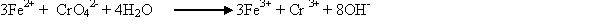
CES TECHNICAL REPORT - 110
ENERGY AND WETLANDS RESEARCH GROUP
CENTRE FOR ECOLOGICAL SCIENCES
INDIAN INSTITUTE OF SCIENCE
BANGALORE 560 012
Web : http://ces.iisc.ac.in/energy
http://ces.iisc.ac.in/biodiversity
Open Source GIS : http://ces.iisc.ac.in/grass
Email : cestvr@ces.iisc.ac.in
energy@ces.iisc.ac.in
SUMMARY
Water resources are of critical importance to both natural ecosystem and human developments. Increasing environmental pollution from industrial wastewater particularly in developing countries is of major concern. Heavy metal contamination exists in aqueous waste streams of many industries, such as metal plating facilities, mining operations, tanneries, etc. Some metals associated with these activities are cadmium, chromium, iron, nickel, lead and mercury. Heavy metals are not biodegradable and tend to accumulate in living organisms causing diseases and disorders. Many industries like dye industries, textile, paper and plastics use dyes in order to colour their products and also consume substantial volumes of water. As a result they generate a considerable amount of coloured wastewater. The presence of small amount of dyes (less than 1 ppm) is highly visible and undesirable. Many of these dyes are also toxic and even carcinogenic and pose a serious threat to living organisms. Hence, there is a need to treat the wastewaters containing toxic dyes and metals before they are discharged into the waterbodies. Many physico-chemical methods like coagulation, flocculation, ion exchange, membrane separation, oxidation, etc are available for the treatment of heavy metals and dyes. Major drawbacks of these methods are high sludge production, handling and disposal problems, high cost, technical constraints, etc. This necessitates cost effective and environmentally sound techniques for treatment of watsewaters containing dyes and metals. During the 1970s, the increasing awareness and concern about the environment motivated research for new efficient technologies that would be capable of treating inexpensively, waste waters polluted by metals and dyes. This search brought biosorption/adsorption to the foreground of scientific interest as a potential basis for the design of novel wastewater treatment processes. Several adsorbents are currently used which are by-products from agriculture and industries, which include seaweeds, molds, yeast, bacteria, crabshells, agricultural products such as wool, rice, straw, coconut husks, peat moss, exhausted coffee waste tea leaves, walnut skin, coconut fibre, etc. Adsorption/Biosorption using low cost adsorbents could be technically feasible and economically viable sustainable technology for the treatment of wastewater streams. Low cost adsorbents are nothing but materials that require little processing, are abundant in nature or is a byproduct or waste material from another industry.
| |
 |
INTRODUCTION
Water has the central role in mediating global-scale ecosystem processes, linking atmosphere, lithosphere and biosphere by moving substances between them and enabling chemical reactions to occur. Natural waters are never pure H2O but a complex and ever-changing mixture of dissolved inorganic and organic molecules and suspended particles.
1.1 DISTRIBUTION OF WATER IN THE WORLD
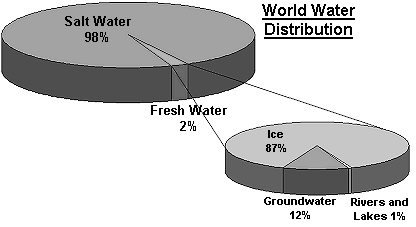
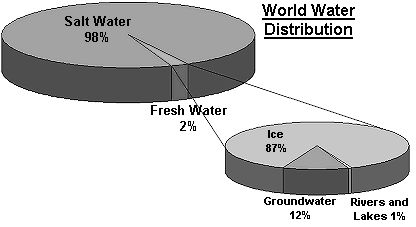
Water is essential to human life and to the health of the environment. As a valuable natural resource, it comprises marine, estuarine, freshwater (river and lakes) and groundwater environments, across coastal and inland areas. Most of the water found on this planet is held within the oceans (~97%). The use of this sink of water by humans is limited because of the dissolved salts it contains. Table 1 below describes the major reservoirs of water found on the Earth. Icecaps and glaciers contain about 2 % of the world's total water, and about 60 % of the freshwater supply. The use of this water by humans is very restricted because of its form and location. Humans primarily use the freshwater found in groundwater, lakes, rivers, etc., which is less than 1% of the earth's supply.
Table 1 : Inventory of water at the Earth's surface.
Reservoir |
Volume (cubic km x 10,000,000) |
Percent of Total |
| Oceans |
1370 |
97.25 |
| Ice Caps/Glaciers |
29 |
2.05 |
| Deep Groundwater |
5.3 |
0.38 |
| Shallow Groundwater |
4.2 |
0.30 |
| Lakes |
0.125 |
0.01 |
| Soil Moisture |
0.065 |
0.005 |
| Atmosphere |
0.013 |
0.001 |
| Rivers |
0.0017 |
0.0001 |
| Biosphere |
0.0006 |
0.00004 |
Figure 1 : World water distribution
Water has two dimensions that are closely linked - quantity and quality. Water quality is commonly defined by its physical, chemical, biological and aesthetic (appearance and smell) characteristics. A healthy environment is one in which the water quality supports a rich and varied community of organisms and protects public health (Ramachandra et al., 2002)
Water quality in a body of water influences the way in which communities use the water for activities such as drinking, swimming or commercial purposes. More specifically, the water may be used by the community for:
- supplying drinking water
- recreation (swimming, boating)
- irrigating crops and watering stock
- industrial processes
- navigation and shipping
- production of edible fish, shellfish and crustaceans
- wildlife habitats
1.2 TYPES OF AQUATIC ECOSYSTEM
The aquatic ecosystem can be broken down into two basic regions, freshwater (i.e, ponds and rivers) and marine (i.e, oceans and estuaries) (Ramachandra and Ahalya, 2001).
1.2.1 Freshwater Regions
Freshwater is defined as having a low salt concentration—usually less than 1%. Plants and animals in freshwater regions are adjusted to the low salt content and would not be able to survive in areas of high salt concentration (i.e, ocean). There are different types of freshwater regions: ponds and lakes, streams and rivers, and wetlands. The following sections describe the characteristics of these three freshwater zones (Ramachandra and Ahalya, 2001).
- Ponds and Lakes : These regions range in size from just a few square meters to thousands of square kilometers.. Many ponds are seasonal, lasting just a couple of months (such as sessile pools) while lakes may exist for hundreds of years or more. Ponds and lakes may have limited species diversity since they are often isolated from one another and from other water sources like rivers and oceans.
- Streams and Rivers : These are bodies of flowing water moving in one direction. Streams and rivers can be found everywhere—they get their starts at headwaters, which may be springs, snowmelt or even lakes, and then travel all the way to their mouths, usually another water channel or the ocean. The characteristics of a river or stream change during the journey from the source to the mouth.
- Wetlands : Wetlands are areas of standing water that support aquatic plants. Marshes, swamps, and bogs are all considered wetlands. Plant species adapted to the very moist and humid conditions are called hydrophytes. These include pond lilies, cattails, sedges, tamarack, and black spruce. Marsh flora also include such species as cypress and gum. Wetlands have the highest species diversity of all ecosystems. Many species of amphibians, reptiles, birds (such as ducks and waders), and furbearers can be found in the wetlands.
1.2.2 Marine Ecosystems
Marine regions cover about three-fourths of the Earth’s surface and include oceans, coral reefs, and estuaries. Marine algae supply much of the world’s oxygen supply and take in a huge amount of atmospheric carbon dioxide. The evaporation of the seawater provides rainwater for the land.
- Oceans : The largest of all the ecosystems, oceans are very large bodies of water that dominate the Earth’s surface.
- Coral Reefs : Coral reefs are widely distributed in warm shallow waters. They can be found as barriers along continents , fringing islands, and atolls. Naturally, the dominant organisms in coral reefs are corals. Corals are interesting since they consist of both algae (zooanthellae) and tissues of animal polyp. Since reef waters tend to be nutritionally poor, corals obtain nutrients through the algae via photosynthesis and also by extending tentacles to obtain plankton from the water. Besides corals, the fauna include several species of microorganisms, invertebrates, fishes, sea urchins, octopuses, and sea stars.
- Estuaries : Estuaries are areas where freshwater streams or rivers merge with the ocean. This mixing of waters with such different salt concentrations creates a very interesting and unique ecosystem. Microflora like algae, and macroflora, such as seaweeds, marsh grasses, and mangrove trees (only in the tropics), can be found here. Estuaries support a diverse fauna, including a variety of worms, oysters, crabs, and waterfowl.
| |
 |
1.3 THREATS TO AQUATIC ECOSYSTEMS
Increasingly, aquatic ecosystems are under increasing stress due to the rapidly growing population, technological development, urbanisation and economic growth.Human activities are causing aquatic species to disappear at an alarming rate. It has been estimated that between 1975 and 2015, species extinction will occur at a rate of 1 to 11 percent per decade. Aquatic species are at a higher risk of extinction than mammals and birds. Losses of this magnitude impact the entire ecosystem, depriving valuable resources used to provide food, medicines, and industrial materials to human beings (Ramachandra et al., 2005). Runoff from agricultural and urban areas, the invasion of exotic species, and the creation of dams and water diversion have been identified as the greatest challenges to freshwater environments. Some of the threats and causes to aquatic ecosystem are presented in Table 2.
Table 2 : Causes and type of threats to aquatic ecosystem
Type of Threat |
Causes |
| Water Regime |
Flooding; reclamation; water diversion; erosion/siltation; roads; irrigation; water works (floods) |
| Water Pollution |
Solid waste refuse; siltation; sewage/fecal; mining; pesticides; fertilizers; salinization of soils |
| Physical Modification |
Erosion; flooding; clearance and fire; sedimentation; infrastructure/housing; quarrying and sand winning; hunting; recreation; agriculture |
| Over-exploitation |
Fishing; fuel wood; hunting of birds and mammals; grazing |
Human impacts on the quality and quantity of fresh water can threaten economic prosperity, social stability, and the resilience of ecological services that aquatic systems provide. Rising demand for fresh water can sever ecological connections in aquatic systems, fragmenting rivers from floodplains, deltas, and coastal marine environments. It also can change the quantity, quality, and timing of freshwater supplies on which terrestrial, aquatic, and estuarine ecosystems depend (Ramachandra et al., 2002). Fresh water is already a limiting resource in many parts of the world. In the next century, it will become even more limiting due to increased population, urbanization, and climate change. This limitation will be caused not just by increased demand for water, but also by pollution in freshwater ecosystems. Pollution decreases the supply of usable water and increases the cost of purifying it. Some pollutants, such as heavy metals or chlorinated organic compounds, contaminate aquatic resources and affect food supplies. This nutrient pollution, combined with human demand for water, affects biodiversity, ecosystem functioning, and the natural services of aquatic systems upon which society depends. Point sources are 'pipeline' discharges of pollutants to receiving waters, e.g. domestic sewage discharges or industrial waste effluents from factories or plants. They are relatively easy to identify and isolate. In contrast, non-point pollution results from storm runoff, which transports polluting materials diffusely and over land.
Major water pollutants include a variety of organic and inorganic chemicals such as heavy metals and industrial compounds. They can affect human health and/or interfere with industrial or agricultural water use. If the level of a pollutant in the water supply exceeds an acceptable level for a given water use (e.g., domestic or industrial water supply), the water is considered unsafe or too degraded for that use. Solutions to such pollution problems, therefore, usually focus on reduction of pollution at the source and/or treatment of the polluted water prior to use (Ahalya and Ramachandra, 2002). It is clear that inland aquatic ecosystems are under increasing threat. As the pervasive and intractable nature of threats makes them difficult to manage, avoidance through protection mechanisms is hugely cost-effective and beneficial. Given that aquatic ecosystem degradation is ubiquitous and increasing, the identification and protection of ecosystems value, is urgent. As most of the industrial processes are located near the water bodies, they are increasingly polluted by a number of organic and inorganic materials. Two of the most hazardous pollutants that are affecting them include heavy metals and dyes.
| |
 |
1.4 METALS AND DYES IN THE AQUATIC ECOSYSTEMS
Metals, a major category of globally-distributed pollutants, are natural elements that have been extracted from the earth and harnessed for human industry and products for millenia. Metals are notable for their wide environmental dispersion from such activity; their tendency to accumulate in select tissues of the human body; and their overall potential to be toxic even at relatively minor levels of exposure. Today heavy metals are abundant in our drinking water, air and soil due to our increased use of these compounds. They are present in virtually every area of modern consumerism from construction materials to cosmetics, medicines to processed foods; fuel sources to agents of destruction; appliances to personal care products. It is very difficult for anyone to avoid exposure to any of the many harmful heavy metals that are so prevalent in our environment. The distribution of heavy metals in manufacturing industries is given in Table 3. Some metals, such as copper and iron, are essential to life and play irreplaceable roles in, for example, the functioning of critical enzyme systems. Other metals are xenobiotics, i.e., they have no useful role in human physiology (and most other living organisms) and, even worse, as in the case of lead and mercury, may be toxic even at trace levels of exposure. Even those metals that are essential, however, have the potential to turn harmful at very high levels of exposure, a reflection of a very basic tenet of toxicology-- “the dose makes the poison.”
Table 3 : General Distribution of Heavy metals in Particular Industrial Effluents
Industries |
Ag |
As |
Cd |
Cr |
Cu |
Fe |
Hg |
Mn |
Ni |
Pb |
Se |
Ti |
Zn |
| General Industry and Mining |
|
|
|
X |
X |
X |
|
X |
|
X |
|
|
X |
| Plating |
|
|
X |
X |
X |
|
|
|
X |
X |
|
|
X |
| Paint Products |
|
|
|
X |
|
|
|
|
|
X |
|
X |
|
| Fertilizers |
|
|
X |
X |
X |
X |
X |
X |
X |
X |
|
|
X |
| Insecticides / Pesticides |
|
X |
|
|
X |
|
X |
|
|
|
|
|
|
| Tanning |
|
X |
|
X |
|
|
|
|
|
|
|
|
|
| Paper Products |
|
|
|
X |
X |
|
X |
|
X |
X |
|
X |
X |
| Photographic |
X |
|
|
X |
|
|
|
|
|
|
|
|
|
| Fibers |
|
|
|
|
X |
|
|
|
|
|
|
|
X |
| Printing / Dyeing |
|
|
|
X |
|
|
|
|
|
X |
|
|
|
| Electronics |
X |
|
|
|
|
|
|
|
|
|
X |
|
|
| Cooling Water |
|
|
|
X |
|
|
|
|
|
|
|
|
|
| Pipe Corrosion |
|
|
|
|
X |
|
|
|
|
X |
|
|
|
Note : Ag - Silver;, As – Arsenic; Cd – Cadmium; Cr – Chromium; Cu –Copper; Fe –Iron, Hg – Mercury; Mn – Manganese; Ni – Nickel; Pb – Lead; Se – Selenium; Zn-Zinc.
Another group of pollutants that are increasingly causing pollution in fresh water bodies are dyes. Dyes are basically chemical compounds that can attach themselves to fabrics or surfaces to impart colour. Most dyes are complex organic molecules and are need to be resistant to many things such as the weather and the action of detergents. Synthetic dyes are extensively used in many fields of up-to-date technology, e.g., in various branches of the textile industry (Gupta et al., 1992; Shukla and Gupta, 1992 and Sokolowska-Gajda et al., 1996), of the leather tanning industry (Tünay et al., 1999 and Kabadasil et al., 1999) in paper production (Ivanov et al., 1996), in food technology (Bhat and Mathur, 1998 and Slampova et al., 2001), in agricultural research (Cook and Linden, 1997 and Kross et al., 1996), in light-harvesting arrays (Wagner and Lindsey, 1996), in photoelectrochemical cells (Wrobel et al., 2001), and in hair colourings (Scarpi et al., 1998). Moreover, synthetic dyes have been employed for the control of the efficacy of sewage (Morgan-Sagastume et al., 1997) and wastewater treatment (Hsu and Chiang, 1997 and Orhon et al., 1999), for the determination of specific surface area of activated sludge (Sorensen and Wakeman, 1996) for ground water tracing (Field et al., 1995), etc.
Dyes can be classified according to their chemical structure or according to their use. However, classifications vary from country to country though there are some fundamental categories that are common to all.
According to the central pollution control board (CPCB), India there are approximately a million known dyes and dye intermediates out of which 5,000 are produced commercially. Based on their use based classification, the dyes are divided into 15 groups.
Table 4 : Classification of dyes based on their use.
Type of Dye |
Application |
According to CPCB1 |
According to World Bank2 |
| Acid dyes |
Wool, silk, nylon |
Animal fibres |
| Azo dyes |
Cotton |
Cotton |
| Basic dyes |
Acrylic |
Paper |
| Direct dyes |
Cotton, leather, paper and synthetics |
Cotton wool or cotton silk |
| Disperse dyes |
Polyster |
|
| Food dyes |
Food, cosmetics |
|
| Metal complexes |
Cotton |
|
| Mordant dyes |
Wool |
|
| Whitening agent |
Plastics, paper, soap |
|
| Pigment dyes |
Paints and plastics |
Paints and inks |
| Reactive dyes |
Wool and cotton |
|
| Solvent dyes |
Synthetics |
|
| Sulphur dyes |
Cotton and Synthetics |
|
| Vat dye |
Cotton and Synthetics |
|
Source : 1 Anon 2002, Effluent toxicity status in water polluting industries, Part 1 – Dye and dye intermediate, bulk drugs and textile industries, Central Pollution Control Board, Ministry of Environment and Forests, Government of India, p7.
2 Pollution prevention and abatement handbook, World Bank, p 298
Unfortunately, the exact amount of dyes produced in the world is not known. It is estimated to be over 10,000 tonnes per year. Exact data on the quantity of dyes discharged in the environment are also not available. It is assumed that a loss of 1–2% in production and 1–10% loss in use are a fair estimate. For reactive dyes, this figure can be about 4%. Due to large-scale production and extensive application, synthetic dyes can cause considerable environmental pollution and are serious health-risk factors. The growing concern of environmental protection has influenced industrial development promoting the development of ecofriendly technologies (Desphande, 2001), reduced consumption of freshwater and lowers output of wastewater (Knittel and Schollmeyer, 1996 and Petek and Glavic, 1996), etc. However, the release of important amounts of synthetic dyes to the environment has posed challenges to environmental scientists apart from increased public concern and legislation problems.
Due to the commercial importance of dyes, their impact (Guaratini and Zanoni, 2000) and toxicity (Walthall and Stark, 1999 and Tsuda et al., 2001) when released in the environment have been extensively studied during the last decade (Hunger, 1995 and Calin and Miron, 1995). Traditional wastewater treatment technologies have proven to be markedly ineffective for handling wastewater of synthetic textile dyes because of the chemical stability of these pollutants. Thus, it has been verified that, of the 18 azo dyes studied 11 compounds passed through the activated sludge process practically untreated, 4 (Acid Blue 113, Acid Red 151, Direct Violet 9, and Direct Violet 28) were adsorbed on the waste activated sludge and only 3 (Acid Orange 7, Acid Orange 8, and Acid Red 88) were biodegraded (Shaul et al., 1991).
Table 5 : Estimation degree of fixation for different dye-fibre combination and loss to effluent
Dye application class |
Fibre |
Degree of fixation (%) |
Loss of effluent (%) |
| Acid |
Polymide |
89 – 95 |
5 - 20 |
| Basic |
Acrylic |
95 – 100 |
0 - 5 |
| Direct |
Cellulose |
70 –95 |
5 - 30 |
| Disperse |
Polyester |
90 – 100 |
0 - 10 |
| Metal - complex |
Wool |
90 – 98 |
2 - 10 |
| Reactive |
Cellulose |
50 – 90 |
10 -50 |
| Sulphur |
Cellulose |
60 – 90 |
10 - 40 |
| Vat |
Cellulose |
80 – 95 |
5 - 20 |
1.5 TOXICOLOGICAL ASPECTS OF METALS AND DYES
1.5.1 Toxicological Aspects of Heavy metals
Due to their mobility in aquatic ecosystems and their toxicity to higher life forms, heavy metals in surface and groundwater supplies have been prioritised as major inorganic contaminants in the environment. Even if they are present in dilute, undetectable quantities, their recalcitrance and consequent persistence in water bodies imply that through natural processes such as biomagnification, concentrations may become elevated to such an extent that they begin exhibiting toxic characteristics. These metals can either be detected in their elemental state, which implies that they are not subject to further biodegradative processes or bound in various salt complexes. In either instance, metal ions cannot be mineralized. Apart from environmental issues, technological aspects of metal recovery from industrial waters must also be considered (Wyatt, 1988).
