The escalating prices of crude oil (from US$80 per barrel in 2006 to US$ 108.01 per barrel in 2013 which is likely to shoot up in future due to unrest in oil producing countries), and rising pollution threatening to engulf the planet with climate change compelled the European Commission to look into alternatives. The EC targeted substituting 5.75% fossil fuels with alternate fuels in 2010 and subsequently aimed raising such substitution to 20% by 2020. Efforts are currently under way in many parts of the world to reduce dependence on fossil fuels, especially coal and petroleum, and find more of renewable and sustainable energy alternatives, which could be cheaper as well as with lower GHG emission.
In this context, in the quest of humans towards production of more viable alternative energy sources, biofuels have captured much attention from researchers. Today we are getting increasingly familiar with the booming prospects of eco-friendlier alternative fuels, of which biofuels like bioethanol and biodiesel are gaining prominence. Biofuels from sugars, starches and oil crops may be considered first generation biofuels. Biofuels from lingo cellulosic biomass and residues belong to the second generation biofuels, whereas those under development from algae belong to the third generation. Sourcing biofuels from sugars, starches and lignocellulosic biomass are becoming contentious issues in the ongoing food fuel debate (Allen et al.,2013). In this context, the macroalgae with its relative ease for cultivation, with higher yield potential faster multiplication, and certainly not at the cost of land or food or other conventional fuel resources are emerging as some of the most prospective candidates for biofuel production.
This work mainly focuses on the occurrence, season-wise, of seaweeds in the various salinity zones of Aghanashini estuary, which is a basic need before any viable project towards their commercially utilisation potential can be explored, especially as regards their potential for biofuel production, research leading to which is our ultimate aim for the next phase, after the current exploratory surveys.
- MATERIALS AND METHODS:
Study site:
Present study was conducted in Aghanashini Estuary (Lat 14.391° to 14.585° N; Long 74.304° to 74.516° E) of Kumta taluk in the Uttara Kannada district of central west coast of India in Karnataka State. Six stations in Aghanashini estuary were selected, namely Divigi Hegde, Bargi, Kagal, Belekan, and Kirubele (Table 1).
Table 1: Sampling locations GPS points |
Location |
Station_id |
Station Name |
Latitude |
Longitude |
Upper estuarine stations |
S1 |
Divigi |
14°26'39.36"N |
74°26'13.44"E |
S2 |
Hegde |
14°28'29.22"N |
74°25'41.04"E |
Mid estuarine stations |
S3 |
Kagal |
14°30'28.33"N |
74°22'37.78"E |
S4 |
Bargi |
14°31'46.13"N |
74°22'40.14"E |
Estuarine mouth stations |
S5 |
Belekan |
14°31'8.76"N |
74°20'40.14"E |
S6 |
Kirubele |
14°30'50.29"N |
74°21'31.57"E |
Tide: The tidal cycle in Aghanashini estuary is semidiurnal, with two high and two low waters with unequal tidal amplitudes each day. The area of intertidal exposure depends on the tidal amplitudes each day. Thus, duration and area of exposure in turn not only depend on the tidal amplitude but also on topography of the coast.
Seaweed sampling: Monthly sampling during low tide was carried out for identification of Seaweeds. At each station a variable transect length depending upon the tide and a quadrat of 0.25m2 was placed along the transect and all the seaweed species present within the quadrat were collected. The samples were washed thoroughly to remove adhered epiphytes and sand and sorted out species wise. Collected seaweed samples were identified using standard identification keys from www.algaebase.com.
RESULTS:
Totally 9 species of 8 genera were recorded of which 2 belong to Chlorophyta, 5 to Rhodophyta and only 1 to Phaeophyta. Table 2 & 3 summarizes the distribution of these seaweeds in the stations. The seaweeds thrive well in saline waters and on rocky shores as they serve as the best substratum for algal spores’ attachment. Many organisms such as Crab species and Gastropods feed on these seaweeds, whereas some polychaetes worms and amphipods hide and live in the nooks and crannies and shady corners of seaweeds. Seaweeds grow abundantly in the intertidal and subtidal regions of the sea, estuaries and backwaters. They flourish well on rocky substratum; corals or where suitable substrata are available for their attachment. The substratum of the estuary studied was muddy or sandy with or without oyster beds. Rocky shores are found towards the estuarine mouth, where the waves are stronger and to the north and south are hills.