1.5.1.1 EFFECTS OF HEAVY METALS ON HUMAN HEALTH
The heavy metals hazardous to humans include lead, mercury, cadmium, arsenic, copper, zinc, and chromium. Such metals are found naturally in the soil in trace amounts, which pose few problems. When concentrated in particular areas, however, they present a serious danger. Arsenic and cadmium, for instance, can cause cancer. Mercury can cause mutations and genetic damage, while copper, lead, and mercury can cause brain and bone damage. Next section presents the harmful effects to the four heavy metals that are prevalent in the environment.
Chromium : Humans are exposed to chromium through breathing, eating or drinking and through skin contact with chromium or chromium compounds. The level of chromium in air and water is generally low. In drinking water the level of chromium is usually low as well, but contaminated well water may contain the dangerous chromium (VI); hexavalent chromium. For most people eating food that contains chromium (III), it is the main route of chromium uptake, as chromium (III) occurs naturally in many vegetables, fruits, meats, yeasts and grains. Various ways of food preparation and storage may alter the chromium contents of food, as in the case of food stored in steel tanks or cans leading to enhanced chromium concentrations.Chromium (VI) is a danger to human health, mainly for people who work in the steel and textile industry. Chromium (VI) is known to cause various health effects. When it is a compound in leather products, it can cause allergic reactions, such as skin rash. Inhaling chromium (VI) can cause nose irritations and nosebleeds.
Other health problems that are caused by chromium (VI) are skin rashes, respiratory problems, weakened immune systems, kidney and liver damage, alteration of genetic material, lung cancer and death. The health hazards associated with exposure to chromium are dependent on its oxidation state. The metal form (chromium as it exists in this product) is of low toxicity. The hexavalent form is toxic. Adverse effects of the hexavalent form on the skin may include ulcerations, dermatitis, and allergic skin reactions. Inhalation of hexavalent chromium compounds can result in ulceration and perforation of the mucous membranes of the nasal septum, irritation of the pharynx and larynx, asthmatic bronchitis, bronchospasms and edema. Respiratory symptoms may include coughing and wheezing, shortness of breath, and nasal itch.
Carcinogenicity- Chromium and most trivalent chromium compounds have been listed by the National Toxicology Program (NTP) as having inadequate evidence for carcinogenicity in experimental animals. According to NTP, there is sufficient evidence for carcinogenicity in experimental animals for the following hexavalent chromium compounds; calcium chromate, chromium trioxide, lead chromate, strontium chromate, and zinc chromate.
Mercury : Mercury is generally considered to be one of the most toxic metals found in the environment (Serpone et al., 1988). Once mercury enters the food chain, progressively larger accumulation of mercury compounds takes place in humans and animals. The major sources of mercury pollution in environment are industries like chlor-alkali, paints, pulp and paper, oil refining, rubber processing and fertilizer (Namasivayam and Periasamy, 1993), batteries, thermometers, fluorescent light tubes and high intensity street lamps, pesticides, cosmetics and pharmaceuticals (Krishnan and Anirudhan, 2002).
Methyl mercury causes deformities in the offspring, mainly affecting the nervous system (teratogenic effects). Children suffer from mental retardation, cerebral palsy and convulsions. Mercury also brings about genetic defects causing chromosome breaking and interference in cell division, resulting in abnormal distribution of chromosome. Mercury causes impairment of pulmonary function and kidney, chest pain and dyspnoea (Beglund and Bertin, 2002; WHO, 1990). The harmful effect of methyl mercury on aquatic life and humans was amply brought out by the Minamata episode in Japan (WHO, 1991).
Nickel : Electroplating is one important process involved in surface finishing and metal deposition for better life of articles and for decoration. Although several metals can be used for electroplating, nickel, copper, zinc and chromium are the most commonly used metals, the choice depending upon the specific requirement of the articles. During washing of the electroplating tanks, considerable amounts of the metal ions find their way into the effluent. Ni (II) is present in the effluents of silver refineries, electroplating, zinc base casting and storage battery industries (Sitting, 1976).
Higher concentrations of nickel cause cancer of lungs, nose and bone. Dermatitis (Ni itch) is the most frequent effect of exposure to Ni, such as coins and jewellery. Acute poisoning of Ni (II) causes headache, dizziness, nausea and vomiting, chest pain, tightness of the chest, dry cough and shortness of breath, rapid respiration, cyanosis and extreme weakness (Al-Asheh and Duvnjak 1997; Kadirvelu, 1998; Beliles1979).
Iron : Iron exists in two forms, soluble ferrous iron (Fe2+) and insoluble ferric particulate iron (Fe3+). The presence of iron in natural water may be attributed to the dissolution of rocks and minerals, acid mine drainage, landfill leachate sewage or engineering industries. Iron in water is generally present in the ferric state. The concentration of iron in well aerated water is seldom high but under reducing conditions, which may exist in some groundwater, lakes or reservoirs and in the absence of sulphate and carbonate, high concentrations of soluble ferrous iron may be found. The presence of iron at concentrations above 0.1mg/l will damage the gills of the fish. The free radicals are extremely reactive and short lived. The free radicals formed by the iron on the surface of the gills will cause oxidation of the surrounding tissue and this will lead to massive destruction of gill tissue and anaemia.The presence of iron in drinking water supplies is objectionable for a number of reasons. Under the pH condition existing in drinking water supply, ferrous sulphate is unstable and precipitates as insoluble ferric hydroxide, which settles out as a rust coloured silt. Such water often tastes unpalatable even at low concentration (0.3 mg/L) and stains laundry and plumbing fixtures. Iron is an essential element in human nutrition. It is contained in a number of biologically significant proteins, but ingestion in large quantities results in haemochromatosis where in tissue damage results from iron accumulation.
1.5.1.2 EFFECTS OF HEAVY METALS ON AQUATIC ORGANISMS
Aquatic organisms are adversely affected by heavy metals in the environment. The toxicity is largely a function of the water chemistry and sediment composition in the surface water system (Ahalya, et al., 2005).
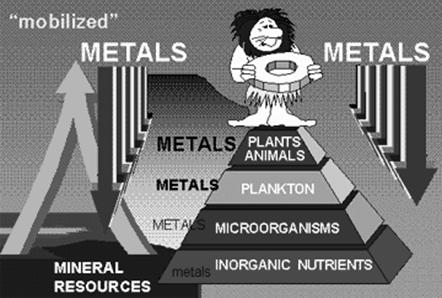
The above illustration (Source: Volesky, 2005) shows how metal ions can become bioaccumulated in an aquatic ecosystem. The metals are mineralised by microorganisms, which in turn are taken up by plankton and further by the aquatic organisms. Finally, the metals by now, several times biomagnified is taken up by man when he consumes fish from the contaminated water.
- Slightly elevated metal levels in natural waters may cause the following sublethal effects in aquatic organisms: histological or morphological change in tissues;
- changes in physiology, such as suppression of growth and development, poor swimming performance, changes in circulation;
- change in biochemistry, such as enzyme activity and blood chemistry;
- change in behaviour; and
- and changes in reproduction (Connell et al., 1984).
Many organisms are able to regulate the metal concentrations in their tissues. Fish and crustacea can excrete essential metals, such as copper, zinc, and iron that are present in excess. Some can also excrete non-essential metals, such as mercury and cadmium, although this is usually met with less success (Connell et al., 1984).
Research has shown that aquatic plants and bivalves are not able to successfully regulate metal uptake (Connell et al., 1984). Thus, bivalves tend to suffer from metal accumulation in polluted environments. In estuarine systems, bivalves often serve as biomonitor organisms in areas of suspected pollution (Kennish, 1992). Shellfishing waters are closed if metal levels make shellfish unfit for human consumption.
In comparison to freshwater fish and invertebrates, aquatic plants are equally or less sensitive to cadmium, copper, lead, mercury, nickel, and zinc. Thus, the water resource should be managed for the protection of fish and invertebrates, in order to ensure aquatic plant survivability (USEPA, 1987). Metal uptake rates will vary according to the organism and the metal in question. Phytoplankton and zooplankton often assimilate available metals quickly because of their high surface area to volume ratio. The ability of fish and invertebrates to adsorb metals is largely dependent on the physical and chemical characteristics of the metal (Kennish, 1992). With the exception of mercury, little metal bioaccumulation has been observed in aquatic organisms (Kennish, 1992). Metals may enter the systems of aquatic organisms via three main pathways:
- Free metal ions that are absorbed through respiratory surface (e.g., gills) are readily diffused into the blood stream.
- Free metal ions that are adsorbed onto body surfaces are passively diffused into the blood stream.
Metals that are sorbed onto food and particulates may be ingested, as well as free ions ingested with water (Connell et al., 1984). For eg: Chromium is not known to accumulate in the bodies of fish, but high concentrations of chromium, due to the disposal of metal products in surface waters, can damage the gills of fish that swim near the point of disposal.
1.5.1.3 IRRIGATION EFFECTS OF HEAVY METALS
Irrigation water contaminated with sewage or industrial effluents may transport dissolved heavy metals to agricultural fields. Although most heavy metals do not pose a threat to humans through crop consumption, cadmium may be incorporated into plant tissue. Accumulation usually occurs in plant roots, but may also occur throughout the plant (De Voogt et al., 1980). Most irrigation systems are designed to allow for up to 30 percent of the water applied to not be absorbed and to leave the field as return flow. Return flow either joins the groundwater or runs off the field surface (tailwater). Sometimes tailwater are rerouted into streams because of downstream water rights or a necessity to maintain streamflow. However, usually the tailwater is collected and stored until it can be reused or delivered to another field (USEPA 1993a).
Tailwater is often stored in small lakes or reservoirs, where heavy metals can accumulate as return flow is pumped in and out. These metals can adversely impact aquatic communities. An extreme example of this is the Kesterson Reservoir in the San Joaquin Valley, California, which received subsurface agricultural drainwater containing high levels of selenium and salts that had been leached from the soil during irrigation. Studies in the Kesterson Reservoir revealed elevated levels of selenium in water, sediments, terrestrial and aquatic vegetation, and aquatic insects. The elevated levels of selenium were cited as relating to the low reproductive success, high mortality, and developmental abnormalities in embryos and chicks of nesting aquatic birds (Schuler et al. 1990).
1.5.2 Toxicological aspects of dyes
Dyeing industry effluents are one of the most problematic wastewaters to be treated not only for their high chemical oxygen demand, but also for high biological oxygen demand, suspended solids, turbidity, toxic constituents but also for colour, which is the first contaminant discernible by the human eye. Dyes may affect the photosynthetic activity in aquatic life due to reduced light penetration and may also be toxic to some aquatic life due to the presence of aromatics, metals, etc. in them (Clarke and Anliker 1980; Zollinger 1987; Mishra and Tripathy 1993; Banat et al1996; Fu and Viraraghvan 2001; Robinson et al2001).
Dyes usually have a synthetic origin and complex aromatic molecular structure, which make them more stable and more difficult to biodegrade. Dyes are classified as follows: anionic – direct, acid and reactive dyes; cationic – basic dyes; non-ionic – disperse dyes (Mishra and Tripathy 1993; Fu and Viraraghvan 2001). The chromophores in anionic and non-ionic dyes are mostly azo groups or anthroquinone types. The reductive cleavage of azo linkages is responsible for the formation of toxic amines in the effluent. Anthraquinone based dyes are more resistant to degradation due to their fused aromatic structures and thus remain coloured in the wastewater. Reactive dyes are typically azo-based chromophore combined with different types of reactive groups e.g, vinyl sulphone, chlorotriazine, trichloropyrimidine, difluorochloropyrimidine. They differ from all other dyes in that they bind to textile fibers like cotton to form covalent bonds. They are used extensively in textile industries regarding favourable characteristics of bright colour, water fast, simple application techniques with low energy consumption.Water soluble reactive and acid dyes are problematic; as they pass through the conventional treatment system unaffected, posing problems. Hence, their removal is also of great importance (Robinson et al 2001; Hu 1992; Juang et al 1997; Karcher et al1999; Sumathi and Manju 2000; Aksu and Tezer et al 2000; O’Mahony et al 2002; Moran et al 1997).
Basic dyes have high brilliance and intensity of colours and are highly visible even in very low concentration (Clarke and Anliker, 1980; Banat et al., 1996; Fu and Viraraghavan, 2001; Mittal and Gupta, 1996; Chu and Chen, 2002; Fu and Viraraghavan, 2002) Metal complex dyes are mostly chromium based, which is carcinogenic (Clarke and Anliker, 1980; Banat et al., 1996; Mishra and Tripathy 1993; Gupta et al., 1990). Disperse dyes do not ionize in an aqueous medium and some disperse dyes have also been shown to have a tendency to bioaccumulate (Banat et al., 1996). Due to the chemical stability and low biodegradability of these, conventional biological wastewater treatment systems are inefficient in treating dye wastewater.
Dyes have generated much concern regarding its use, due to its toxic effects. It has been reported to cause carcinogenesis, mutagenesis, chromosomal fractures, teratogenecity and respiratory toxicity. McGeorge et al. (1985) reported the mutagenic activity of textile wastewater effluents, using the salmonella/microsome assay and contributed the highest percentage (67%) of mutagenic effluents. Costan et al. (1993) found that a textile effluent ranked second in toxicity, among eight industrial sectors represented, by using a series of bioassays assessing the acute, sublethal and chronic toxicity at various trophic levels.
Estimation of LC50 values of many commercial dyes at different time intervals on fish was done earlier by Clarke and Anliker 1980. Srivastava et al. (1995a) also observed changes in LC50 values of malachite green in a fresh water catfish. Gambusia affinis was used to find the LC50 value for acid red 73 and showed higher toxicity (Muthukumar et al., 2005). Over 90% of some 4000 dyes tested in an ETAD (Ecological and Toxicological Association of the Dyestuffs Manufacturing Industry) Survey had LD50 values greater than 2 X 103 mg/kg. The highest rates of toxicity were found amongst basic and diazo direct dyes (Shore, 1996).
Sub – chronic exposure (13 week) to benzidine – based dyes resulted in hepatocellular carcinomas and hepatic neoplastic nodules in rats (National Cancer Institute 1978) and carcinomas in very short duration (National Institute for Occupational Safety, 1980). Histopathological changes in the testes of textile wastewater exposed rats (sub – chronic) included a reduction in the number of germ and Leydig cells, resulting in impaired spermatogenesis (Mathur, et al. 2003).
Umbuzeiro et al. (2005) analysed the mutagenic activity of dyes in environmental samples of the Cristais River, Sao Paulo, Brazil. A low level mutagencity of textile/dye industries in the underground water of Sanganer, Jaipur (India) were also investigated (Mathur et al2005). A number of studies have demonstrated mutagenic activity in effluents from textile and dye- related industries (Mcgeorge, et al. 1985; Sanchez, et al., 1988; Wells, et al. 1994).
1.6 NEED FOR THE REMOVAL OF DYES AND HEAVY METALS
Continuous discharge of industrial, domestic and agricultural wastes in rivers and lakes causes deposit of pollutants in sediments. Such pollutants include heavy metals, which endanger public health after being incorporated in food chain. Heavy metals cannot be destroyed through biological degradation, as is the case with most organic pollutants. Incidence of heavy metal accumulation in fish, oysters, mussels, sediments and other components of aquatic ecosystems have been reported from all over the world (Naimo, 1995; Sayler et al., 1975, Ahalya et al., 2005).
Excessive amounts of some heavy metals can be toxic through direct action of the metal or through their inorganic salts or via organic compounds from which the metal can become easily detached or introduced into the cell. Exposure to different metals may occur in common circumstances, particularly in industrial setting. Accidents in some environments can result in acute, high level exposure. Some of the heavy metals are toxic to aquatic organisms even at low concentration. The problem of heavy metal pollution in water and aquatic organisms including fish, needs continuous monitoring and surveillance as these elements do not degrade and tend to biomagnify in man through food chain. Hence, there is a need to remove the heavy metals from the aquatic ecosystems.
India produces 64,000 tonnes of dyes, 2 per cent of which - 7,040 tonnes - are directly discharged into the environment. With the Indian dyestuff industry growing by over 50 per cent during the last decade, India is now the second largest producer of dyes and intermediaries in Asia. The CPCB puts their number at 900 units. The production is estimated to be around 60,000 tonnes or about 6.6 per cent of the world production. There are around 700 varieties of dyes and dye intermediaries produced in India. In India only a third of the dyestuff producing industries are in organised sector. The rest come from the unregulated small-scale sector, which produces more than half of India's aggregate volumes. Located mainly in Gujarat and Maharashtra, this sector pays no heed to environmental concerns. The domestic textile industry, which consumes up to 80 per cent of the dyestuffs produced, looks for manageable costs rather than consistent quality. So the bulk of its demand for dyes is met by the small- scale sector. The small-scale sector's substantially lower investment in pollution control measures also makes it more economical.
Dyes and colour pigments also contain metals such as copper, nickel, chromium, mercury and cobalt. Metals are difficult to remove from wastewater and may escape the capacities of the effluent treatment system. Moreover, the unused dyes and colour released in effluent from dyeing vats, interferes with the transmission of light in the water bodies that receives the effluent.
This in turn inhibits the photosynthesis activity of aquatic biota besides direct toxic effects on biota. Several textile and food dyes have been linked to carcinogenicity, such as dye intermediaries like benzidines. Hence the ubiquitous colour needs to be regulated. The new drinking water standards prescribed by the Bureau of Indian Standards (IS 10500) set colour standards at five colour units as the desirable limit and 25 colour units as the permissible limit in the absence of alternate source. But removing the colour from effluents is extremely difficult. There is no universally applicable technique for all conditions.
Research and development, therefore focuses on sector-specific methods and technologies to remove colour and heavy metals from different kinds of waste streams. In view of the above toxicological effects of dyes and heavy metals on environment, animals and human beings, it becomes imperative to treat these toxic compounds in wastewater effluents before they are discharged into freshwater bodies.
1.7 CONVENTIONAL METHODS FOR THE TREATMENT OF METALS
Over the last few decades, several methods have been devised for the treatment and removal of heavy metals. Numerous industries (e.g., electroplating, metal finishing operations, electronic –circuit production, steel and non-ferrous processes and fine-chemical and pharmaceutical production) discharge a variety of toxic metals into the environment.
For several years now, it is mandatory that industry is required to remove metal pollutants from liquid discharges. The commonly used procedures for removing metal ions from aqueous streams include chemical precipitation, lime coagulation, ion exchange, reverse osmosis and solvent extraction (Rich and Cherry, 1987, Ahalya et al., 2005, 2006). The process description of each method is presented below.
1.7.1 Chemical precipitation :
Precipitation of metals is achieved by the addition of coagulants such as alum, lime, iron salts and other organic polymers. The large amount of sludge containing toxic compounds produced during the process is the main disadvantage.
- Hydroxide precipitation : Chemical precipitation of heavy metals as their hydroxides using lime or sodium hydroxide is widely used. Lime is generally favoured for precipitation purposes due to the low cost of precipitant, ease of pH control in the range of 8.0 –10.0 and the excess of lime also serves as an adsorbent for the removal of metal ions. The efficiency of the process depends on a number of factors, which include the ease of hydrolysis of the metal ion, nature of the oxidation state, pH, presence of complex forming ions, standing time, degree of agitation and settling and filtering and characteristics of the precipitate. The limitations of this method include difference between metals in the optimum pH for hydroxide formation may lead to the problems in the treatment of effluents containing combined metal ions. Variability in metal hydroxide solubility at a fixed pH is another drawback.
- Carbonate precipitation : Carbonate precipitation of metals using calcium or sodium carbonate is very limited. Patterson et al., 1997 reported improved results using carbonate precipitate for Cd (II) and Pb (II) from electroplating effluents. When the pH was brought to 7.5, residual concentration of Pb (II) and Cd (II) were 0.60 and 0.25 mg/L respectively.
- Sulphide precipitation : Since most of the heavy metals form stable sulphides, excellent metal removal can be obtained by sulphide precipitation. Treatment with sulphides is most advantageous when used as a polishing step after conventional hydroxide precipitation or when very high metal removals are required.