A definite zonation pattern was observed with regard to several species of seaweeds. On the rocks of the upper littoral zone, i.e. towards the top line where the surging tides reach, grow Ulva lactuca, Enteromorpha intestinalis, both of green algae (Phylum Chlorophyta) along with Grateloupia filicina, a red alga (Phylum Rhodophyta). Gelidium pusillium, another red alga grows in tufts in the mid littoral zone which experiences constant variations in water submersion during high and low tide. A red alga Gracilaria corticata, strongly fixed to the substratum, was found in the deeper mid littoral zone. Padina tetrastromatica, a flat, fan shaped brown alga (Phaeophyta)sensitive to prolonged exposure and desiccation was more associated with the were the Lower littoral zone. Enteromorpha sp. were observed in all the stations on sandy shores, in mangrove roots and on oyster beds, forming tangled masses of bright green.
Of all these algae, the green macroalgae occurred in abundance during monsoon and Post monsoon seasons. The brown macroalgae begins towards the end of the rainy season and flourishes in the post monsoon. The euryhaline Enteromorpha grows tolerant of wide variations in salinity having its range from the upstream Divigi and mid-estuarine Kagal and Bargi stations growing with ease tolerant of salinity nearly down to fresh water conditions during the peak of the South-west Monsoon, to the estuarine mouth stations of Belekan and Kirubele, where it occurs firmly moored to rocky base resisting strong waves and higher saltiness, where it occurs in association with the more stenohaline Ulva lactuca which withstands salinity variations to lesser extent only. Both are dominant on the rocks Monsoon and Post Monsoon, where the red algae like Gelidium pusillium, G. micropterum Grateloupia filicinia, Gracilaria corticata and Chondria armata begins in the Monsoon and reaches profusion in the Post monsoon. On the contrary, the brown alga Padina tetrastromatica is a more Post monsoon entrant along the seaward rocky shore.
Such distribution pattern of seaweeds is expressive of variations in limiting factors viz. salinity, temperature and relative submersion in the intertidal zone and even under constant flooding in the estuary, as is the case with Enteromorhpa in particular (Lobban,1984). Salinity is a crucial factor for seaweeds at developing stage, as freshwater intrusion can rupture their embryonic stages, especially so for the brown seaweeds which emerge on the intertidal rocks during the post-monsoon, and that too in the lower littoral zone where the lesser variability in temperature, salinity and near constant submersion favour their optimal growth. The Table-2 makes explicitly clear that a single seaweed (Enteromorpha) alone is capable of reaching the mid-and upstream portion of estuary, where the salinity dips to nearly zero levels during several days of peak monsoon rains, even tolerant of fresh water flushing during floods which are not infrequent in Aghanashini. Further, phyletic consideration shows that it is the green alga alone that occurs the upper reaches (Figure 4, Tables 2 and 3).
Table 2:Number of algal genera and species recorded from Aghanashini estuary |
LOCATION |
Chlorophyta |
Rhodophyta |
Phaeophyta |
Total |
|
Genera |
species |
Genera |
species |
Genera |
species |
Genera |
Species |
Divigi |
1 |
- |
- |
- |
- |
- |
1 |
1 |
Hegde |
1 |
- |
- |
- |
- |
- |
1 |
- |
Kagal |
1 |
- |
- |
- |
- |
- |
1 |
- |
Bargi |
1 |
- |
- |
- |
- |
- |
1 |
- |
Belekan |
2 |
2 |
4 |
5 |
1 |
1 |
7 |
8 |
Kirubele |
2 |
2 |
2 |
2 |
1 |
1 |
5 |
5 |
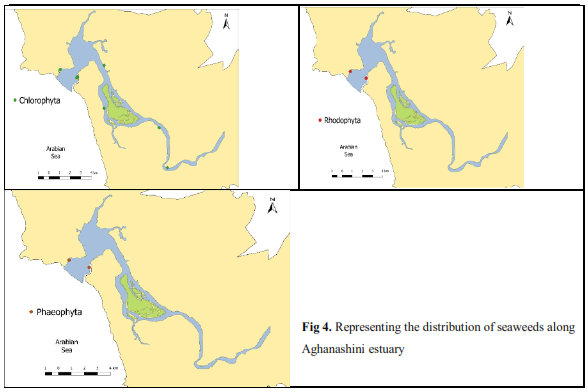
Table 3. Distribution of macroalgae along Aghanashini estuary sampling station |
Sl no. |
Algae |
Locations |
Divigi |
Hegde |
Kagal |
Bargi |
Belekan |
Kirubele |
|
Green algae |
1 |
Ulva lactuca |
|
|
|
|
+ |
+ |
2 |
Enteromorpha intestinalis |
|
|
|
|
+ |
+ |
3 |
Enteromorpha sp. |
+ |
+ |
+ |
+ |
|
|
|
Red algae |
4 |
Gracillaria corticata |
|
|
|
|
+ |
|
5 |
Gelidium pusillium |
|
|
|
|
+ |
|
6 |
Gelidium micropterum |
|
|
|
|
+ |
+ |
7 |
Grateloupia filicinia |
|
|
|
|
+ |
+ |
8 |
Chondria armata |
|
|
|
|
+ |
|
|
Brown algae |
9 |
Padina tetrastromatica |
|
|
|
|
+ |
+ |
Note: + presence |

2. POTNETIAL FOR SEAWEED UTILISATION
2.1 Overall commercial prospects: Seaweeds are known for their commercial values as sources of certain unique products like agar, carrageenan, alginate, which have various applications in dye industries, and as emulsifier and stabilizer, in dairy products. Smit (2004) estimates the global utilisation of (non-fuel) products from marine macroalgae as a multi-billion-dollar industry.
Seaweeds contain more than 60 trace elements along with protein, iodine, bromine, vitamins and substances of stimulatory and antibiotic nature. Around 221 seaweed species are known to be exploited by mankind with 66% of them being used as food. Edible seaweeds such as Ulva species popularly known as “Sea lettuce" are consumed as fresh salads or cooked as vegetables along with rice (FAO, 2003). Containing 16-30% protein on dry weight basis which is higher than cereals, or at par with or higher than eggs or fish, their proteins can be extracted as dry powders and added to foods deficient proteins (CMFRI, 1987). Enetromorpha sp. known as ‘green laver’ and Gracilaria sp. known as ‘sea moss’ are also consumed in Japan (Khan, S. 2003) (Table 4). In India, seaweeds are not utilized for food items directly, except sparingly; for eg. Gracilaria edulis for making gruel in coastal areas of Tamil Nadu. Seaweeds washed ashore are utilized to manure coconut plantations in some coastal regions in India. Manure from seaweeds maintains high level of available nitrogen in soil and the easy decomposability of seaweed organic matter is beneficial for the growth of soil microorganisms. Fresh seaweeds are cleaned and ground to prepare seaweed meal, used along with fish meal for use as poultry feed. Reviews and feeding trials on the use of seaweeds as supplementary animal feed, for chicks, sheep and cattle, are conducted in Japan, Germany, UK, Norway etc. Their medicinal uses in China and Japan, especially to treat goiter and other glandular diseases, probably date back to 3000 B.C. Ancient voyagers used seaweeds to prevent scurvy during long voyages. Red seaweeds were used to treat various intestinal disorders, and due to their gelatinous property, helps in relieving constipation and related discomforts. Seaweeds are known as prolific producers of biologically active compounds Most of these substances exhibit an appreciable number of distinct biological activities such as antitumoral, antiviral, antifungal, insecticidal, cytotoxic, phytotoxic, and antiproliferative actions, of which terpenes and polyphenols are the majority (Peres et.al, 2012). These seaweed substances are recognized as potential sources of the antibiotic substances and synthesis of different metabolites from seaweeds is an indicator of the presence of antimicrobial active compounds. Seaweeds also contain bioactive components which affects germination of pathogenic bacteria (Shafay et. al, 2016).
2.2 Third generation biofuels
Apart from these various commercial uses existing, macroalgal species in recent years, have gained considerable global attention as source of third generation biofuels (Horn et al., 2000; Goh and Lee, 2010; Wang et al., 2011; Khambhaty et al., 2012; Meinita et al., 2012), which is our primary field of interest and the motivation for the ongoing study. Gaseous fuel production from seaweed was first considered in 1978 in US. The major advantages offered by seaweeds over terrestrial biomass are (1) higher biomass production rate per unit area, (2) easier depolymerisation as it does not contain lignin in their cell wall (3) do not compete with agricultural plants for land, and (3) require no agricultural input such as fertilizer, pesticides and water (Jones and Mayfield, 2012).