1.7.2 Chemical reduction :
Reduction of hexavalent chromium can also be accomplished with electro-chemical units. The electrochemical chromium reduction process uses consumable iron electrodes and an electric current to generate ferrous ions that react with hexavalent chromium to give trivalent chromium as follows (USEPA, 1979)
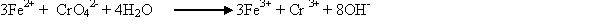
Another application of reduction process is the use of sodium borohydride, which has been considered effective for the removal of mercury, cadmium, lead, silver and gold (Kiff, 1987).
1.7.3 Xanthate process :
Insoluble starch xanthate (ISX) is made from commercial cross linked starch by reacting it with sodium hydroxide and carbon disulphide. To give the product stability and to improve the sludge settling rate, magnesium sulphate is also added. ISX works like an ion exchanger, removing the heavy metals from the wastewater and replacing them with sodium and magnesium. Average capacity is 1.1-1.5 meq of metal ion per gram of ISX (Anon, 1978).
ISX is most commonly used by adding to it the wastewater as slurry for continuous flow operations or in the solid form for batch treatments. It should be added to the effluent at pH ≥ 3. Then the pH should be allowed to rise above 7 for optimum metal removal (Wing, 1978). Residual metal ion level below 50 μg/L has been reported (Hanway et al., 1978, Wing et al., 1978). The effectiveness of soluble starch xanthate (SSX) for removal of Cd (II), Cr (VI) and Cu (II) and insoluble starch xanthate (ISX) for Cr (VI) and Cu (II) have been evaluated under different aqueous phase conditions. Insoluble starch xanthate had better binding capacity for metals. The binding capacity of SSX and ISX respectively for different metal ions follows the sequence of Cr (VI)> Cu (II)> Cd(II) and Cr (VI)> Cu (II) (Tare et al., 1988).
1.7.4 Solvent extraction :
Liquid-liquid extraction (also frequently referred as solvent extraction) of metals from solutions on a large scale has experienced a phenomenal growth in recent years due to the introduction of selective complexing agents (Beszedits, 1988). In addition to hydrometallurgical applications, solvent extraction has gained widespread usage for waste reprocessing and effluent treatment.
Solvent extraction involves an organic and an aqueous phase. The aqueous solution containing the metal or metals of interest is mixed with the appropriate organic solvent and the metal passes into the organic phase. In order to recover the extracted metal, the organic solvent is contacted with an aqueous solution whose composition is such that the metal is stripped from the organic phase and is reextracted into the stripping solution. The concentration of the metal in the strip liquor may be increased, often 110 to 100 times over that of the original feed solution. Once the metal of interest has been removed, the organic solvent is recycled either directly or after a fraction of it has been treated to remove the impurities.
1.7.5 Membrane process :
Important examples of membrane process applicable to inorganic wastewater treatment include reverse osmosis and eletrodialysis (EPA, 1980). These processes involve ionic concentration by the use of selective membrane with a specific driving force. For reverse osmosis, pressure difference is employed to initiate the transport of solvent across a semipermeable membrane and electro dialysis relies on ion migration through selective permeable membranes in response to a current applied to electrodes. The application of the membrane process described is limited due to pretreatment requirements, primarily, for the removal of suspended solids. The methods are expensive and sophisticated, requiring a higher level of technical expertise to operate.
A liquid membrane is a thin film that selectively permits the passage of a specific constituent from a mixture (Beszedits, 1988). Unlike solid membranes, however liquid membranes separate by chemistry rather than size, and thus in many ways liquid membrane technology is similar to solvent extraction.
Since liquid membrane technology is a fairly recent development, a number of problems remain to be solved. A major issue with the use of supported membranes is the long term stability of the membranes, whereas the efficient breakup of microspheres for product recovery is one of the difficulties encountered frequently with emulsion membranes.
1.7.6 Evaporators :
In the electroplating industry, evaporators are used chiefly to concentrate and recover valuable plating chemicals. Recovery is accomplished by boiling sufficient water from the collected rinse stream to allow the concentrate to be returned to the plating bath. Many of the evaporators in use also permit the recovery of the condensed steam for recycle as rinse water. Four types of evaporators are used throughout the elctroplating industry (USEPA, 1979a) (I) Rising film evaporators; (ii) Flash evaporators using waste heat; (iii) submerged tube evaporators; (iv) Atmospheric evaporators.
Both capital and operational costs for evaporative recovery systems are high. Chemical and water reuse values must offset these costs for evaporative recovery to become economically feasible.
1.7.7 Cementation :
Cementation is the displacement of a metal from solution by a metal higher in the electromotive series. It offers an attractive possibility for treating any wastewater containing reducible metallic ions. In practice, a considerable spread in the electromotive force between metals is necessary to ensure adequate cementation capability. Due to its low cost and ready availability, scrap iron is the metal used often. Cementation is especially suitable for small wastewater flow because a long contact time is required. Some common examples of cementation in wastewater treatment include the precipitation of copper from printed etching solutions and the reduction of Cr (VI) in chromium plating and chromate-inhibited cooling water discharges (Case, 1974). Removal and recovery of lead ion by cementation on iron sphere packed bed has been reported (Angelidis et al., 1988, 1989). Lead was replaced by a less toxic metal in a harmless and reusable form.
1.7.8 Ion exchange :
Ion exchange resins are available selectively for certain metal ions. The cations are exchanged for H+ or Na+. The cation exchange resins are mostly synthetic polymers containing an active ion group such as SO3H. The natural materials such as zeolites can be used as ion exchange media (Van der Heen, 1977). The modified zeolites like zeocarb and chalcarb have greater affinity for metals like Ni and Pb (Groffman et al., 1992). The limitations on the use of ion exchange for inorganic effluent treatment are primarily high cost and the requirements for appropriate pretreatment systems. Ion exchange is capable of providing metal ion concentrations to parts per million levels. However, in the presence of large quantities of competing mono-and divalent ions such as Na and Ca, ion exchange is almost totally ineffective.
1.7.9 Electrodeposition :
Some metals found in waste solution can be recovered by electrodeposition using insoluble anodes. For example, spent solutions resulting from sulphuric acid cleaning of Cu may be saturated with copper sulphate in the presence of residual acid. These are ideal for electro-winning where the high quality cathode copper can be electrolytically deposited while free sulphuric acid is regenerated.
1.7.10 Adsorption :
Since activated carbon also possesses an affinity for heavy metals, considerable attention has been focussed on the use of carbon for the adsorption of hexavalent chromium, complexed cyanides and metals present in various other forms from wastewaters. Watonabe and Ogawa first presented the use of activated carbon for the adsorption of heavy metals in 1929.
The mechanism of removal of hexavalent and trivalent chromium from synthetic solutions and electroplating effluents has been extensively studied by a number of researchers. According to some investigators, the removal of Cr (VI) occurs through several steps of interfacial reactions (Huang and Bowers, 1979).
- The direct adsorption of Cr6+ onto carbon surface.
- The reduction of Cr6+ species to Cr3+ by carbon on the surface.
- The adsorption of the Cr3+ species produced, which occurs to a much lesser extent than the adsorption of the Cr6+ species.
Adsorption of Cr (III) and Cr (VI) on activated carbon from aqueous solutions has been studied (Toledo, 1994). Granular activated carbon columns have been used to treat wastewaters containing lead and cadmium (Reed and Arunachalam, 1994, Reed et al., 1994). Granular activated carbon was used for the removal of Pb (II) from aqueous solutions (Cheng et al., 1993). The adsorption process was inhibited by the presence of humic acid, iron (III), aluminum (III) and calcium (II).
1.8 DISADVANTAGES OF CONVENTIONAL METHODS FOR TREATMENT OF WASTEWATER CONTAINING HEAVY METALS
Metals are a class of pollutants, often toxic and dangerous, widely present in industrial and household wastewaters. Electroplating and metal finishing operations, electronic circuit production, steel and aluminum processes to name but a few industries, produce large quantities of wastewater containing metals. Although metal precipitation using a cheap alkali such as lime (calcium hydroxide) has been the most favoured option, other separation technologies are now beginning to find favour. Precipitation, by adjusting the pH value is not selective and any iron (ferric ion) present in the liquid effluent will be precipitated initially followed by other metals. Consequently precipitation produces large quantities of solid sludge for disposal, for example precipitation as hydroxides of 100 mg/l of copper (II), cadmium (II) or mercury (II) produces as much as 10-, 9- and 5 fold mg/l of sludges respectively. The metal hydroxide sludge resulting from treatment of electroplating wastewater has been classified as a hazardous waste.
The performance characteristics of heavy metal waste water treatment technologies are identified in Table 6. The versatility, simplicity and other technology characteristics will contribute to the overall process costs, both capital and operational. At present many of these technologies such as ion exchange represent significant capital investments by industry.
Table 6 : Performance characteristics of various heavy metal removal /recovery technologies
Technology |
pH change |
Metal selectivity |
Influence of
Suspended solids |
Tolerance of
organic molecules |
Working level for
appropriate metal (mg/I) |
Adsorption, e.g.
Granulated
Activated carbon |
Limited tolerance |
Moderate |
Fouled |
Can be poisoned |
<10 |
Electro
chemical |
Tolerant |
Moderate |
Can be engineered
to tolerate |
Can be accommodated |
>10 |
| Ion exchange |
Limited tolerance |
Chelate - resins can
be selective |
Fouled |
Can be poisoned |
<100 |
| Membrane |
Limited tolerance |
Moderate |
Fouled |
Intolerant |
>10 |
| Precipitation |
| (a) Hydroxide |
Tolerant |
Non-selective |
Tolerant |
Tolerant |
>10 |
| (b) Sulphide |
Limited tolerance |
Limited selective
pH dependent |
Tolerant |
Tolerant |
>10 |
| Solvent extraction |
Some systems |
Metal selective |
Fouled |
Intolerant |
>100 |
| |
pH tolerant |
extractants available |
|
|
|
As seen from the table above, conventional methods are ineffective in the removal of low concentrations of heavy metals and they are non-selective. Moreover, it is not possible to recover the heavy metals by the above mentioned methods.
1.9 CONVENTIONAL METHODS FOR TREATMENT OF WASTEWATER CONTAINING DYES
Synthetic dyes often receive considerable attention from researchers interested in textile wastewater effluents treatment processes. As discharge standards are becoming more stringent, the development of technological systems for minimizing concentration of dyes and their break down products in wastewater are nowadays necessary. The following are generally used for the removal of colour from wastewaters.
1.9.1 Physicochemical methods for dye removal :
Adsorptive bubble separation techniques (ion flotation, solvent sublation and adsorbing colloid flotation) resulted in the efficient removal (99%) of Direct Blue from wastewater (Horng and Huang, 1993). The application of coagulation processes for the removal of dyes from wastewater has also been assessed. The efficiencies dependent on the type of flocculant and on the pH of the medium (Koprivanac et al., 1993). Electrocoagulation was used for the effective removal of Acilan Blue from the wastewater of an operating textile plant in a bipolar packed-bed electrochemical reactor (Ogutveren et al., 1992).
1.9.2 Photocatalytic decolourisation and oxidation of synthetic dyes :
Commercial dyes are designed to resist photodegradation, so the selection of optimal photocatalytic conditions for the decolourisation of dyes requires considerable expertise. Due to the significant commercial and environmental interest the efficacy of a large number of catalysts and irradiation conditions has been established for the decolourisation of various synthetic dyes.
1.9.2.1 PHOTOCATALYSIS AND OXIDATION WITH HYDROGEN PEROXIDE :
Hydrogen peroxide has been frequently applied to the decolourisation of synthetic dyes in waters. Hydrogen peroxide can effectively decolourize dye wastewaters in the presence of Fe (II) sulphate, with the higher rates of decolourisation at higher concentrations of the reagents (Kuo, 1992). Iron (III) with hydrogen peroxide was successfully employed for the degradation of the dye intermediate anthraquinone-2-sulphonic acid sodium salt (Kiwi et al., 1993). The results indicated that the method could be successfully used for the decolourisation of acid dyes, direct dyes, basic dyes and reactive dyes but it proved to be inadequate for vat dyes and disperse dyes (Yang et al., 1998).
1.9.2.2 OZONATION :
Ozonation, as an effective oxidation process, has found application in the decolourisation of synthetic dyes. The technique employed in the decolouration of Orange II. Oxalate, formate and benzene sulphonate ions were the most important decomposition products (Tang and An, 1995a and Tang and An, 1995b). It was reported that ozone effectively decomposed azo dyes in textile wastewater. The decomposition rate was considerably higher at acidic pH. However, the influence of temperature and UV irradiation on the decomposition rate was negligible (Koyuncu and Afsar, 1996). The negligible influence of UV irradiation on the decomposition rate of azo dyes by ozone has been supported by other authors. The effect of chemical structure on the decomposition rate has been demonstrated (Davis et al., 1994).
1.9.2.3 OTHER OXIDIZING SYSTEMS :
The photodecomposition of five dyes (Reactive Red 2, Reactive Blue 4, Reactive Black 8, Basic Red 13 and Basic Yellow 2) under UV irradiation in the presence of trivalent iron-oxalato complexes was also reported (Nansheng et al., 1997a). It has been established that the rate of photodegradation is highly dependent on the chemical structure of the dye.
1.9.2.4 MEMBRANE PROCESSES :
Membrane filtration is used by many process industries for product purification. Water entering the membrane is called feed water and the water passing through the membrane is called permeate, treated or product water. Membrane technologies have the potential either to remove the dyestuff or allow reuse of the auxillary chemicals used for dyeing or to concentrate the dyestuffs and auxiliaries and produce purified water.
This method has the ability to clarify, concentrate and most importantly to separate dye continuously from effluent (Mishra and Tripathy, 1993; Xu and Lebrun, 1999). It has some special features unrivalled by other methods; resistance to temperature, adverse chemical environments and microbial attack. The concentrated residue left after separation poses disposal problems, high capital cost, possibility of clogging and membrane replacements are its disadvantages.
(i) Ultrafiltration : Ultrafiltration has many good points such as the recovery of dyes and water or the possibility of reusing them. Membrane transport properties are influenced by casting parameters and membrane thickness. The coefficient of dye separation increases when the time of solvent evaporation increases and the temperature of the casting solution decreases. Membranes prepared from casting solutions of an initial temperature of 318K at a solvent evaporation time of 60 s yield a dye separation between 95 and 100 per cent irrespective of the pressures and dye concentrations applied. Polysulphone membranes 90 to 100 μm thick exhibit the best transport properties as reported in the literature (Pawlowski, 1982).
(ii) Reverse osmosis : In the water treatment industry, reverse osmosis is sometimes referred to as hyperfiltration, is a process in which water is forced through a semipermeable membrane. Reverse osmosis is suitable for removing ions and larger species from dye bath effluents (Marcucci, et al. 2001). Majority of commercial reverse osmosis plants are used for the desalination of seawater and brackish water, while the number of reverse osmosis plants treating municipal and industrial wastewater for reuse is still limited (Mavrov, et al. 2001; Abdel – Jawad, and Al- Sulaimi, 2002; Durham and Walton, 1999).
(iii) Nanofiltration : Nanofiltration is a process of separation with membrane and performance characteristics between reverse osmosis and ultrafiltration. Nanofiltration membranes present an asymmetric structure, which consists of a filtering skin supported by a sub-layer of high porosity with thickness varying from 100 to 300 μm. Studies by Stoyko and Pencho (Stoyko and Pencho, 2003) on the purification of water contaminated with reactive dye, using nanofiltration, considered a dye retention of 85 – 90 % and a permeate flux of 30 – 45 L/h. m2, showed satisfactory for the reuse of the water. Colour and COD retention present in textile industry were reported and the results showed that the colour retention were around 99 % for the DK 1073 (Lopes, et al. 2005).
1.9.3. Microbiological decomposition of synthetic dyes
The application of microorganisms for the biodegradation of synthetic dyes is an attractive and simple method by operation. However, the biological mechanisms can be complex. Large number of species has been tested for decolouration and mineralisation of various dyes. The use of microorganisms for the removal of synthetic dyes from industrial effluents offers considerable advantages. The process is relatively inexpensive, the running costs are low and the end products of complete mineralisation are not toxic. The various aspects of the microbiological decomposition of synthetic dyes have been reviewed by Stolz (2001). Besides the traditional wastewater cleaning technologies, other methods have been employed in the microbial decolourisation of dyes.
The application of microorganisms for the biodegradation of synthetic dyes is an attractive and simple method. Unfortunately, the majority of dyes are chemically stable and resistant to microbiological attack. The isolation of new strains or the adaptation of existing ones to the decomposition of dyes will probably increase the efficacy of microbiological degradation of dyes in the near future.
1.9.4 Enzymatic decomposition of synthetic dyes
The character of enzymes and enzyme systems in microorganisms that are suitable for the decomposition of dyes has been extensively investigated. Effort has been devoted to the separation, isolation and testing of these enzymes. Exact knowledge of the enzymatic processes governing the decomposition of dyes is important in the environmental protection both from theoretical and practical points of view.
Lignin peroxidase isoenzymes were isolated from P. chrysosporium and purified by chromatofocusing. The activity of isoenzymes towards decolouring triphenylmethane dyes, heterocyclic dyes, azo dyes and polymer dyes was compared with that of a crude enzyme preparation. Optimum pH values for the decolourisation of dyes by various isozymes were markedly different. According to the results, the decomposition capacity of crude enzyme preparation and purified isoenzymes showed marked differences while variations in the structure of dyes exerted slight influence (Ollikka et al., 1993). Horseradish peroxidase has been successfully employed for the decomposition and the precipitation of azo dyes. The degradation rate was dependent on the pH (Bhunia et al., 2001). Another study revealed that the enzymes of white rot fungus degraded Crystal Violet via N-demethylation (Bumpus et al., 1991). Interestingly, lignin peroxidase from B. adusta showed very low degradation capacity towards azo dyes and phthalocyanine dyes. However, veratryl alcohol considerably increased the decomposition rate (Heinfling et al., 1998). Similar investigations proved that pure laccase was also unable to decolourize Remazol Brilliant Blue R but the decolouration rate was facilitated by the presence of a mediator (violuric acid) (Soares et al., 2001).
The employment of enzyme preparations shows considerable benefits over the direct use of microorganisms. Commercial enzyme preparations can be easily standardized, facilitating accurate dosage. The application is simple and can be rapidly modified according to the character of the dye or dyes to be removed. But the cost of such enzyme preparations is quite high.
1.9.5 Adsorption :
Adsorption techniques employing solid sorbents are widely used to remove certain classes of chemical pollutants from waters, especially those that are practically unaffected by conventional biological wastewater treatments. However, amongst all the sorbent materials proposed, activated carbon is the most popular for the removal of pollutants from wastewater (Babel and Kurniawan, 2003, Derbyshire et al., 2001 and Ramakrishna and Viraraghavan, 1997). In particular, the effectiveness of adsorption on commercial activated carbons (CAC) for removal of a wide variety of dyes from wastewaters has made it an ideal alternative to other expensive treatment options (Ramakrishna and Viraraghavan, 1997). Table 7 shows a non-exhaustive list of examples of CAC used in wastewater treatment. Because of their great capacity to adsorb dyes, CAC are the most effective adsorbents. This capacity is mainly due to their structural characteristics and their porous texture, which gives them a large surface area, and their chemical nature which can be easily modified by chemical treatment in order to increase their properties. However, activated carbon presents several disadvantages (Babel and Kurniawan, 2003). It is quite expensive, the higher the quality, the greater the cost, non-selective and ineffective against disperse and vat dyes. The regeneration of saturated carbon is also expensive, not straightforward, and results in loss of the adsorbent. The use of carbons based on relatively expensive starting materials is also unjustified for most pollution control applications (Streat et al., 1995). This has led many workers to search for more economic adsorbents.