Table 4. Economically important Seaweed species in Aghanashini estuary |
|
Seaweeds |
Agar |
Alginate |
Carrageenan |
Edible |
Fertilizers |
Animal / poultry Feed |
Medicinal |
Ulva |
|
|
|
+ |
+ |
+ |
|
Enteromorpha |
|
|
|
+ |
+ |
+ |
+ |
Gracilaria |
+ |
|
|
+ |
|
+ |
|
Gelidium |
+ |
|
+ |
|
|
|
+ |
Grateloupia |
|
|
|
|
|
|
|
Chondria |
+ |
|
+ |
+ |
|
|
+ |
Padina |
|
+ |
|
|
|
+ |
+ |
Macroalgae provides huge amounts of carbohydrate year around (Matsumoto et al., 2003), which can be utilised for bioethanol production by fermentation process as summarized in fig 4. The process involves identification of seaweed followed by chemical characterization i.e. determining percentage carbohydrate composition for bioethanol production. After the characterization seaweeds are dewatered and macerated to fine powder and subjected to pre-treatment: acid hydrolysis or enzymatic hydrolysis method is applied in order to break down the complex polysaccharide synthesized by the seaweed. In fermentation process, the reduced sugars are fed to the fermentative organisms (i.e. yeast) to convert the liquefied seaweed substrate (reduced sugars) to alcohol (bioethanol) and carbon dioxide, and other value added products. Harvesting the wild stocks and utilizing it for commercial or biofuel production purpose is energy intensive. Cultivation of seaweeds for commercial exploitation is taken up by countries like Japan, Philippines, and China at very large scale. In India, Kappaphycus alvarezii one of the economically important red algae, yielding carrageenan, a commercially important polysaccharide being cultivated along Tamil Nadu coast, Gracilaria verrucosa from Chilka lake, Odisha (Kumar, S. 2013), red seaweed, Enteromorpha compressa and Ulva lactuca, green alga from Gulf of Mannar, Mandapam coast (Suganya, T. et al, 2013) etc. are being used for production of agar and biofuel.
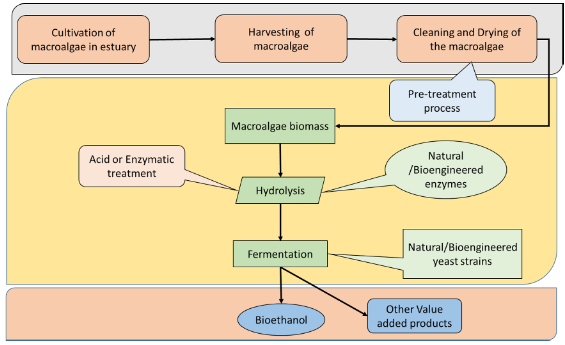
Fig 4: Schematic representation of Bioethanol production from Macroalgae from estuary.
Table 9. Bioethanol production from Seaweed distributed along Indian coast
|
Seaweed |
Conditions of hydrolysis |
Sugars produced |
Yield of sugar
[g-sugar/g-seaweed] |
Concentration of
sugar [g/L] |
Cultivated area |
Reference |
Kappaphycus alvarezii |
0.9 N H2 SO4 100°C for 1 h |
Reducing sugar |
0.30 |
|
Mandapam, Tamil Nadu coast |
Khambhaty et al., 2012 |
Gracillaria verrucosa |
1.0 L of 5.0% NaOH solution at 80°C for 2h
1.5% H2SO4 for 2 h
Commercial cellulase from Trichoderma reesei (ATCC 26921)and b-glucosid ase from Aspergillus niger |
Glucose |
|
0.43 g/g |
Chilika lake, Orrisa |
Kumar, S. 2013 |
Biodiesel production from Seaweed distributed along Indian coast |
|
|
Seaweed |
Condition |
Biodiesel yield (%) |
Cultivated area |
Reference |
Enteromorpha compressa |
Acid esterification was carried out to reduce Free Fatty Acid from 6.3% to 0.34% with optimized parameters of 1.5% H2SO4, 12:1 methanol–oil ratio, 400 rpm at
60 _C and 90 min of reaction time |
90.6% |
Gulf of Mannar, along Mandapam coast |
Suganya, T. et al, 2013 |
Ulva lactuca |
Ultra sonication at 24kHz temp 50oC for 5 min, autoclave at 121oC,15lbs for 5 min, oil extraction by solvent method |
10.88% |
Gulf of Mannar, along Mandapam coast |
Suganya, T. et al, 2013 |
CONCLUSION
India with its vast coastline and a significant number of estuaries has potential for cultivation and production of economically important seaweeds and rank among the leading producers of seaweed, especially biofuels. Seaweeds apart from satisfying the Industrial need for phycocolloids, has a potential to satisfy the need for advanced biofuels in India. A viable seaweed industry would require an updated study on available seaweed resources from Indian coast, including the estuaries, where seaweeds with high potential biomass production per hectare, high percentage of carbohydrate synthesis are to be found out, suitable site characteristics for massive production and cost-effective method for cultivation are to be investigated. A viable, seaward based, energy industry will be a great boon for the country, which has low production, but escalating import needs of fossil fuels, the rising use of which is not only draining our financial resources but also creating alarming levels of transport and industrial pollution within the country and for the planet as a whole.