Table 7 : Recent reported adsorption capacity qmax (mg/g) for commercial activated carbons
Dye |
Qmax (mg/g) |
Sources |
| Acid yellow |
1179 |
Chern and Wu (2001) |
| Remazol yellow |
1111 |
AI-Degs et al. (2000) |
| Basic yellow 21 |
860 |
Allen et al. (2003) |
| Basic red 22 |
720 |
Allen et al. (2003) |
| Reactive orange 107 |
714 |
Aksu and Tezer (2005) |
| Reactive red 2 |
712.3 |
Chiou et al. (2004) |
| Basic dye |
309.2 |
Meshko et al. (2001) |
| Basic blue 9 |
296.3 |
Kannan and Sundaram (2001) |
| Reactive red 5 |
278 |
Aksu and Tezer (2005) |
| Direct red 81 |
240.7 |
Chiou et al. (2004) |
| Acid yellow 117 |
155.8 |
Choy et al. (2000) |
| Acid blue 40 |
133.3 |
Ozacar and Sengil (2002) |
| Acid blue 80 |
112.3 |
Choy et al. (2000) |
| Acid red 88 |
109 |
Venkata Mohan et al. (1999) |
| Basic red 46 |
106 |
Martin et al. (2003) |
| Acid red 114 |
103.5 |
Choy et al. (2000) |
| Acid yellow 17 |
57.47 |
Ozacar and Sengil (2002) |
| Direct red 28 |
16.81 |
Fu and Viraraghavan (2002a) |
| Direct brown 1 |
7.69 |
VenkataMohan et al. (2002) |
1.10 DISADVANTAGES OF USING CONVENTIONAL METHODS FOR DYE REMOVAL
Some of the disadvantages of conventional methods for dye removal are listed in Table 8.
Table 8 : Disadvantages of conventional methods for dye removal
Treatment Process |
Technology |
Disadvantages |
| Conventional treatment processes |
Coagulation |
High sludge production, handling and disposal problems |
| Flocculation |
| Biodegradation |
Slow process, necessary to create an optimal favorable environment, maintenance and nutrition requirements |
| |
Adsorption on activated carbons |
Ineffective against disperse and vat dyes, the regeneration is expensive and results in loss of the adsorbent, non-destructive process |
| Established recovery process |
Membrane separations |
High pressures, expensive, incapable of treating large volumes. |
| Ion-exchange |
Economic constraints, not effective for disperse dyes |
| Oxidation |
High energy cost, chemicals required |
| Emerging removal process |
Advanced oxidation process |
Economically unfeasible, formation of by-products, technical constraints |
Although, some of these techniques have been shown to be effective, they have limitations. Among these are: excess amount of chemical usage, or accumulation of concentrated sludge with obvious disposal problems; expensive plant requirements or operational costs; lack of effective colour reduction; and sensitivity to a variable wastewater input.
In view of these disadvantages, biosorption or removal by heavy metals/dyes by biological materials has gained momentum from 1990’s.
1.11 BIOSORPTION
During the 1970’s increasing environmental awareness and concern led to a search for new techniques capable of inexpensive treatment of polluted wastewaters with metals. The search for new technologies involving the removal of toxic metals from wastewaters has directed attention to biosorption, based on binding capacities of various biological materials.
Till date, research in the area of biosorption suggests it to be an ideal alternative for decontamination of metal/dye containing effluents. Biosorbents are attractive since naturally occurring biomass/adsorbents or spent biomass can be effectively used. Biosorption is a rapid phenomenon of passive metal/dye sequestration by the non-growing biomass/adsorbents. Results are convincing and binding capacities of certain biomass/adsorbents are comparable with the commercial synthetic cation exchange resins.
The biosorption process involves a solid phase (sorbent or biosorbent; adsorbent; biological material) and a liquid phase (solvent, normally water) containing a dissolved species to be sorbed (adsorbate, metal/dyes). Due to the higher affinity of the adsorbent for the adsorbate species, the latter is attracted and bound there by different mechanisms. The process continues till equilibrium is established between the amount of solid-bound adsorbate species and its portion remaining in the solution. The degree of adsorbent affinity for the adsorbate determines its distribution between the solid and liquid phases.
There are many types of adsorbents; Earth’s forests and plants, ocean and freshwater plankton, algae and fish, all living creatures, that including animals are all “biomass/ adsorbents”. The renewable character of biomass that grows, fuelled directly or indirectly by sunshine, makes it an inexhaustible pool of chemicals of all kinds.
Biosorption has advantages compared with conventional techniques (Volesky, 1999). Some of these are listed below:
- Cheap: the cost of the biosorbent is low since they often are made from abundant or waste material.
- Metal/Dye selective: the metal/dye sorbing performance of different types of biomass can be more or less selective on different metals. This depends on various factors such as type of biomass, mixture in the solution, type of biomass preparation and physico-chemical treatment.
- Regenerative: biosorbents can be reused, after the metal is recycled.
- No sludge generation: no secondary problems with sludge occur with biosorption, as is the case with many other techniques, for example, precipitation.
- Metal recovery possible: In case of metals, it can be recovered after being sorbed from the solution.
- Competitive performance: biosorption is capable of a performance comparable to the most similar technique, ion exchange treatment. Ion exchange is, as mentioned above, rather costly, making the low cost of biosorption a major factor.
Biosorbents intended for bioremediation environmental applications are waste biomass of crops, algae, fungi, bacteria, etc., which are the naturally abundant. Numerous chemical groups have been suggested to contribute to biosorption. A review of biosorption of heavy metals by microorganisms is presented below followed by biosorption of dyes by microorganisms. Biosorption by microorganisms have various disadvantages, and hence many low cost adsorbents (industrial/agricultural waste products/byproducts) are increasingly used as biosorbents. This report provides review of the low cost adsorbents used for removal of heavy metals (Ahalya et al., 2004; Ahalya et al., 2006) and dyes (in the later part of the Section).
REFERENCES
Acemioglu B., 2004 Adsorption of Congo red from aqueous solution onto calcium-rich fly ash, J. Colloid Int. Sci. 274, pp. 371–379.
Ahalya N. and Ramachandra T.V. (2002) Restoration of wetlands - Feasibility Aspects of Biological Restoration presented at the National Conference on Aquatic Restoration and Biodiversity – Feb 15-16 2002 in Kongunadu Arts and Science College, Coimbatore, India.
Ahalya N, Kanamadi RD and Ramachandra TV 2006, Biosorption of Iron (III) using the husk of Cicer arientinum. Indian Journal of Chemical Technology , 13, pp 122-127
Ahalya N, Ramachandra T.V., Kanamadi R.D 2004, Biosorption of heavy metals, Journal of Chemistry and Environment, 7(4): 71-79.
Ahalya N, Ramachandra TV and Kanamadi RD, 2005. Biosorption of Chromium (VI) from aqueous solutions by the husk of bengal gram (Cicer arientinum). Electronic Journal of Biotechnology (Online) 15 December 2005.
Ahalya N, Ramachandra TV and Kanamadi RD, 2006. Removal of hexavalent chromium using coffee husk. Bioresource Technology (Communicated)
Ahmed M.N and Ram R.N., 1992, Removal of basic dye from wastewater using silica as adsorbent, Environ. Pollut. 77, pp. 79–86.
Ajmal M., Khan A.H., Ahmad S.and Ahmad A. 1998. Role of sawdust in the removal of copper(II) from industrial wastes Water Res. 32, pp. 3085–3091.
Aksu Z and Tezer S., 2000, Equilibrium and kinetic modelling of biosorption of Remazol Black B by R. arrhizus in a batch system: effect of temperature. Process Biochem. 36, pp. 431–439.
Aksu Z and Tezer S., 2000, Equilibrium and kinetic modelling of biosorption of Remazol Black B by R. arrhizus in a batch system: effect of temperature. Process Biochem. 36, pp. 431–439.
Aksu Z. and Dönmez G., 2003. A comparative study on the biosorption characteristics of some yeasts for Remazol Blue reactive dye. Chemosphere 50, pp. 1075–1083.
Aksu Z., 2001. Biosorption of reactive dyes by dried activated sludge: equilibrium and kinetic modelling. Biochem. Eng. J. 7, pp. 79–84.
Al-Asheh S. and Duvnjak Z., 1997, Sorption of cadmium and other heavy metals by pine bark, Adv. Environ. Res. 1 pp. 194.
Al-Ghouti M.A., Khraisheh M.A.M., Allen S.J. and Ahmad M.N., 2003, The removal of dyes from textile wastewater: a study of the physical characteristics and adsorption mechanisms of diatomaceous earth, J. Environ. Manage. 69, pp. 229–238.
Allen S.J., Gan Q., Matthews R and Johnson.P.A., 2003, Comparison of optimised isotherm models for basic dye adsorption by kudzu, Bioresour. Technol. 88, pp. 143–152.
Alves M. M., Gonzaa lez C. G., Guedes de Carvalho R., Castanheira J. M., Sol Pereira M. C. and Vasconcelos L. A. T. (1993) Chromium removal in tannery wastewaters polishing' by Pinus sylverstris bark. Water Res. 27(8), 1333-1338.
Angelidis T, Fytianos, K. and Vasilikiotics, G, 1989. Lead recovery from aqueous solution and wastewater by cementation utilising an iron rotating disc. Resources, Conservation and Recycling, 2, pp131-138.
Angelidis, T., Fytianos, K. and Vasilikiotics, G. 1988. Lead removal from wastewater by cementation utilising a fixed bed of iron spheres. Environ. Pollut., 50, pp 243-251.
Anita Iyer, and Bhavanath Jha, 2005. Biosorption of heavy metals by a marine bacterium. Marine Pollution Bulletin, 50(3), pp 340-343.
Annachhatre A.P., Win N.N., Chandrkrachan S.G, in: W.J. Stevens, M.S. Rao, S. Chandrkrachang (Eds.), Proceedings of the Second Asia-Pacific Symposium, Asian Institute of Technology, Bangkok, Thailand, 1996, pp. 169–173.
Annadurai G, Juang R.S. and Lee D.J., 2002 Use of cellulose-based wastes for adsorption of dyes from aqueous solutions, J. Hazard. Mater. B92, pp. 263–274.
Annadurai G. and Krishnan M.R.V, 1997, Adsorption of acid dye from aqueous solution by chitin: equilibrium studies, Indian J. Chem. Tech. 4, pp. 217–222.
Annadurai G., Chellapandian M. and Krishnan M.R.V., 1999, Adsorption of reactive dye on chitin, Environ. Monitor. Assessment 59, pp. 111–119.
Anon 1978, Heavy Metal Removal? Try starch xanthate. Prod. Finishing, 31, 72-74
Atun G and Hisarli G., 2003, Adsorption of carminic acid, a dye onto glass powder, Chem. Eng. J. 95, pp. 241–249.
Aygün A., Yenisoy-Karakas S. and Duman I., 2003, Production of granular activated carbon from fruit stones and nutshells and evaluation of their physical, chemical and adsorption properties, Micropor. Mesopor. Mater. 66, pp. 189–195.
Babel S. and Kurniawan, T.A. 2003. Low-cost adsorbents for heavy metals uptake from contaminated water: a review, J. Hazardous Mater. B97, pp. 219–243.
Bagane M and Guiza S., 2000, Removal of a dye from textile effluents by adsorption, Ann. Chim. Sci. Mater. 25, pp. 615–626.
Banat F., Al-Asheh S. and Al-Makhadmeh L, 2003, Evaluation of the use of raw and activated date pits as potential adsorbents for dye containing waters, Process Biochem. 39, pp. 193–202.
Banat Fawzi, Sameer Al-Asheh and Leema Al-Makhadmeh 2003. Evaluation of the use of raw and activated date pits as potential adsorbents for dye containing waters. Process Biochemistry. 39(2), pp 193-202.
Banat I.M., Nigam P., Singh D. and Marchant R., 1996 Microbial decolourization of textile-dye containing effluents: a review. Bioresour. Technol. 58, pp. 217–227.
Basci Nurgul, Kocadagistan Erdem and Kocadagistan Beyhan, 2004, Biosorption of copper (II) from aqueous solutions by wheat shell, Desalination, 164(2), pp 135-140.
Basibuyuk M. and. Forster C.F, 2003. An examination of the adsorption characteristics of a basic dye (Maxilon Red BL-N) on to live activated sludge system. Process Biochem. 38, pp. 1311–1316.
Beliles R.P., The lesser metals. In: F.W. Oehme, Editor, Toxicity of Heavy Metals in the Environment, Part 2, Marcel Dekker, New York (1979), p. 383.
Beveridge, T. J. and Doyle, R. J. (1989). Metal Ions and Bacteria. John Wiley Sons, New York.
Beveridge, T.J. 1986. The immobilisation of soluble metals by bacterial walls, in Biotechnology and Bioengineering Symposium No. 16: Biotechnology for the Mining, Metal –Refining and Fossil-Fuel Processing Industries, Ehrlich, H.L. and Holmes, D.S., (eds), J.Wiley Interscience, New York, 127-140.
Bhat, R.V. and Mathur, P., 1998. Changing scenario of food colours in India. Curr. Sci. 74, pp. 198–202.
Bhunia, A., Durani, S. and Wangikar, P.P., 2001. Horseradish peroxidase catalyzed degradation of industrially important dyes. Biotechnol. Bioeng. 72, pp. 562–567.
Bouzaida I and Rammah M.B., 2002 Adsorption of acid dyes on treated cotton in a continuous system, Mater. Sci. Eng. C 21, pp. 151–155.
Brady and Duncan, J.R. 1993. Bioaccumulation of cations by Sacchromyces cerevisae in Biohydrometallurgical Technologies. Proceedings of the international biohydrometallurgical symposium, Torma, A.E., Apel, M.L. and Brierley, C.L., (eds), The Minerals, Metals and Materials Society, warrendale, PA, 35-44.
Brady, D. and Duncan, J. R., in Biohydrometallurgical Technologies(eds Torma, A. E., Apel, M. L. and Brierley, C. L.), The Minerals, Metals and Materials Society, TMS Publication, Wyoming, USA, 1993, vol. II, pp. 711–723.
Brahimi-Horn M.C., Lim K.K., Liany S.L and Mou D.G., 1992. Binding of textile azo dyes by Mirothecium verrucaria Orange II, 10B (blue) and RS (red) azo dye uptake for textile wastewater decolourization. J. Ind. Microbiol. 10, pp. 245–261.
Brierley, C.L. and Brierley, J.A. 1993. Immobilisation of biomass for industrial application of biosorption in Biohydrometallurgical Technologies. Proceedings of the international biohydrometallurgical symposium, Torma, A.E., Apel, M.L. and Brierley, C.L., (eds), The Minerals, Metals and Materials Society, warrendale, PA, 35-44.
Brierley, J.A. and Vance, D.B. 1988. Recovery of precious metalsby microbial biomass, in BioHydrometallurgy: Proceedings Internat. Symp., Norris, P.R. and Kelly D.P., (eds), Sci Technol Letters, Kew, Surrey, U.K., 477-86.
Brierley, J.A., Brierley, C.L. and Goyak G.M.1986. AMT-BIOCLAIM: A new wastewater treatment and metal recovery technology, in Fundamental and Applied Biohydrometallurgy, Lawrence, R.W., Branion, R.M.R. and Ebner, H.G., (eds), Elsevier, Amsterdam, The Netherlands, 291-304.
Brierley, J.A.,Brierley, C.L. and Goyak G.M.1986. AMT-BIOCLAIM: A new wastewater treatment and metal recovery technology, in Fundamental and Applied Biohydrometallurgy, Lawrence, R.W., Branion, R.M.R. and Ebner, H.G., (eds), Elsevier, Amsterdam, The Netherlands, 291-304.
Brigatti M.F., Campana G., Medici L. and Poppi L. 1996. Influence of layer charge on Zn2+ and Pb2+ sorption by smectites. Clay Miner. 31, pp. 477–483.
Brown P.A., Gill S.A and Allen S.J., 2000, Metal removal from wastewater using peat, Water Res. 34, pp. 3907–3916.
Brown Pauline A., Brown Joseph M. and Allen Stephen J. 2001, The application of kudzu as a medium for the adsorption of heavy metals from dilute aqueous wastestreams. Bioresource Technology, 78(2) pp 195-201.
Brown Pauline, Atly Jefcoatb I, Dana Parrisha, Sarah Gilla and Elizabeth Graham.2000. Evaluation of the adsorptive capacity of peanut hull pellets for heavy metals in solution. Advances in Environmental Research, 4 (1), pp 19-29.
Bryant P. S., Petersen J. N., Lee J. M. and Brouns T. M. 1992 Sorption of heavy metals by untreated red sawdust. Appl. Biochem. Biotechnol. 34-35, pp 777-788.
Bumpus, J.A., Mileski, G., Brock, B., Ashbaugh, W. and Aust, S.D., 1991. Biological oxidations of organic compounds by enzymes from a white rot fungus. Innovative Hazard. Waste Treat. Technol. Ser. 3, pp. 47–54.
Bustard M., McMullan G and McHale A.P., 1998, Biosorption of textile dyes by biomass derived from Kluveromyces marxianus IMB3. Bioprocess Eng. 19, pp. 427–430.
Cadena F., Rizvi R. and Peters R. W. 1990, Feasibility studies for the removal of heavy metals from solution using tailored bentonite. In Hazardous and Industrial Wastes, Proceedings of the Twenty-Second Mid-Atlantic Industrial Waste Conference, Drexel University, pp. 77-94.
Carrasco-Marin F., Alvarez-Merino M.A and Moreno-Castilla C., 1996 Microporous activated carbons from a bituminous coal, Fuel 75, pp. 966–970.
Case, O.P. Metallic recovery from wastewaters utilising cementation, EPA-270/2-74-008, U.S. Washington DC (1974).
Chandra Sekhar K, Subramanian S, Modak JM and Natarajan KA, 1998, Removal of metal ions using an industrial biomass with reference to environmental control. Int.J.Miner.Process. 53, pp 107-120.
Chen B., Hui C.W. and McKay G., 2001, Film-pore diffusion modelling and contact time optimisation for the adsorption of dyestuffs on pith, Chem. Eng. J. 84 , pp. 77–94.
Cheng J., Subramanian, K.S., Chakrabarti, C.L., Guo, R., Ma, X., Lu, Y.J. and Pickering, W.F. 1993 Adsorption of low levels of Pb (II) by the granular activated carbon. J. Environ. Sci. Hlth., A28, pp 51-72.
Chiou M.S and Li H.Y., 2003, Adsorption behaviour of reactive dye in aqueous solution on chemical cross-linked chitosan beads, Chemosphere 50, pp. 1095–1105.
Chiou M.S andLi H.Y., 2002, Equilibrium and kinetic modelling of adsorption of reactive dye on cross-linked chitosan beads, J. Hazard. Mater. B93, pp. 233–248.
Chiou M.S., Ho P.Y and Li H.Y., 2004, Adsorption of anionic dyes in acid solutions using chemically cross-linked chitosan beads, Dyes Pigments 60, pp. 69–84.
Chojnackaa K, Chojnackib A and Górecka H, 2005, Biosorption of Cr3+, Cd2+ and Cu2+ ions by blue–green algae Spirulina sp.: kinetics, equilibrium and the mechanism of the process. Chemosphere, 59(1), pp 75-84.
Chou K.S., Tsai J.C and Lo C.T., 2001, The adsorption of Congo red and vacuum pump oil by rice hull ash, Bioresour. Technol. 78, pp. 217–219.
Chu H.C and Chen K.M., 2002. Reuse of activated sludge biomass: I. Removal of basic dyes from wastewater by biomass. Process Biochem. 37, pp. 595–600.
Chu H.C and. Chen K.M. 2002. Reuse of activated sludge biomass: II. The rate processes for the adsorption of basic dyes on biomass. Process Biochem. 37, pp. 1129–1134.
Chubara Natalia, Carvalho Jorge R and Correia M. Joana Neiva., 2003, Cork biomass as biosorbent for Cu(II), Zn(II) and Ni(II).Colloids and Surfaces A: Physicochemical and Engineering Aspects, 230(1-3), pp 57-65.
Clarke EA, Anliker R. Organic dyes and pigments. In: Handbook of environmental chemistry, anthropogenic compounds, vol. 3, part A. New York: Springer-Verlag, 1980. p. 181–215.
Connell, D.W., Miller. G.J 1984. Chemistry and Ecotoxicology of Pollution. John Wiley & Sons, NY.
Cook, S.M.F. and Linden, D.R., 1997. Use of rhodamine WT to facilitate dilution and analysis of atrazine samples in short-term transport studies. J. Environ. Qual. 26, pp. 1438–1441.
Costan, G., N. Bermingham, C. Blaise and J.F. Ferard. 1993. Potential ecotoxic effect probe (PEEP); a novel index to assess and compare the toxic potential of industrial effluents. Environ. Toxic. Water Qual. 8, pp 115-140.
Cotoras, D., Viedma, P. and Pimentel, J. 1993. Biosorption of metal ions by attached bacterial cells in a packed bed reactor in Biohydrometallurgical Technologies. Proceedings of the international biohydrometallurgical symposium, Torma, A.E., Apel, M.L. and Brierley, C.L., (eds), The Minerals, Metals and Materials Society, Warrendale, PA.
Couillard D., 1994, The use of peat in wastewater treatment, Water Res. 28, pp. 1261–1274.
Crini G. and Morcellet M., 2002 Synthesis and applications of adsorbents containing cyclodextrins, J. Sep. Sci. 25, pp. 1–25.
Crini G., 2003 Studies of adsorption of dyes on beta-cyclodextrin polymer, Bioresour. Technol. 90, pp. 193–198.
Crini G., Janus L., Morcellet M., Torri G. and Morin N., 1999, Sorption properties toward substituted phenolic derivatives in water using macroporous polyamines beta-cyclodextrins polymers, J. Appl. Polym. Sci. 73, pp. 2903–2910.
Crini G., Morin-Crini N. and Badot P.M., 2002, Adsorption of toxic aromatic derivatives on polysaccharide gels, Hydrosciences 133, pp. 58–61.
Darnall, D.W., Greene, B. and Gradea-Torresday, J. 1988. Gold Binding to algae in BioHydrometallurgy: Proceedings Internat. Symp., Norris, P.R. and Kelly D.P., (eds), Sci Technol Letters, Kew, Surrey, U.K., 487-98.
Darnall, D.W.; Greene, B.; HenziI, M.T.; Hosea, J.M.; Mcpherson, R.A.; Sneddon, J. and Alexander, M.D. Selective recovery of gold and other metal ions from an algal biomass. Environment Science and Technology, 1986, 20, pp. 206-208.
Davis J A and Leckie JO, 1978. Surface ionisation and complexation at the oxide/water interface, II. Surface properties of amorphous ironoxyhydroxide and adsorption of metal ions. J. Colloid and Interface Sci., 67, pp 90-107.
Davis, R.J., Gainer, J.L., O'Neal, G. and Wu, I.W., 1994. Photocatalytic decolorization of wastewater dyes. Water Environ. Res. 66, pp. 50–53.
Davis, T.A., Volesky, B. and Mucci, A. 2003. A review of Biochemistry of heavy metal biosorption by brown algae. Wat.Res. 37 (18), pp 4311-4330.
Davis, TA, Volesky, B. and Vieira, RHSF. 2000. Sargassum seaweed as biosorbent for heavy metals. Wat.Res . 34, pp 4270-4278.
De Voogt, P., B. Van Hattum, J.F. Feenstra, and J.W. Copius Peereboom. 1980. Exposure and health effects of cadmium. Toxicol. Environ. Chem. Rev. 3:89-109.
Delval F., Crini G., Janus L., Vebrel J. and Morcellet M., 2001, Novel crosslinked gels with starch derivatives. Polymer-water interactions. Applications in waste water treatment, Macromol. Symp. 166, pp. 103–108.
Delval F., Crini G., Morin N., Vebrel J., Bertini S. and Torri G., 2002, The sorption of several types of dye on crosslinked polysaccharides derivatives, Dyes Pigments 53, pp. 79–92.
Derbyshire F., Jagtoyen M., Andrews R., Rao A., Martin-Gullon I and Grulke E. Carbon materials in environmental applications. In: L.R. Radovic, Editor, Chemistry and Physics of Carbon Vol. 27, Marcel Dekker, New York (2001), pp. 1–66.
Desai M., Dogra A., Vora S., Bahadur P and Ram R.N., 1997 Adsorption of some acid dyes from aqueous solutions onto neutral alumina, Indian J. Chem. A 36 (1997), pp. 938–944.
Deshkar A. M., Bokade S. S. and Dara S. S. 1990, Modified Hardwickia Binata bark for adsorption of mercury (II) from water. Water Res. 24(8), pp 1011-1016.
Desphande, S.D., 2001. Ecofriendly dyeing of synthetic fibres. Ind. J. Fibre Text. Res. 26, pp. 136–142.
Dikshit V. P. 1989, Removal of chromium (VI) by adsorption using saw-dust. Nat. Acad. Sci. Lett. 12(12), pp 419-421.
Dilek F. B. Erbay A and Yetis U 2002, Ni(II) biosorption by Polyporous versicolor. Process Biochemistry, 37(7), pp 723-726
Dinesh Mohan and Kunwar P. Singh, 2002. Single- and multi-component adsorptionnext term of cadmium and zinc using activated carbon derived from bagasse—an agricultural waste. Water Research, 36 (9), pp 2304-2318.
EPA, Development document for effluent limitation guidelines and standards for the inorganic chemicals manufacturing point source category, USEPA, Effluent Guidelines Division, Office of Water and Waste Management, Washington, DC (1980).
Erdem Mehmet and Özverdi Arzu. 2005, Lead adsorption from aqueous solution onto siderite. Separation and Purification Technology, 42(3) , pp 259-264.
Erdem, N. Karapinarb and R. Donat. 2004. The removal of heavy metal cations by natural zeolites. Journal of Colloid and Interface Science, 280 (2), pp 309-314.
Espantaleon A.G., Nieto J.A., Fernandez M. and. Marsal A, 2003. Use of activated clays in the removal of dyes and surfactants from tannery waste waters, Appl. Clay Sci. 24 , pp. 105–110.
Farajzadeh Mir Ali and Monji Akbar Boviery, 2004, Adsorption characteristics of wheat bran towards heavy metal cations. Separation and Purification Technology. 38(3), pp197-207.
Fattahpour Sedeh I., Igsell P., Ringqvist L and Lindström E.B, 1996, Comparison of metal adsorption properties and determination of metal adsorption capacities of different peat samples. Resource Environ Biotechnol 1, pp. 111–128.
Field, M.S., Wilhelm, R.G., Quinlan, J.F. and Aley, T.J., 1995. An assessment of the potential adverse properties of fluorescent tracer dyes used for groundwater tracing. Environ. Monit. Assess. 38, pp. 75–97.
Figueira, MM, Volesky, B, Azarian, K & Ciminelli, VST. 1999. Multimetal biosorption in a column using Sargassum biomass in Biohydrometallurgy and the Environment Toward the Mining of the 21st Century, Internat. Biohydrometallurgy Symposium Proceedings, 1999, volume B, Ballester, A. & Amils, R. (eds.) Elsevier Sciences, Amsterdam, The Netherlands pp.503-512.
Figueira, MM, Volesky, B, Ciminelli, VST and Roddick, FA. 2000. Biosorption of metals in brown seaweed biomass. Wat. Res . 34, pp 196-204.
Figueira, MM, Volesky, B. & Ciminelli, VST. 1997. Assessment of interference in biosorption of heavy metals. Biotechnol. Bioeng. 54, pp 344-349.
Figueira, MM, Yang, J, Volesky, B & Camargos, ERS. 1995. Interference of Fe in the Cd uptake by Sargassum biomass. Biohydrometallurgical Processing, Vol.2, Proc. Internat. Biohydromet. Symp., Jerez, CA, Vargas, T, Toledo, H & Wiertz, JV (eds.) Univ. Chile, Santiago, Chile, p. 187-194.
Fourest, E. and Roux, J.C. 1992. Heavy metal biosorption by fungal mycelial byproducts: mechanism and influence of pH. Appl. Microbiol. Biotechnol. 37, pp 399-403.
Fourest, E. and Roux, J.C. 1992. Heavy metal biosorption by fungal mycelial byproducts: mechanism and influence of pH. Appl. Microbiol. Biotechnol. 37, 399-403.
Freeland G. N., Hoskinson R. M. and Mayfeld R. J. 1974, Adsorption of mercury from aqueous solutions by polyethylenimine-modified wool fibers. Envir. Sci. Technol. 8(10), pp 943-944.
Friis, N. and Myers-Keith, P. 1986. Biosorption of uranium and lead by Streptomyces longwoodensis. Biotechnol. Bioeng. 28, pp 21-28.
Fu Y and Viraraghavan T, 2000, Removal of a dye from an aqueous solution by the fungus Aspergillus niger. Water Quality Res. J. Can. 35, pp. 95–111.
Fu Y and Viraraghavan T, 2002, Removal of Congo Red from an aqueous solution by fungus Aspergillus niger. Advances Environ. Res. 7, pp. 239–247.
Fu Y and Viraraghavan T., 2001, Fungal decolourization of wastewaters: a review. Bioresour. Technol. 79, pp. 251–262.
Fu Y and Viraraghavan T., 2001, Removal of C.I. Acid Blue 29 from an aqueous solution by Aspergillus niger. AATCC Mag 1, pp. 36–40.
Fu Y. and Viraraghavan T, 2001 Fungal decolourization of wastewaters: a review. Bioresour. Technol. 79, pp. 251–262.
Fu Y. and Viraraghavan T., 2002, Removal of Congo red from an aqueous solution by fungus Aspergillus niger, Adv. Environ. Res. 7, pp. 239–247.
Fu Y.and Viraraghavan T., 2002, Dye biosorption sites in Aspergillus niger. Bioresour. Technol. 82, pp. 139–145.
Fu, Y.Z. and Viraraghavan, T., 2001. Fungal decolorization of dye wastewaters: a review. Bioresour. Technol. 79, pp. 251–262.
Gadd GM and Griffiths AJ, 1978. Microorganisms and heavy metal toxicity. Microb. Ecol. 4, pp303-317.
Gadd GM and White C 1993, Microbial treatment of metal pollution - A working biotechnology. TIBTech.11, pp 353-359.
Gadd, G. M. (1988). “Accumulation of metals by microorganisms and algae.” In: Biotechnology – A Comprehensive Treatise (H.J. Rehm, ed.). VCH Verlagsgesellschaft, Weinheim, pp. 401–433
Gadd, G.M., White, C. and de Rome, L. 1988. Heavy metal and radionucleide uptake by fungi and yeasts, in BioHydrometallurgy: Proceedings Internat. Symp., Norris, P.R. and Kelly D.P., (eds), Sci Technol Letters, Kew, Surrey, U.K., 421-36.
Gadd, Geoffrey M. 1990 Heavy metal accumulation by bacteria and other microorganisms. Experientia, 46, pp 834-840.
Gallagher KA, Healy MG, Allen SJ. Biosorption of synthetic dye and metal ions from aqueous effluents using fungal biomass. In: Wise DL. (Ed.). Global Environmental Biotechnology. UK: Elsevier; 1997. p. 27–50.
Galun, M., Keller, P., Malki, D., Feidstein, H., Galun, E., Siegel, S. and Siegel, B., Water Air Soil Pollut. 21, pp 411–414.
Garg V.K., Amita M., Kumar R and Gupta R., 2004, Basic dye (methylene blue) removal from simulated wastewater by adsorption using Indian Rosewood sawdust: a timber industry waste, Dyes Pigments 63, pp. 243–250.
Garg V.K., Gupta R., Yadav A.B. and Kumar R., 2003, Dye removal from aqueous solution by adsorption on treated sawdust, Bioresour. Technol. 89, pp. 121–124.
Garg V.K., Kumar R and Gupta R., 2004, Removal of malachite green dye from aqueous solution by adsorption using agro-industry waste: a case study of Prosopis cineraria, Dyes Pigments 62, pp. 1–10.
Gee, A.R. and Dudeney, A.W.L. 1988. Adsorption and crystallisation of gold at biological surfaces, in BioHydrometallurgy: Proceedings Internat. Symp., Norris, P.R. and Kelly D.P., (eds), Sci Technol Letters, Kew, Surrey, U.K., 437-451.
Grant D.C., Skriba M.C and Saha A.K. 1987. Environ. Prog. 6, pp. 104–109.
Greene B. and Darnall D.W. 1990. Microbial oxygenic photoautotrophs for metal-ion binding. In: Microbial mineral recovery (Ed. by H.L. Ehrlich & C.L. Brierley), pp. 277-302. McGraw-Hill, New York.
Groffman, A., Peterson, S and Brookins, D. 1992 Removing lead from wastewater using zeolite. Wat. Environ. Technol., 4, 54-59.
Guo Y., Yang S., Fu W., Qi J., Li R., Wang Z and Xu H., Adsorption of malachite green on micro- and mesoporous rice husk-based active carbon, Dyes Pigments 56.
Gupta D.C and Tiwari U.C. 1985. Aluminum oxide as adsorbent for removal of hexavalent chromium from aqueous waste Ind. J. Environ. Health 27, pp. 205–215.
Gupta G.S., Prasad G. and Singh V.H., 1990, Removal of chrome dye from aqueous solutions by mixed adsorbents: fly ash and coal, Water Res. 24, pp. 45–50.
Gupta G.S., Shukla S.P., Prasad G and Singh V.N., 1992, China clay as an adsorbent for dye house wastewater, Environ. Technol. 13, pp. 925–936.
Gupta V. K., Shrivastava A. K. and Neeraj Jain. 2001. Biosorption of Chromium(VI) From Aqueous solutions by green algae spirogyra species. Water Research. 35 (17), pp 4079-408.
Gupta V.K., Ali I., Suhas and Mohan D., 2003, Equilibrium uptake and sorption dynamics for the removal of a basic dye (basic red) using low-cost adsorbents, J. Colloid Int. Sci. 265, pp. 257–264.
Gupta V.K., Mohan D., Sharma S and Sharma M., 2000, Removal of basic dye (Rhodamine B and Methylene blue) from aqueous solutions using bagasse fly ash, Sep. Sci. Technol. 35, pp. 2097–2113.
Gupta, G.S., Prasad G, Panday, K.K and Singh V.N. 1988, Removal of chrome dye from aqueous solutions by fly ash. Water, Air and Soil Pollu., 37, pp 13-24.
Gupta, G.S., Shukla, S.P., Prasad, G. and Singh, V.N., 1992. China clay as an adsorbent for dye house wastewaters. Environ. Technol. 13, pp. 925–936.
Gürses A., Karaca S., Dogar C., Bayrak R, Acikyildiz M and Yalcin M., 2004, Determination of adsorptive properties of clay/water system: methylene blue sorption, J. Colloid Int. Sci. 269, pp. 310–314.
Hall K.R., Eagleton L.C., Acrivos A and Ver Meulen T, 1966, Pore- and Solid-Diffusion kinetics in fixed bed adsorption under constant pattern conditions. Ind. Eng. Chem. Fund. 5 (2) pp 212 – 223.
Hanway, J.E., Mumford, R.G. and Mishra P.N. Treatment of industrial effluents for heavy metal removal using the cellulose xanthat process in 71st Annual meeting of the American Institute of Chemical Engineeres, Miami, Florida (1978).
Harris R.G., Wells J.D. and Johnson B.B., 2001, Selective adsorption of dyes and other organic molecules to kaolinite and oxide surfaces, Colloid Surf. A: Physicochem. Eng. Aspects 180, pp. 131–140.
Heinfling, A., Martinez, M.J., Martinez, A.T., Bergbauer, M. and Szewczyk, U., 1998. Transformation of industrial dyes by manganese peroxidases from Bjerkandera adusta and Pleurotus eryngii in a manganese-independent reaction. Appl. Environ. Mircobiol. 64, pp. 2788–2793.
Heinfling-Weidtmann, A., Reemtsma, T., Storm, T. and Szewczyk, U., 2001. Sulfophtalimide as major metabolite formed from sulfonated phtalocyanine dyes by the white-rot fungus Bjerkandera adusta. FEMS Microbiol. Lett. 203, pp. 179–183.
Ho Y. S., Wase D. A. J. and Forster C. F. 1994. The adsorption of divalent copper ions from aqueous solution by sphagnum moss peat. Trans. IChemE 72B(3), pp 185-194.
Ho Y.S. and McKay G., 1998, Kinetic models for the sorption of dye from aqueous solution by wood, Trans. IChemE B 76, pp. 183–191.
Ho Y.S., Chiang C.C and Hsu Y.C., 2001, Sorption kinetics for dye removal from aqueous solution using activated clay, Sep. Sci. Technol. 36, pp. 2473–2488.
Ho Y.S., Chiang T.H and Hsueh Y.M., 2005, Removal of basic dye from aqueous solutions using tree fern as a biosorbent, Process Biochem. 40, pp. 119–124.
Ho Y.S., John Wase Y.S. and Forster C.F., 1995, Batch nickel removal from aqueous solution by Sphagnum moss peat, Water Res. 29(5), pp. 1327–1332.
Holan Z. R., Volesky B. and Prasetyo I. 1993. Biosorption of cadmium by biomass of marine algae. Biotechnol. Bioeng. 41(8), pp 819-825.
Holan, Z.R. and Volesky, B. 1995, Accumulation of cadmium, lead and nickel by fungal and wood biosorbents. Appl. Biochem. Biotechnol. 53, pp 133-146.
Holan, ZR and Volesky, B., 1994. Biosorption of lead and nickel by biomass of marine algae. Biotech Bioeng. 43, pp 1001-9.
Horikoshi T, Nakajima A, Sakaguchi T 1981, Studies on the accumulation of heavy metal elements in biological systems, XIX: Accumulation of uranium by microorganisms. Eur J Appl Microbiol Biotechnol 12, pp 90.
Horng, J.Y. and Huang, S.O., 1993. Removal of organic dye (direct blue) from synthetic wastewater by adsorptive bubble separation techniques. Environ. Sci. Technol. 27, pp. 1169–1175.
Hsien T.Y, Rorrer G. L., in: R.-H. Chen, H.-S. Chen (Eds.), Proceedings of the Third Asia-Pacific Symposium on Chitin and Chitosan, National Taiwan Ocean University, Keelung, Taiwan, 1998, pp. 111–120.
Hsu, T.C. and Chiang, C.S., 1997. Activated sludge treatment of dispersed dye factory wastewater. J. Environ. Sci. Health. Part A, Environ. Sci. Eng. Toxic Hazard. Substance Control A 32, pp. 1921–1932.
Hsuan-Liang Liu, Bor-Yann Chen, Yann-Wen Lana and Yang-Chu Cheng, 2004, Biosorption of Zn(II) and Cu(II) by the indigenous Thiobacillus thiooxidans. Chemical Engineering Journal, 97(2-3), pp 195-201.
Hu T.L., 1992 Sorption of reactive dyes by Aeromonas biomass. Water Sci. Technol. 26 , pp. 357–366.
Hu T.L., 1996, Removal of reactive dyes from aqueous solution by different bacterial genera. Water Sci. Technol. 34, pp. 89–95.
Huang C.P, Chung Y.C.and Liou. M.R 1996, Adsorption of Cu(II) and Ni(II) by pelletized biopolymer J. Hazard. Mater. 45, pp. 265–277.
Huang C.P., and Bowers A.R., The development of an activated carbon process for the treatment of chromium (VI) containing plating wastewater. In: 2nd Conference on Advanced Pollution Control for the metal finishing industries. EPA – 600/8-79-014, Cincinnati, Ohio, (1979). pp:114-122.
Huang, M.C and Chen, K M 1993. The removal of colour from effluents using polyamide-epichlorohydrin-cellulose polymer, III. Use in anionic dye removal in a batch process. J. Appl. Polym. Sci., 50, pp 735-744.
Illan Gomez M.J., Garcia-Garcia A., Salinas-Martinez de Lecea C and Linares-Solano A., 1996, Activated carbon from Spanish coal. 2. Chemical activation, Energy Fuel 10, pp. 1108–1114.
Iqbal M., Saeed A.and Akhtar N. 2002, Petiolar felt-sheath of palm: a new biosorbent for the removal of heavy metals from contaminated water.Bioresource Technology. 81(2) , pp 151-153.
Ivanov, K., Gruber, E., Schempp, W. and Kirov, D., 1996. Possibilities of using zeolite as filler and carrier for dyestuffs in paper. Das Papier 50, pp. 456–46.
Jain A.K., Gupta V.K., Bhatnagar A. and Suhas, 2003, Utilization of industrial waste products as adsorbents for the removal of dyes, J. Hazardous Mater. B101, pp. 31–42.
Janos P., Buchtova H and Ryznarova M., 2003, Sorption of dyes from aqueous solutions onto fly ash, Water Res. 37, pp. 4938–4944.
Jasinski S.M., USGS Minerals Information, US Geological Survey Mineral Commodity Summary 2001, January 2002, ftp://minerals.usgs.gov/minerals/pubs/commodity/peat/510302.pdf.