REFERENCES
- Allen, E., Browne, J., Hynes, S., & Murphy, J. D. (2013). The potential of algae blooms to produce renewable gaseous fuel. Waste management,33(11), 2425-2433.
-
Boyer, K. E., & Fong, P. (2005). Macroalgal-mediated transfers of water column nitrogen to intertidal sediments and salt marsh plants. Journal of Experimental Marine Biology and Ecology, 321(1), 59-69.
- CMFRI, K. (1987). Seaweed research and utilization in India. CMFRIBulletin, 41, 1-116.
- Goh, C. S., Tan, K. T., Lee, K. T., & Bhatia, S. (2010). Bio-ethanol from lignocellulose: status, perspectives and challenges in Malaysia. Bioresource Technology, 101(13), 4834-4841.
- Horn, S. J., Aasen, I. M., & Østgaard, K. (2000). Production of ethanol from mannitol by Zymobacter palmae. Journal of Industrial Microbiology and Biotechnology, 24(1), 51-57
- Jones, C. S., & Mayfield, S. P. (2012). Algae biofuels: versatility for the future of bioenergy. Current opinion in biotechnology, 23(3), 346-351.
- Kumar, S., Gupta, R., Kumar, G., Sahoo, D., & Kuhad, R. C. (2013). Bioethanol production from Gracilaria verrucosa, a red alga, in a biorefinery approach. Bioresource technology, 135, 150-156.
- Khan, S. I., & Satam, S. B. (2003). Seaweed mariculture: scope and potential in India. Aquaculture Asia, 8(4), 26-29.
- Khambhaty, Y., Mody, K., Gandhi, M. R., Thampy, S., Maiti, P., Brahmbhatt, H., ... & Ghosh, P. K. (2012). Kappaphycus alvarezii as a source of bioethanol. Bioresource technology, 103(1), 180-185.
- Lobban, C. S., & Harrison, P. J. (1994). Seaweed ecology and physiology. Cambridge University Press.
- McHugh, D. J. (2003). A guide to the seaweed industry. Rome: Food and Agriculture Organization of the United Nations.
- Meinita, M. D. N., Kang, J. Y., Jeong, G. T., Koo, H. M., Park, S. M., & Hong, Y. K. (2012). Bioethanol production from the acid hydrolysate of the carrageenophyte Kappaphycus alvarezii (cottonii). Journal of applied phycology, 24(4), 857-862.
- Matsumoto, M., Yokouchi, H., Suzuki, N., Ohata, H., & Matsunaga, T. (2003). Saccharification of marine microalgae using marine bacteria for ethanol production. In Biotechnology for Fuels and Chemicals (pp. 247-254). Humana Press.
- Oza R.M. & Zaidi S.H., 2001. A Revised checklist of Indian marine algae. Central Salt and Marine Chemicals Research Institute, Bhavnagar, 296 pp.
- Rao, P. S., & Mantri, V. A. (2006). Indian seaweed resources and sustainable utilization: scenario at the dawn of a new century. CURRENT SCIENCE-BANGALORE-, 91(2), 164.
- Sharma, Y. C., & Singh, V. (2017). Microalgal biodiesel: A possible solution for India’s energy security. Renewable and Sustainable Energy Reviews, 67, 72-88
- Suganya, T., Gandhi, N. N., & Renganathan, S. (2013). Production of algal biodiesel from marine macroalgae Enteromorpha compressa by two step process: optimization and kinetic study. Bioresource technology, 128, 392-400.
-
Shimaa M. El Shafay, Samh S. Ali, Mostafa M. El-Sheekh,2016. Antimicrobial activity of some seaweeds species from Red sea, against
|