Jha I.N., Iyengar L and Rao A.V.S.P. 1988. Removal of cadmium using chitosan. J. Environ. Eng. 114, pp. 962–974.
Johnson P. D., Watsona M. A., Browna J and Jefcoat I. A., 2002, Peanut hull pellets as a single use sorbent for the capture of Cu (II) from wastewater. Waste Management, 22(5), pp 471-480.
Jolley, R.A. and Forstner, C.F.1985 The kinetics of sulphide oxidation. Environ. Technol. Lett., 6, 1-10.
Juang R.S., F.C. Wu F.C. and Tseng R.L., 2002, Characterization and use of activated carbons prepared from bagasses for liquid-phase adsorption, Colloid Surf. A: Physicochem. Eng. Aspect 201, pp. 191–199.
Juang R.S., Tseng R.L. and Wu F.C., 2001, Role of microporosity of activated carbons on their adsorption abilities for phenols and dyes, Adsorption 7, pp. 65–72.
Juang R.S., Tseng R.L., Wu F.C and Lee S.H., 1997, Adsorption behavior of reactive dyes from aqueous solutions on chitosan, J. Chem. Technol. Biotechnol. 70 (1997), pp. 391–399.
Juang R.S., Tseng. R. L, Wu F.C and Lin S.J., 1996, Use of chitin and chitosan in lobster shell wastes for colour removal from aqueous solutions, J. Environ. Sci. Health A 31, pp. 325–338.
Juang R.S., Wu F.C. and R.L. Tseng, 2002, Use of chemically modified chitosan beads for sorption and enzyme immobilization, Adv. Environ. Res. 6, pp. 171–177.
Kabadasil, I., Tünay, O. and Orhon, D., 1999. Wastewater control and management in a leather tanning district. Water Sci. Technol. 40/1, pp. 261–267.
Kadirvelu K., Preparation and characterization of activated carbon, from coir pith and its application to metal bearing wastewater, Ph.D. Thesis, Bharathiar University, Coimbatore, India, 1998.
Kannan N and Sundaram M.M, 2001, Kinetics and mechanism of removal of methylene blue by adsorption on various carbons—a comparative study, Dyes Pigments 51, pp. 25–40.
Karaca S., Gürses A and Bayrak R., 2004, Effect of some pre-treatments on the adsorption of methylene blue by Balkaya lignite, Energy Convers. Manage. 45, pp. 1693–1704.
Karcher S., Kornmuller A and Jekel M., 1999 Removal of reactive dyes by sorption/complexation with cucurbituril. Water Sci. Technol. 40, pp. 425–433.
Kennish, M.J. 1992. Ecology of Estuaries: anthropogenic effects. CRC Press: Boca Raton.
Kertman S. V., Kertman G. M. and Chibrikova Zh. S. 1993, Peat as a heavy-metal sorbent. J. Appl. Chem.U.S.S.R. 66(2), pp 465-466.
Kesenci Kemal, Sayb Ridvan and Denizl Adil. 2002, Removal of heavy metal ions from water by using poly(ethyleneglycol dimethacrylate-co-acrylamide) beads European Polymer Journal, 38(7) pp 1443-1448.
Khalid, A.M., Ashfaq, S.R., Bhatti, T.M., Anwar, M.A., Shemsi, A.M. and Akhtar, K. 1993. The uptake of microbially leached uranium by immobilised microbial biomass, in Biohydrometallurgical Technologies. Proceedings of the international biohydrometallurgical symposium, Torma, A.E., Apel, M.L. and Brierley, C.L., (eds), The Minerals, Metals and Materials Society, Warrendale, PA.
Khan S. A., Riaz-ur-Rehman A. and Khan M. A. 1995, Adsorption of chromium (III), chromium (VI) and silver (I) on bentonite. Waste Manage. 15(4),pp 271-282.
Khan S.A., Rehman R and Khan M.A. 1995. Sorption of strontium on bentonite. Waste Manage. 15, pp. 641–650.
Khattri S.D. and Singh M.K., 2000, Colour removal from synthetic dye wastewater using a bioadsorbent, Water Air Soil Pollut. 120, pp. 283–294.
Kiwi, J., Pulgarin, C., Peringer, P. and Grätzel, M., 1993. Beneficial effects of homogeneous photo-Fenton pretreatment upon the biodegradation of anthraquinone sulfonate in waste water treatment. Appl. Catal., B Environ. 3, pp. 85–89.
Knittel, D. and Schollmeyer, E., 1996. Prevention of water pollution in dyeing processes of synthetic textiles. Eur. Water Pollut. Control 6, pp. 6–10.
Kobya M., Demirbas E., Senturka E. and Incea M., 2005, Adsorption of heavy metal ions from aqueous solutions by activated carbon prepared from apricot stone Bioresource Technology,96 (3), pp 1518-1521.
Koprivanac, N., Bosanac, G., Grabaric, Z. and Papic, S., 1993. Treatment of wastewaters from dye industry. Environ. Technol. Lett. 14, pp. 385–390.
Koyuncu, I. and Afsar, H., 1996. Decomposition of dyes in the textile wastewater with ozone. J. Environ. Sci. Health. Part A, Environ. Sci. Eng. Toxic Hazard. Substance Control A 31, pp. 1035–1041.
Krishnan A.K and Anirudhan T.S., 2002 Removal of mercury(II) from aqueous solutions and chlor-alkali industry effluent by steam activated and sulphurised activated carbons prepared from bagasse pith: kinetics and equilibrium studies, J. Hazard. Mater. 92 pp. 161.
Kross, B.C., Nicholson, H.F. and Ogilvie, L.K., 1996. Methods development study for measuring pesticide exposure to golf course workers using video imaging techniques. Appl. Occup. Environ. Hyg. 11, pp. 1346–1351.
Krysztafkiewicz A., Binkowski S. and Jesionowski T., 2002, Adsorption of dyes on a silica surface, Appl. Surf. Sci. 199, pp. 31–39.
Kuo, W.G., 1992. Decolorizing dye wastewater with Fenton's reagent. Water Res. 26, pp. 881–886.
Kuyucak, N and Volesky, B. 1988. New algal biosorbent for a gold recovery process in BioHydrometallurgy: Proceeding of the Internat. Symp.,Norris, P.R. and Kelly D.P., (eds), Sci Technol Letters, Kew, Surrey, U.K., 453-464.
Kuyucak, N. and Volesky, B. 1989. Accumulation of cobalt by marine alga, Biotechnol.Bioeng. 33(7), pp 809-14.
Kuyucak, N. and Volesky, B. 1988. Biosorbents for recovery of metals from industrial solutions. Biotechnol. Lett. 10, pp 137--42.
Lazaridis N.K., Karapantsios T.D and Geogantas D., 2003, Kinetic analysis for the removal of a reactive dye from aqueous solution onto hydrotalcite by adsorption, Water Res. 37, pp. 3023–3033.
Lee S.M and Davis A.P., 2001. Removal of Cu (II) and Cd (II) from aqueous solution by seafood processing waste sludge Water Res. 35, pp. 534–540.
Leppert D. 1990, Heavy metal sorption with clinoptilolite zeolite: alternatives for treating contaminated soil andwater. Mining Eng. 42(6), pp 604-608.
Low K. S. and Lee C. K. 1991, Cadmium uptake by the moss, Calymperes delessertii. Besch. Bioresource Technol.38(1),pp 1-6.
Low K. S., Lee C. K., Cheong F. Y. and Lim G. S. 1993. Enhancement effect of dye-coating on the sorption of copper by moss. J. Environ. Sci. Health. A 28(8),pp 1689- 1704.
Low K.S and Lee C.K 1990. The removal of cationic dyes using coconut husk as an adsorbent. Pertanika, 13, pp 221-228.
Low K.S., Lee C.K and Tan B.F (2000), Quaternised wood as sorbent for reactive dyes, Appl. Biochem. Biotechnol. 87, pp. 233–245.
Low K.S., Lee, C.K and Heng L.L 1994, Sorption of basic dyes by Hydrilla verticallata. Environ. Technol., 14, pp 115-124.
Low K.S., Lee, C.K and Toh BL, 1994 Binding of basic dyes by the algae, chara aspera. Pertanika J. Sci. Technol., 2, 85-92.
Luef, E.; Prey, T.; Kubicek, C.P. 1991 Biosorption of zinc by fungal micelial wastes. Appl. Microbiol. Biotechnol., 14, pp 688-692.
Macaskie L.E., Empson, R.M., Cheetham, A.K., Grey, C.P. and Skarnaulis, A.J. 1992. Uranium bioaccumulation by a Citrobacter sp. as a result of enzymatically mediated growth of polycrystalline HUO2PO4. Science 257, pp 782-84.
Macaskie L.E., van der lelie, D and Gutnick, D. 1997. Bacterial interaction with heavy metals in the environment. Res. Microbiol. 148,pp 513-533.
Mackaskie, L.E., 1990. An immobilised cell bioprocess for the removal of heavy metals from aqueous flows. J. Chem Technol Biotechnol. 49,pp 357-79.
Magdy Y.H and Daifullah A.A.M., 1998, Adsorption of a basic dye from aqueous solutions onto sugar-industry mud, Waste Manage. 18, pp. 219–226.
Majeda A.M. Khraisheh, Yahya S. Al-degs and Mcmin Wendy A. M. 2004, Remediation of wastewater containing heavy metals using raw and modified diatomite.Chemical Engineering Journal, 9(2), pp 177-184.
Malik P.K., 2003, Use of activated carbons prepared from sawdust and rice-husk for adsorption of acid dyes: a case study of acid yellow 36, Dyes Pigments 56, pp. 239–249.
Malkoc E and Nuhoglu 2005, Investigations of nickel (II) removal from aqueous solutions using tea factory waste. Journal of Hazardous Materials, 127(1-3), pp 120-128.
Manju G. N., Anoop Krishnan K.,. Vinod V. P and Anirudhan T. S. 2002. An investigation into the sorption of heavy metals from wastewaters by polyacrylamide-grafted iron (III) oxide. .Journal of Hazardous Materials, 91(1-3), pp 221-238.
Mann H, 1990. Biosorption of heavy metals by bacterial biomass in Biosorption of heavy Metals, Volesky, B (ed). CRC Press, Boca Raton, FL.pp 93-137.
Martel B., Devassine M, Crini G., Weltrowski M., Bourdonneau M. and Morcellet M., 2001, Preparation and sorption properties of a beta-cyclodextrin-linked chitosan derivative, J. Polym. Sci. Part A: Polym. Chem. 39, pp. 169–176.
Masri M. S. and Friedman M. 1974. Effect of chemical modification of wool on metal ion binding. J. Appl.Polymer Sci. 18, pp 2367-2377.
Masri M. S., Reuter F. W. and Friedman M. 1974, Binding of metal cations by natural substances. J. Appl. Polymer Sci. 18,pp 675-681.
Matheickal Jose T. and Qi Ming Yu. 1996. Biosorption of lead from aqueous solutions by marine algae ecklonia radiata Water Science and Technology.34 (9), pp 1-7.
Mathur N, Krishnatrey R, Sharma S, Pathak S, Sharma KP. 2003 Certain haematological responses in Swiss albino mice following exposure to textile dye wastewater. J Environ Biol.; 24(2):161-4.
Matos G. D and Arruda M. A. Z. 2003. Vermicompost as natural adsorbent for removing metal ions from laboratory effluents, Process Biochemistry, 39(1) pp 81-88.
Mattuschka, B., Junghaus, K. and Straube G. 1993. Biosorption of metals by waste biomass, in Biohydrometallurgical Technologies. Proceedings of the international biohydrometallurgical symposium, Torma, A.E., Apel, M.L. and Brierley, C.L., (eds), The Minerals, Metals and Materials Society, Warrendale, PA.
McDonald, C.N. and Bajwa, R.S. 1977 Removal of toxic metal ions from metal finishing wastewater by solvent extraction. Sep. Sci., 12, pp 435-445.
McGeorge, L. J., Louis, J. B., Atherholt, T. B. and McGarrity, G. J. 1985. Mutagenicity analyses of industrial effluent: Results and considerations for integration into water pollution control programs.-In: Short-Term Bioassays in the Analysis of Complex Environmental Mixtures IV (eds M. D. Waters et al.), Plenum Press, New York.
McKay G., Blair H. S. and Findon A. 1989, Equilibrium studies for the sorption of metal ions onto chitosan .Ind. J. Chem. A 28, pp 356-360.
McKay G., Blair H.S and A. Findon Ind. J. Chem. 28A (1989), pp. 356–360.
McKay G., Blair H.S. and Gardner J.R., 1989, The adsorption of dyes onto chitin in fixed bed column and batch adsorbers, J. Appl. Polym. Sci. 28 , pp. 1499–1544.
McKay G., Porter J.F. and Prasad G.R., 1999, The removal of dye colours from aqueous solutions by adsorption on low-cost materials, Water Air Soil Pollut. 114, pp. 423–438.
McLelland J. K. and Rock C. A. 1988, Pretreating landffll leachate with peat to remove metals. Water, Air Soil Poll. 37, pp 203-215.
Meena Ajay Kumar, Mishraa G.K.,. Raia P.K, Chitra Rajagopala, and Nagarb P.N. 2005 Journal of Hazardous Materials, 122(1-2), pp 161-170.
Merrifield John D. Davids William G, MacRae Jean D. and Aria Amirbahman. 2004, Water Research, 38 (13) , pp 3132-3138.
Meshko V., Markovska L., Mincheva M and Rodrigues A.E., 2001, Adsorption of basic dyes on granular activated carbon and natural zeolite, Water Res. 35, pp. 3357–3366.
Meuniera N., Laroulandieb J., Blais J. F and Tyagi J. F., 2003, Cocoa shells for heavy metal removal from acidic solutions. Bioresource Technology, 90 (3), pp 255-263.
Mineral Resources Institute Technical Report Series 1985, Use of Alabama peat as an adsorbent for heavy metals. MRI Technical Report Series, T.R. No. 12.
Mishra G and Tripathy M., 1993 A critical review of the treatment for decolourization of textile effluent. Colourage 40, pp. 35–38.
Mittal A.K. and Gupta S.K., 1996 Biosorption of cationic dyes by dead macro fungus Fomitopsis carnea: batch studies. Water Sci. Technol. 34, pp. 157–181.
Mittal A.K. and Gupta S.K., 1996, Biosorption of cationic dyes by dead macro fungus Fomitopsis carnea: batch studies. Water Sci. Technol. 34, pp. 157–181.
Mohan D., Singh K.P., Singh G. and Kumar K., 2002, Removal of dyes from wastewater using fly ash, a low-cost adsorbent, Ind. Eng. Chem. Res. 41, pp. 3688–3695.
Morais L.C., Freitas O.M., Gonçalves E.P, Vasconcelos L.T. and Gonzalez Beça C.G., 1999, Reactive dyes removal from wastewaters by adsorption on eucalyptus bark: variables that define the process, Water Res. 33, pp. 979–988.
Moran C., Hall M.E. and Howell R.C., 1997, Effects of sewage treatment on textile effluent. J. Soc. Dyers Colour 11, pp. 272–274.
Morgan-Sagastume, J.M., Jimenez, B. and Noyola, A., 1997. Tracer studies in a laboratory and pilot scale UASB reactor. Environ. Technol. 18, pp. 817–826.
Munther Kandah. 2001. Zinc adsorption from aqueous solutions using disposal sheep manure waste (SMW). Chemical Engineering Journal, 84(3), pp 543-549.
Namasivayam C and Arası D.J.S.E., 1997, Removal of Congo red from wastewater by adsorption onto waste red mud, Chemosphere 34 (2), pp. 401–417.
Namasivayam C and Kadirvelu K., 1994, Coir pith as an agricultural waste by-product, for the treatment of dyeing wastewater, Biores. Technol. 48, pp. 79–81.
Namasivayam C and Kanchana N 1992, Waste banana pith as an adsorbent for colour removal from wastewaters. Chemosphere, 25 (1), pp 1691-1705.
Namasivayam C and Kanchana N 1993, Removal of Congo red from aqueous solution by waste banana pith. Pertanika, 1, pp 33-42.
Namasivayam C and Kavitha D., 2002 Removal of Congo red from water by adsorption onto activated carbon prepared from coir pith, an agricultural solid waste, Dyes Pigments 54, pp. 47–58.
Namasivayam C and Sumithra S, 2005, Removal of direct red 12B and methylene blue from water by onto Fe (III)/Cr (III) hydroxide, an industrial solid waste. Journal of Environmental Management, 74 (3), pp 207-215.
Namasivayam C. and Arasi D.J.S.E., 1997 Removal of Congo red from wastewater by adsorption onto red mud, Chemosphere 34, pp. 401–471.
Namasivayam C. and. Periasamy, K 1993, Bicarbonate treated peanut hull carbon for Hg(II) removal from aqueous solution, Water Res. 27, pp. 1663–1668.
Namasivayam C. and Ranganathan K. 1992, Environ. Pollut. 82, pp. 255–261
Namasivayam C. and Sumithra S.,2005 Removal of direct red 12B and methylene blue from water by adsorption onto Fe(III)/Cr(III) hydroxide, an industrial solid waste, J. Environ. Manage. 74, pp. 207–215.
Namasivayam C., Dinesh Kumar M., Selvi K., Begum Ashruffunissa R., Vanathi T and Yamuna R.T., 2001, Waste coir pith—a potential biomass for the treatment of dyeing wastewaters, Biomass Bioenergy 21, pp. 477–483.
Namasivayam C., Jeyakumar R and Yamuna R.T., 1994, Dye removal from wastewater by adsorption on waste Fe (III)/Cr (III) hydroxide, Waste Management 14, pp. 643–648.
Namasivayam C., Kanchana N and Yamuna R.T 1993. Waste banana pith as adsorbent for removal of Rhodamine B from aqueous solution. Waste management, 13, pp 89-95.
Namasivayam C., Prabha D. and Kumutha M., 1998, Removal of direct red and acid brilliant blue by adsorption on to banana pith, Bioresour. Technol. 64 , pp. 77–79.
Namasivayam C., Radhika R. and Suba S. 2001, Uptake of dyes by a promising locally available agricultural solid waste: Coir Pith, Waste Managt. 21, pp. 381–387.
Nansheng, D., Feng, W., Fan, L. and Zan, L., 1997. Photodegradation of dyes in aqueous solutions containing Fe(III)-oxalato complexes. Chemosphere 35, pp. 2697–2706.
Nansheng, D., Feng, W., Shizhong, T. and Tao, F., 1997. Photodegradation of dyes in aqueous solutions containing Fe(III)-hydroxy complex II. Solar photodegradation kinetics. Chemosphere 34, pp. 2725–2735.
Nawar, S and Doma H.S. 1989, Removal of dyes from effluents using low cost agricultural by-products. The Sci. of the Total Environ., 79, pp 271-279.
Nemec, P., Prochazka, H., Stamberg, K., Katzer, J., Stamberg, J., Jilek, R. and Hulak, P. 1977. Process of treating mycelia of fungi for retention of metals. (U.S. Patent 4 021 368).
Netpradit S., Thiravetyan P and Towprayoon S., 2004, Evaluation of metal hydroxide sludge for reactive dye adsorption in a fixed-bed column system, Water Res. 38, pp. 71–78.
Netpradit S., Thiravetyan P and Towprayoon S., 2003, Application of “waste” metal hydroxide sludge for adsorption of azo reactive dyes, Water Res. 37, pp. 763–772.
Netpradit S., Thiravetyan P and Towprayoon S., 2004, Adsorption of three azo reactive dyes by metal hydroxide sludge: effect of temperature, pH and electrolytes, J. Colloid Int. Sci. 270, pp. 255–261.
Niu H., Xu X. S. and Wang J. H. 1993, Removal of lead from aqueous solutions by penicillium biomass.Biotechnol. Bioeng. 42, 785-787.
Niu, H, Xu, XS, Wang, JH and Volesky, B. 1993. Removal of lead from aqueous solutions by Penicillium biomass. Biotechnol. Bioeng. 42, 785-787.
Nuhoglu Y., Malkoca E., Gürsesb A.and Canpolat N.2002, The removal of Cu(II) from aqueous solutions by Ulothrix zonata. Bioresource Technology. 85 (3), pp 331-333.
O’Mahony T., Guibal E and. Tobin J.M, 2002. Reactive dye biosorption by Rhizopus arrhizus biomass. Enzyme Microbial. Technol. 31, pp. 456–463.
Ogutveren, U.B., Gonen, N. and Koparal, S., 1992. Removal of dye stuffs from waste water: electrocoagulation of acilan blau using soluble anode. J. Environ. Sci. Health. Part A, Environ. Sci. Eng. 27, pp. 1237–1247.
Okada K., Yamamoto N., Kameshima Y and Yasumori A., 2003, Adsorption properties of activated carbon from waste newspaper prepared by chemical and physical activation, J. Colloid Int. Sci. 262, pp. 194–199.
Ollikka, P., Alhonmäki, K., Leppänen, V.M., Glumoff, T., Raijola, T. and Suominen, I., 1993. Decolorization of azo, triphenyl methane, heterocyclic, and polymeric dyes by lignin peroxidase isoenzymes form Phanerochaete chrysosporium. Appl. Environ. Microbiol. 59, pp. 4010–4016.
O'Neill, C., Hawkes, F.R., Esteves, S.R.R., Hawkes, D.L. and Wilcox, S.J., 1999. Anaerobic and aerobic treatment of a simulated textile effluent. J. Chem. Technol. Biotechnol. 74, pp. 993–999.
Onsùyen E. and Skaugrudé 1990, Metal recovery using chitosan. J. Chem. Tech. Biotechnol. 49, pp 395-404.
Orhan Y.1993, The removal of heavy metals by using agricultural wastes. Water Sci.Technol. 28(2), pp 247-255.
Orhon, D., Sözen, S., Görgün, E., Cokgör, E. and Artan, N., 1999. Technological aspects of wastewater management in coastal tourist areas. Water Sci. Technol. 39/8, pp. 177–184.
Otero M., Rozada F., Calvo L.F., Garcia A.I and Moran A., 2003, Elimination of organic water pollutants using adsorbents obtained from sewage sludge, Dyes Pigments 57.
Otero M., Rozada F., Calvo L.F., Garcia A.I and Moran A., 2003, Kinetic and equilibrium modelling of the methylene blue removal from solution by adsorbent materials produced from sewage sludges, Biochem. Eng. J. 15, pp. 59–68.
Özacar M and Sengil A.I., 2003, Adsorption of reactive dyes on calcined alunite from aqueous solutions, J. Hazardous Mater. B98, pp. 211–22
Özacar M. and Sengil A.I, 2005 Adsorption of metal complex dyes from aqueous solutions by pine sawdust, Bioresour. Technol. 96, pp. 791–795.
Özcan A.S., Erdem B and Özcan A., 2004 Adsorption of acid blue193 from aqueous solutions onto Na-bentonite and DTMA-bentonite, J. Colloid Int. Sci. 280, pp. 44–54.
Ozdemir O., Armagan B., Turan M and Celik M.S., 2004, Comparison of the adsorption characteristics of azo-reactive dyes on mezoporous minerals, Dyes Pigments 62, pp. 49–60.
Ozer Ayla, Ozer Dursun and H. Ibrahim Ekiz, 1999, Process Biochemistry 34 (1999) pp 919–927.
Padmavathy V, Vasudevan P and Dhingra S.C 2003, Biosorption of nickel (II) ions on Baker's yeast, Process Biochem. 38 (10), pp. 1389–1395.
Paknikar, K.M., Palnitkar, U.S and Puranik, P.R. 1993. Biosorption of metals from solution by mycelial waste of Pencillium chrysogenum, Proceedings of the international biohydrometallurgical symposium, Torma, A.E., Apel, M.L. and Brierley, C.L., (eds), The Minerals, Metals and Materials Society, Warrendale, PA.
Pala A. and Tokat E., 2002, Color removal from cotton textile industry wastewater in an activated sludge system with various additives, Water Res. 3, pp. 2920–2925.
Panday K. K., Prasad G. and Singh V. N. 1984, Removal of Cr(VI) from aqueous solutions by adsorption on fly ash-wollastonite. J. Chem. Tech. Biotechnol.,A 34, pp 367-374.
Panday K.K. Prasad , G and Singh V.N., 1984Removal of Cr(VI) from aqueous solutions by adsorption on fly ash-wollastonite. J. Chem. Technol. Biotechnol. 34A, pp. 367–374.
Panday K.K., Prasad G and. Singh V.N, 1985. Copper(II) removal from aqueous solutions by fly ash Water Res. 19, pp. 869–873.
Panday K.K., Prasad G. and Singh. V.N. 1986. Mixed adsorbents for Cu(II) removal from aqueous solutions Environ. Technol. Lett. 7, pp. 547–554.
Parab H, Joshi S, Shenoy N, Lali A, Sarma U S and Sudersanan M. 2006, Determination of kinetic and equilibrium parameters of the batch adsorption of Co(II), Cr(III) and Ni(II) on to coir pith. Process Biochemistry, 41(3), pp 609-615.
Patterson, J.W. 1975 wastewater Treatment Technology. Ann Arbor Science, Ann Arbor, Michigan 1975.
Peniche-Covas C., Alvarez L. W. and Arguelles-Monal W. 1992 The adsorption of mercuric ions by chitosan. J.Appl. Polymer Sci. 46,pp 1147-1150.
Peniche-Covas C., Alvarez L.W. and. Arguella-Monal W, 1991, J. Appl. Polym. Sci. 46, pp. 1147–1150.
Periasamy K. and Namasivayam C., 1994, Process development for removal and recovery of cadmium from wastewater by a low-cost adsorbent: adsorption rates and equilibrium studies. Ind. Eng. Chem. Res. 33, pp. 317–320.
Petek, J. and Glavic, P., 1996. An integral approach to waste minimization in process industries. Resour. Conserv. Recycl. 17, pp. 169–188.
Petersen J. N., Davison B. H., Scott C. D. and Blankinship S. L. 1991, Size changes associated with metal adsorption onto modified bone gelatin beads. Biotechnol. Bioeng. 38, pp 923-928.
Phan T.N.T., Bacquet M and Morcellet M., 2000, Synthesis and characterization of silica gels functionalized with monochlorotriazinyl beta-cyclodextrin and their sorption capacities towards organic compounds, J. Inclusion Phenom. Macrocyclic Chem. 38, pp. 345–359.
Ping Xin Sheng, Yen-Peng Ting, J. Paul Chen and Liang Hong 2004. Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: characterization of biosorptive capacity and investigation of mechanisms. Journal of Colloid and Interface Science, 275 (1), pp 131-141
Pollard S.J.T., Fowler G.D., Sollars C.J and Perry R., 1992, Low cost adsorbents for waste and waste waster treatment: a review, Sci. Total Environ. 116, pp. 31–52.
Polman J.K.and Breckenridge C.R., 1996, Biomass-mediated binding and recovery of textile dyes from waste effluents. Text. Chem. Colour. 28, pp. 31–35.
Poots V.J.P., McKay G and Healy J.J., 1976, The removal of acid dye from effluent using natural adsorbents—I, Peat. Water Res. 10, pp. 1061–1066.
Pradas E.G., Sanchez M.V., Cruz F.C., Viviana M.S.and Perez M.F, 1994, Adsorption of cadmium and zinc from aqueous solution on natural and activated bentonite. J. Chem. Technol. Biotechnol. 59, pp. 289–295.
Pradhan J., Das S.N. and Thakur R.S. 1999, Adsorption of Hexavalent Chromium from Aqueous Solution by Using Activated Red Mud J. Colloid Interf. Sci. 217, pp. 137–141.
Prakasham, RS, Merrie, JS, Sheela, R, Saraswati N and Ramakrishnan S.V. 1999, Biosorption of Chromium (VI) by free and immobilized Rhizopus arrhizus. Environmental Pollution, 104, pp 421-427.
Puranik, P. R. and Paknikar, K. M., 1997. Biosorption of lead and zinc from solutions using Streptoverticillium cinnamoneum wastebiomass. J. Biotechnol., 55, 113–124.
Radhika, M., Kadirvelu, K., Shanthi, K., Pattabhi, S., 2001, Adsorptive removal of Rhodamine-B from aqueous solution onto activated carbon prepared from low cost material. In: Proceedings of ENVIRO 2001, National Conference on Control of Industrial Pollution and Environmental Degradation, Coimbatore, India.
Rajeshwarisivaraj, Namasivayam C and Kadirvelu K., 2001, Orange peel as an adsorbent in the removal of acid violet 17 (acid dye) from aqueous solutions, Waste Manage. 21, pp. 105–110.
Rajeshwarisivaraj, Sivakumar S., Senthilkumar P. and Subburam V., 2001, Carbon from Cassava peel, an agricultural waste, as an adsorbent in the removal of dyes and metal ions from aqueous solution, Bioresour. Technol. 80, pp. 233–235.
Ramachandra T.V., Ahalya N. and Rajasekara Murthy C., 2005 Aquatic ecosystems: conservation, restoration and management , Capital Publishers, New Delhi.
Ramachandra T.V., Kiran R and Ahalya N (2002) Status, Conservation and Management of Wetlands–– Allied Publishers (P) Ltd, India.
Ramachandra T.V., Rajasekaramurthy C., and Ahalya N. (2002) Restoration of Lakes and Wetlands. Allied Publishers (P) Ltd., India.
Ramakrishna K.R. and Viraraghavan T., 1997, Dye removal using low cost adsorbents, Water Sci. Technol. 36 , pp. 189–196.
Ramakrishna K.R. and Viraraghavan T., 1997, Dye removal using low cost adsorbents, Water Sci. Technol. 36, pp. 189–196.
Randall J. M., Bermann R. L., Garrett V. and Waiss A.C., Jr. 1974a Use of bark to remove heavy metal ionsfrom waste solutions. Forest Prod. J. 24(9),pp 80-84.
Randall J. M., Hautala E. and McDonald G. 1978, Binding of heavy metal ions by formaldehyde-polymerized peanut skins. J. Appl. Polymer Sci. 22(2), pp 379-387.
Randall J. M., Hautala E. and Waiss A. C. Jr. 1974b, Removal and recycling of heavy metal ions from miningand industrial waste streams with agricultural byproducts. Proceedings of the Fourth Mineral WasteUtilization Symposium. Chicago, IL, May 7-8, 1974, pp. 329-334.
Reed, B.E. and Arunachalam S. 1994. Use of granular activated carbon columns for the lead removal. J. Environ. Engg., 120, pp 416-436.
Reed, B.E., Arunachalam , S and Thomas, B. 1994 Removal of lead and cadmium from aqueous waste streams using granular activated carbon (GAC) columns. Environ. Prog., 13, pp 60-64.
Rich G, Cherry K , (1987) Hazardous Waste Treatment Technologies, Pudvan Publishers, New York.
Ricordela S, Taha S, Cisse I and Dorange G. 2001. Heavy metals removal by adsorption onto peanut husks carbon: characterization, kinetic study and modeling, Separation and Purification Technology, 24 (3), pp 389-401.
Roberto Herrero, Pablo Lodeiro, Carlos Rey-Castro, Teresa Vilariño and Manuel E. Sastre de Vicente, 2005. Removal of inorganic mercury from aqueous solutions by biomass of the marine macroalga Cystoseira baccata .Water Res, 39(14), pp 3199-3210
Roberts E. J. and Rowland S. P. 1973, Removal of mercury from aqueous solutions by nitrogen-containing chemically modified cotton. Envir. Sci. Technol. 7(6), pp 552-555.
Robinson T, B. Chandran and P. Nigam, 2002, Removal of dyes from an artificial textile dye effluent by two agricultural waste residues, corncob and barley husk, Environ. Int. 28, pp. 29–33.
Robinson T., Mcmullan G., Marchant R. and Nigam P, 2001 Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 77, pp. 247–255.
Rodriguez-Reinoso F., Activated carbon: structure, characterization, preparation and applications. In: H. Marsh, E.A. Heintz and F. Rodriguez-Reinoso, Editors, Introduction to Carbon Technologies, Universidad de Alicante, Secretariado de Publicaciones (1997).
Rollinson C.L, 1973, Chromium, Molybdenum, Tungsten. In: Trotman-Dickenson, Comprehensive Inorganic Chemistry, Third ed. Pergamon Press, Oxford. pp: 691-694.
Rorrer G. L., Hsien T.-Y. and Way J. D. 1993, Synthesis of porous-magnetic chitosan beads for removal of cadmium ions from waste water. Ind. Eng. Chem. Res. 32, pp 2170-2178.
Rorrer G.L., Way J.D.,2002, Chitosan Beads to Remove Heavy Metal from Wastewater, Dalwoo-ChitoSan, May 2002, ftp://dalwoo.com/chitosan/rorrer.html.
Rosenberger, R. F., in The Filamentous Fungi (eds Smith, J. E. and Berry, D. R.), Edward Arnold, London, 1975, vol. 2, pp. 328–342.
Rozada F., Calvo L.F., Garcia A.I., Martin-Villacorta J. and Otero M., 2003, Dye adsorption by sewage sludge-based activated carbons in batch and fixed-bed systems, Bioresour. Technol. 87, pp. 221–230.
Sağ Y and Kutsal T. 1995 Biosorption of heavy metals by Zoogloea ramigera: use of adsorption isotherms and a comparison of biosorption characteristics, The Chemical Engineering Journal 60 181-188.
Sahoo D.K., Kar R.N and Das R.P, 1992, Bioaccumulation of heavy metal ions by Bacillus circulans, Bioresour. Technol. 41, pp. 177–179.
Santiago I., Worland V. P., Cazares-Rivera E. and Cadena F. 1992, Adsorption of hexavalent chromium onto tailored zeolites. 47th Purdue Industrial Waste Conference Proceedings, pp. 669-710. Lewis Publishers, Inc., Chelsea, MI.
Sawada K. and Ueda M., 2003, Adsorption behavior of direct dye on cotton in non-aqueous media, Dyes Pigments 58, pp. 37–40.
Sayler G. S., Nelson J. D., Jr., and. Colwell R. R 1975 Role of Bacteria in bioaccumulation of mercury in the Oyster Crassostrea virginica. Appl Microbiol.; 30(1): 91–96.
Scarpi, C., Ninci, F., Centini, M. and Anselmi, C., 1998. High-performance liquid chromatography determination of dir Schuler, C.A., Anthony R.G., and Ohlendorf. H.M. 1990. Selenium in Wetlands and Waterfowl Foods and Kesterson Reservoir, California, 1984. Archives of Environmental Contamination and Toxicology 29:845-853.
Schiewer, S and Wong, M.H. 1999. Metal binding stoichiometry and iostherm choice in biosorption. Environ. Sci. Technol. 333, (21), pp 3821-28.
Schiewer, S, Fourest, E, Chong, KH and Volesky, B. 1995. Ion exchange in biosorption by dried seaweed: experiments and model predictions. Biohydrometallurgical Processing, Vol.2, Proc. Internat. Biohydromet. Symp., Jerez, CA, Vargas, T, Toledo, H and Wiertz, JV (eds.) Univ. Chile, Santiago, Chile, p. 219-228.
Schiewer, S. and Volesky, B. 1996. Modeling multi-metal ion exchange in biosorption.
Schiewer, S. and Volesky, B. 1997. Ionic strength and electrostatic effects in biosorption of divalent metal ions and protons. Environ. Sci. Technol. 31, pp 2478-2485.
Schmuhl R., Krieg H.M and Keizer K. 2001, Adsorption of Cu(II) and Cr(VI) ions by chitosan: Kinetics and equilibrium studies Water S.A. 2, pp. 1–7.
Selatnia A, Boukazoula A, Kechid N, Bakhti MZ and Chergui A, 2004. Biosorption of Fe 3+ from aqueous solution by a bacterial dead Strptomyces rimosus biomass. Process Biochemistry, pp 1643-1651.
Sen A K and De A K 1987, Adsorption of mercury (II) by coal fly ash, Water Res. 21, pp. 885–888.
Sen A.K and Arnab K.D., 1987, Adsorption of mercury(II) by coal fly ash Water Res. 21, pp. 885–888.
Serpone N., E. Borgarello and E. Pelizzti, Photoreduction and photodegradation of inorganic pollutants. In: M. Schiavello, Editor, Photocatalysis and Environment, Kluwer Academic, Dordrecht (1988), p. 527.
Seshadri, S., Bishop, P.L. and Agha, A.M., 1994. Anaerobic/aerobic treatment of selected azo dyes in wastewater. Waste Manag. 14, pp. 127–137.
Shao Y., Martel B., Morcellet M., Weltrowski M and Crini G., 1996, Sorption of textile dyes on beta-cyclodextrin-epichlorhydrin gels, J. Inclusion Phenom. Mol. Recogn. Chem. 25 (1996), pp. 209–212.
Sharma C., Gupta G. S., Prasad G. and Rupainwar D. C. 1990 Use of wollastonite in the removal of Ni(II) from aqueous solutions. Water Air Soil Poll. 49, pp 69-79.
Sharma D. C. and Forster C. F. 1993, Removal of hexavalent chromium using sphagnum moss peat. WaterRes. 27(7), 1201-1208.
Sharma D. C. and Forster C. F. 1994, The treatment of chromium wastewaters using the sorptive potential of leaf mould. Bioresource Technol. 49, pp 31-40.
Sharma D. C. and Forster C. F. 1995, Continuous adsorption and desorption of chromium ions by sphagnum moss peat. Process Biochem. 30(4), pp 293-298.
Sharma D.C. and Forster C.F.1993. Removal of hexavalent chromium using sphagnum moss peat. Water Res. 2, pp. 1201–1208.
Sharma D.C.and Forster 1994. Continuous adsorption and desorption of chromium ions by sphagnum moss peat. Process Biochem. 30, pp. 293–298.
Sharma, D.C. and Forster, C.F. 1994. A preliminary examination onto the adsorption of hexavalent chromium using low cost adsorbents. Bioresource Technol., 47, pp 257-264
Shaul, G.M., Holdsworth, T.J., Dempsey, C.R. and Dostal, K.A., 1991. Fate of water soluble azo dyes in the activated sludge process. Chemosphere 22, pp. 107–119.
Shawabkeh R.A. and Tutunji M.F., 2003, Experimental study and modelling of basic dye sorption by diatomaceous clay, Appl. Clay Sci. 24 , pp. 111–120.
.
Shichi T and Takagi K, 2000, Clay minerals as photochemical reaction fields, J. Photochem. Photobiol. C: Photochem. Rev. 1, pp. 113–130.
Shukla A., Zhang Y.H, Dubey P., Margrave J.L. and Shukla S.S., 2002, The role of sawdust in the removal of unwanted materials from water, J. Hazardous Mater. B95, pp. 137–152.
Shukla S. R. and Sakhardande V. D. 1992, Column studies on metal ion removal by dyed cellulosic materials.J. Appl. Polymer Sci. 44, pp 903-910.
Shukla S.R. and Roshan S. Pai,. 2005, Adsorption of Cu(II), Ni(II) and Zn(II) on modified jute fibres, Bioresource Technology, 96 (13), pp 1430-1438.
Shukla S.R.and Roshan S. Pai. 2005, Adsorptionof Cu(II), Ni(II) and Zn(II) on dye loaded groundnut shells and sawdust.Separation and Purification Technology, 43(1), pp 1-8.
Shukla, S.P. and Gupta, G.S., 1992. Toxic effects of omega chrome red ME and its treatment by adsorption. Ecotoxicol. Environ. Saf. 24, pp. 155–163.
Shukla, S.S. and Sakherdande V.D. 1990 Cupric ion removal by dyed cellulosic materials. J. Appl. Poly Sci, 41, pp 2655-2663.
Siegel, S., Keller, P., Galun, M., Lehr, H., Siegel, B. and Galun, B.1986, Biosorption of lead and chromium by penicillium preparations Water Air Soil Pollut. 27, pp 69–75.
Singh V.N., Mishra G and Panday K.K., 1984, Removal of Congo red by wollastonite, Indian Journal of Technology 22, pp. 70–71.
Singh, D.B., Rupainwar, D.C. and Prasad G, 1991. Studies on the removal of Cr (VI) from wastewater by feldspar. J. Chem. Technol. Biotechnol, 53, pp 127-131.
Sitting M., Toxic Metals—Pollution Control and Worker Protection, Noyes Data Corporation, New Jersey (1976).
Smith E.F., McCarthy P., Yu T.C and Mark Jr H.B., 1977, Sulfuric acid treatment of peat for cation exchange, Chem. Eng. J. 49 , pp. 633–638.
Snell F.D and. Snell C.T, Colorimetric Methods of Analysis, 3rd edn, Van Nostrand, New York, 1961.
Soares, G.M.B., de Amorim, M.T.P. and Costa-Ferreira, M., 2001. Use of laccase together with redox mediators to decolourize Remazol Brilliant Blue R. J. Biotechnol. 89, pp. 123–129.
Society of Dyers and Colourists. 1990. Colorants and Auxiliaries Volume 1-Colorants.
Sokolowska-Gajda, J., Freeman, H.S. and Reife, A., 1996. Synthetic dyes based on environmental considerations: 2. Iron complexed formazan dyes. Dyes Pigm. 30, pp. 1–20.
Sorensen, B.L. and Wakeman, R.J., 1996. Filtration characterization and specific surface area measurement of activated sludge by rhodamine B adsorption. Water Res. 30, pp. 115–121.
Srivastava K., Bhattacharjee G., Tyagi R., Pant N and Pal N 1988. Studies on the removal of some toxic metal ions from aqueous solutions and industrial waste. Part I (Removal of lead and cadmium by hydrous iron and aluminium oxide) Environ. Technol. Lett. 9 (1988), pp. 1173–1185.
Srivastava S. K., Singh A. K. and Sharma A. 1994, Studies on the uptake of lead and zinc by lignin obtained from black liquor - a paper industry waste material. Environ. Technol. 15, pp 353-361.
Srivastava S.K, Gupta V.K and Mohan D. 1997. Removal of lead and chromium by activated slag - ablast-furnace waste J. Environ. Eng. 123, pp. 461–468.
Srivastava S.K., Tyagi R. and Pal N. 1989, Studies on the removal of some toxic metal ions. Part II (removal of lead and cadmium by montmorillonite and kaolinite) Environ. Technol. Lett. 10, pp. 275–282.
Srivastava, S.J., Singh, N.D., Srivastava, A.K., Sinha, R., 1995a. Acute toxicity of malachite green and its effects on certain blood parameters of a catfish, Heteropneustes fossilis. Aquat. Toxicol. 31, 241–247.
Staunton S. and Roubaud M. 1997. Adsorption of 137Cs on montmorillonite and illite: Effect of charge compensating cation, ionic strength, concentration of Cs, K and fulvic acid. Clays and Clay Miner. 45, pp. 251–260.
Stolz, A., 2001. Basic and applied aspects in the microbial degradation of azo dyes. Appl. Microbiol. Biotechnol. 56, pp. 69–80.
Strandberg G W, Shumate S E II and Parrot J R Jr, 1981. Microbial cells as biosorbents for heavy metals: accumulation of uranium by Saccharomyces cerevisiae and Pseudomonas aeruginosa. Appl Environ Microbiol 41, pp 237-245.
Stratton, G. W., in Review in Environmental Toxicology (ed. Hodgson, E.), Elsevier, Amsterdam, 1987, pp. 85–94.
Stuetz, R.M. , Madgwick, J.C. and Gee A.R. 1993. Immobilisation of biosorbed metal ions in Proceedings of the international biohydrometallurgical symposium, Torma, A.E., Apel, M.L. and Brierley, C.L., (eds), The Minerals, Metals and Materials Society, Warrendale, PA.
Sumathi S.and. Manju B.S, 2000, Uptake of reactive textile dyes by Aspergillus foetidus. Enzyme Microbial. Technol. 27, pp. 347–352.
Sun Q and Yang L., 2003, The adsorption of basic dyes from aqueous solution on modified peat-resin particle, Water Res. 37, pp. 1535–1544.
Tang, W.Z. and An, H., 1995. Photocatalytic degradation kinetics and mechanism of acid blue 40 by TiO2/UV in aqueous solution. Chemosphere 31, pp. 4171–4183.
Tang, W.Z. and An, H., 1995. UV/TiO2 photocatalytic oxidation of commercial dyes in aqueous solutions. Chemosphere 31, pp. 4158–4170.
Tare, V, Jawed, M and Iyengar, L. 1988 Application of xanthates in heavy metal removal. Indian Assoc. Wate. Pollut. Control. Tech. Annual, 15, pp 88-94.
Tatarko M.and. Bumpus J.A, 1998. Biodegradation of Congo Red by Phanerochaete chrysosporium. Water Res. 32, pp. 1713–1717.
Taty-Costodes Christian V., Fauduet Henri, Catherine Porte and Alain Delacroixa, 2003. Removal of Cd(II) and Pb(II) ions, from aqueous solutions, by adsorption onto sawdust of Pinus sylvestris. Journal of Hazardous Materials. 105 (1-3), pp 121-142.
Teles de Vasconcelos L. A. and Gonzaalez C. G. 1994, Adsorption equilibria between pine bark and several ions in aqueous solution, 1. Pb(II). Eur. Water Poll. Control 4(1), pp 41-51.
Teles de Vasconcelos L. A. and Gonzalez Beca C. G. 1993, Adsorption equilibria between pine bark and several ions in aqueous solution, .Water Poll. Control 3(6), pp 29-39.
Teng L.T., Khor E., Tan T.K., Lim L.Y. and Tan S.C., 2001, Concurrent production of chitin from shrimp shells and fungi, Carbohydr. Res. 332, pp. 305–316.
Teresa J. Naimo 1995, A review of the effects of heavy metals on freshwater mussels, Ecotoxicology, Vol 4, No 6, pp 341 – 362.
Tobin, J.M., Cooper D.G., and Neufield R.J. 1984. Uptake of metal ions by Rhizopus arrhizus. Appl. Envir. Microbiol. 47, 821-24.
Toledo, B.I., Utrilla, J.R., Gracia, M.A. and Cistilla, C.M. Influence of the oxygen surface complexes of activated carbon on the adsorption of chromium ions from aqueous solution: Effect of sodium chloride and humic acid, Carbon, 32, pp 93-100.
Tong P., Baba Y., Adachi Y. and Kawazu K. 1991, Adsorption of metal ions on a new chelating ion-exchange resin chemically derived from chitosan. Chem.Lett, 9, pp 1529-1532.
Townsley, C.C., Ross, I.S., and Atkins A.S., 1986. Biorecovery of metallic residues from various industrial effluents using filamentous fungi, in Fundamental and Applied Biohydrometallurgy, Lawrence, R.W., Branion, R.M.R. Ebner, H.G. (eds). Elsevier, Amsterdam, The Netherlands. 279-289.
Tsai W.T., Chang C.Y., Lin M.C., Chien S.F., Sun H.F and Hsieh M.F., 2001, Adsorption of acid dye onto activated carbon prepared from agricultural waste bagasse by ZnCl2 activation, Chemosphere 45, pp. 51–58.
Tseng R.L., Wu F.C. and Juang R.S., 2003, Liquid-phase adsorption of dyes and phenols using pinewood-based activated carbons, Carbon 41, pp. 487–495.
Tsezos, M and Volesky, B. 1981,Biosorption of uranium and thorium, Biotechnol.Bioeng. 23, pp 583-604.
Tummavuori J. and Aho M. 1980a, On the ion-exchange properties of peat. Part I: On the adsorption of some divalent metal ions (Mn2+, Co2+, Ni2+, Cu2+, Zn2+,Cd2+ and Pb2+) on the peat. Suo 31(4), pp 79-83.
Tummavuori J. and Aho M. 1980b, On the ion-exchange properties of peat. Part II: On the adsorption of alkali, earth alkali, aluminum (III), chromium (III), iron (III), silver, mercury (II) and ammonium ions to the peat. Suo 31(2-3), pp 45-51.
Tünay, O., Kabdasli, I., Ohron, D. and Cansever, G., 1999. Use and minimalization of water in leather tanning processes. Water Sci. Technol. 40/1, pp. 237–244.
Turner D.R., Pabalan R.T. and Bertetti F.P. 1998. Neptunium (V) sorption on montmorillonite: an experimental and surface complexation modeling study. Clays Clay Miner. 46, pp. 256–269.
U.S. EPA. Environmental Pollution : Control Alternatives: Ecnomics of Wastewater Treatment Alternatives for the Electroplating Industry. EPA – 625/5-79-016, Cincinnati, Ohio (1979).
U.S.EPA Control and treatment technology for the metal finishing industry: Sulphide precipitation, EPA – 625/8-80-003, Cincinnati, Ohio (1980).
U.S.EPA. Control Technology for the metal finishing industry – Evaporators, EPA-625/8-79-002, Cincinnati, Ohio (1979a).
Ucun Handan, Kemal Bayhan Y, Yusuf Kaya, Avni Cakici and Faruk Algur O, (2002) Biosorption of chromium (VI) from aqueous solution by cone biomass of Pinus sylvestris. Bioresource Technology, 85, pp 155-158
Udaybhaskar P., Iyengar L. and Rao A.V.S.P., 1990, Hexavalent chromium interaction with chitosan J. Appl. Polym. Sci. 39, pp. 739–747.
USEPA. U.S. Environmental Protection Agency. 1987. Quality Criteria for Water. EPA Publication 440/5-86- 001. U.S. Gov. Prin. Office, Washington D.C.
USEPA. U.S. Environmental Protection Agency. 1993a. Guidance Specifying Management Measures for Sources of Nonpoint Pollution in Coastal Waters. EPA: Washington DC. Available through EPA Wetlands Hotline. 1-800-832-7828.
Valix M., Cheung W.H and McKay G., 2004, Preparation of activated carbon using low temperature carbonisation and physical activation of high ash raw bagasse for acid dye adsorption, Chemosphere 56, pp. 493–501.
Van der Heen, P. The removal of traces of heavy metals from drinking water and industrial effluent with ion exchanger. The Regional Chemical Society Meeting (1977).
Varma A.J., Deshpande S.V and Kennedy J.F., 2004, Metal complexation by chitosan and its derivative: a review, Carbohydr. Polym. 55, pp. 77–93.
Vaszquez G., Antorrena G., Gonzalez J. and Doval M. D. 1994, Adsorption of heavy metal ions by chemically modified Pinus Pinaster bark. Bioresource Technol. 48, 251-255.
Venkata Mohan S., Chandrasekhar Rao N and Karthikeyan J., 2002 Adsorptive removal of direct azo dye from aqueous phase onto coal based sorbents: a kinetic and mechanistic study, J. Hazardous Mater. B 90, pp. 189–204.
Venkata Mohan S., Sailaja P., Srimurali M and Karthikeyan J., 1999, Colour removal of monoazo acid dye from aqueous solution by adsorption and chemical coagulation, Environ. Eng. Policy 1, pp. 149–154.
Vijayaraghavan K., Jegan J., Palanivelu K. and Velan M. 2005, Biosorption of copper, cobalt and nickel by marine green alga Ulva reticulata in a packed column. Chemosphere 60(3), pp 419-426.
Viraraghavan T. and Kapoor A. 1994, Adsorption of mercury from wastewater by bentonite. Appl. Clay Sci, 9, pp 31-49.
Viraraghavan T. and Rao G. A. K. 1993, Adsorption of cadmium and chromium from wastewater by peat. Int.J. Environ. Studies, 44, pp 9-27.
Virta R., USGS Minerals Information, US Geological Survey Mineral Commodity Summary 2002, January 2002, ftp://minerals.usgs.gov/minerals/pubs/commodity/clays/190496.pdf.
Volesky B. and Prasetyo I. 1994, Cadmium removal in a biosorption column. Biotechnol. Bioeng. 43, pp 1010-1015.
Volesky, B and May-Phillips, HA 1995. Biosorption of heavy metals by Saccharomyces cerevisiae. J. Appl. Microbiol. Biotechnol. 42, pp 797-806.
Volesky, B. 1999. Biosorption for the next century , Biohydrometallurgy and the Environment Toward the Mining of the 21st Century, Internat. Biohydrometallurgy Symposium Proceedings, 1999, volume B, Ballester, A. & Amils, R. (eds.) Elsevier Sciences, Amsterdam, The Netherlands : pp.161-170.
Volesky, B., 2003. Sorption and Biosorption, BV Sorbex, St. Lambert, Quebec, pp. xii, 316.
Volesky, B., and Kuyucak, N., Biosorbent for gold, US Patent No. 4,769,223, 1988.
Volesky, B., and Tsezos, M., 1982, Separation of uranium by Biosorption,Biotechnol.Bioeng. 23, pp 583-604.
Volesky, Bohumil. Biosorption of Heavy Metals. CRC Press, Boston, USA, November 1990. 408 p. ISBN 0849349176.
Wagner, R.W. and Lindsey, J.S., 1996. Boron-dipyrromethane dyes for incorporation in synthetic multi-pigment light-harvesting arrays. Pure Appl. Chem. 68, pp. 1373–1380.
Walker G.M., Hansen L., Hanna J.A. and Allen S.J., 2003, Kinetics of a reactive dye adsorption onto dolomitic sorbents, Water Res. 37, pp. 2081–2089.
Wan Ngah W. S, Endud C. S and Mayanar R. 2002, Removal of copper(II) ions from aqueous solution onto chitosan and cross-linked chitosan beads. Reactive and Functional Polymers, 50(2), pp 181-190.
Wan Ngah W.S. and Isa I.M.J. 1998. Comparison study of copper ion adsorption on chitosan, dowex A-1, and zerolit 225. Appl. Polym. Sci. 67, pp. 1067–1070.
Wan Ngah W.S., Endud C.S.andMayanar R. 2002, Removal of copper(II) ions from aqueous solution onto chitosan and cross-linked chitosan beads, Reactive Functional Polym. J. 50, pp. 181–190.
Wang, X.Y. and Yu, J., 1998. Adsorption and degradation of synthetic dyes on the mycelium of Trametese versicolor. Water Sci. Technol. 38, pp. 233–238.
Wase, J., Forster, C.F., 1997. Biosorbents for Metal Ions; Taylor & Francis, London, pp. 238.
Watonabe, T and Ogawa, K. 1929 Activated carbon for purifying copper electrolytes. Chem. Abs. 24: 1037.
Whang, J.S., Young, D and Pressman, M. Design of soluble sulphide precipitation system for heavy metal removal. Ind. Waste.Proc. 13th Mid Atlantic Conference. Ann Arbor Science (1981) pp 63-71.
WHO Environmental Health Criteria 101, Methyl Mercury, Geneva, World Health Organization (1990) 68.
Wilson M. W. and Edyvean R. G. 1994, Biosorption for the removal of heavy metals from industrial wastewaters. Institution of Chemical Engineers Symposium Series 1994. Environ. Biotechnol .pp 89-91.
Wing R. E. 1983, Dissolved heavy-metal removal by insoluble starch xanthate (ISX). Environ. Prog. 2(4),pp 269-272.
Wing R. E. and Rayford W. E. 1977, Heavy metal removal processes for plating rinse waters. Proceedings of the 32nd Industrial Waste Conference May 10-12, 1977. Purdue University, Lafayette, IN.
Wing, R.E. Process for heavy metal removal from plating wastewaters. In Proc. 1st Annual EPA3AES. Conference on Advanced Pollution Control for metal finishing industry, Lake Buena Vista, Florida (1978).
Wing, R.E., Navickis, L.D., Jasberg, B.K., and Rayford, W.E. Removal of heavy metals from industrial wastewaters using insoluble starch xanthate. EPA-600/22-78-085 (1978).
Wong Y.C., Szeto Y.S., Cheung W.H. and McKay G., 2004, Adsorption of acid dyes on chitosan-equilibrium isotherm analyses, Proc. Biochem. 39, pp. 693–702.
Woolard C.D., Strong J and Erasmus C.R., 2002 Evaluation of the use of modified coal ash as a potential sorbent for organic waste streams, Appl. Geochem. 17, pp. 1159–1164.
Wrobel, D., Boguta, A. and Ion, R.M., 2001. Mixtures of synthetic organic dyes in a photoelectronic cell. J. Photochem. Photobiol., A Chem. 138, pp. 7–22.
Wu F.C., Tseng R.L and Juang R.S., 1999 Preparation of activated carbons from bamboo and their adsorption abilities for dyes and phenol, J. Environ. Sci. Health A 34, pp. 1753–1775.
Wu F.C., Tseng R.L and Juang R.S., 2000, Comparative adsorption of metal and dye on flake- and bead-types of chitosan prepared from fishery wastes, J. Hazardous Mater. B73, pp. 63–75.
Wu F.C., Tseng R.L. and R.S. Juang, 2001, Enhanced abilities of highly swollen chitosan beads for color removal and tyrosinase immobilization, J. Hazardous Mater. B81, pp. 167–177.
Wu F.C., Tseng R.L. and Juang R.S., 2001, Kinetic modelling of liquid-phase adsorption of reactive dyes and metal ions on chitosan, Water Res. 35, pp. 613–618.
Xu Y. and R.E. Lebrun, 1999, Investigation of the solute separation by charged nanofiltration membrane: effect of pH, ionic strengh and solute type, J. Membr. Sci., 158 pp 93.
Yadava K. P., Tyagi B. S. and Singh V. N. 1991, Effect of temperature on the removal of lead(II) by adsorption on China clay and wollastonite. J. Chem. Tech. Biotechnol. 51, pp 47-60.
Yadava K.P, Tyagi B.S.and Singh V.N. 1991. Effect of temperature on the removal of lead (II) by adsorption on China clay and wollastonite J. Chem. Biotechnol. 51, pp. 47–60.
Yamuna R.T. Utilisation of biogas waste slurry as adsorbent for removal of dyes. MPhil dissertation. Submitted to Bharathiar University, Coimbatore (1990).
Yang T. C. and Zall R. R. 1984. Absorption of metals by natural polymers generated from seafood processing wastes. Ind.Eng. Chem. Prod. Res. Dev. 23, pp 168-172.
Yang, J. and Volesky, B. 1999. Removal and concentration of uranium by seaweed biosorbent. Internat. Biohydrometallurgy Symposium Proceedings, 1999, volume B, Ballester, A. & Amils, R. (eds.) Elsevier Sciences, Amsterdam, The Netherlands : pp.483-492.
Yang, J. and Volesky, B. 1999. Biosorption of uranium by Sargassum biomass. Wat. Res . 33, 3357-3363.
Yang, J. and Volesky, B. 1999. Cd biosorption rate in protonated Sargassum biomass. Environ. Sci. Technol. 33, pp 751-75
Yang, Y.Q., Wyatt, D.T. and Bahorsky, M., 1998. Decolorization of dyes using UV/H2O2 photochemical oxidation. Text. Chem. Color. 30, pp. 27–35.
Yoshida, H, Fukuda S, Okamoto A and Kataoka, J, 1991. Recovery of direct dye and acid dye by adsorption on chitosan fiber. Wat Sci Technol, 23, pp 1667-1676.
Young, L. and Yu, J., 1997. Ligninase-catalysed decolorization of synthetic dyes. Water Res. 31, pp. 1187–1193.
Youssef, B.M 1993. Adsorption of acid dyes by cellulose derivatives. Amer. Dyestuff Rep., 82, pp 30-33.
Zakaria A. Mohamed 2001, Removal of cadmium and manganese by a non-toxic strain of the freshwater cyanobacterium Gloeothece magna Water Research, 35 (18), pp 4405-4409
Zarraa M. A. 1995, A study on the removal of chromium (VI) from waste solutions by adsorption on to sawdust in stirred vessels. Adsorption Sci. Technol.12(2), pp 129-138.
Zhou J.L and Banks C.J., 1991. Removal of humic acid fraction by Rhizopus arrhizus: uptake and kinetic studies. Environ. Technol. 12, pp. 859–869.
Zhou J.L.and Banks C.J., 1993. Mechanism of humic acid colour removal from natural waters by fungal biomass biosorption. Chemosphere 27, pp. 607–620.
Zhou W. and Zimmermann W.1993. Decolourization of industrial effluents containing reactive dyes by actinomycetes. FEMS Microbiol. Lett. 107, pp. 157–162.
Zollinger H. Azo dyes and pigments. Colour chemistry-synthesis, properties and applications of organic dyes and pigments. New York: VCH, 1987. p. 92–100.
Zouboulis A.I. and Kydros K.A. 1993. Use of red mud for toxic metals removal: The case of nickel J. Chem. Technol. Biotechnol. 58, pp. 95–101.